Q: 1.The lowest first ionisation energy would be associated with which of the following structures?...
-
Upload
elmer-talbert -
Category
Documents
-
view
222 -
download
2
Transcript of Q: 1.The lowest first ionisation energy would be associated with which of the following structures?...
![Page 1: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/1.jpg)
![Page 2: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/2.jpg)
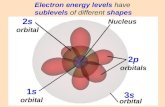
Q: 1. The lowest first ionisation energy would be associated with which of the following
structures?
a] 1s2 2s2 2p6 3s1
b] 1s2 2s2 2p5
c] 1s2 2s2 2p6
d] 1s2 2s2 2p6 3s2 3p2
Ans : a] 1s22s2 2p6 3s1
![Page 3: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/3.jpg)
Q: 2. Which of the following combinations contains only isoelectronic species?
a] N3-, O2-, Cl-
b] F-, Ar, S2-, Cl-
c] P3-,S2-, Cl-
d] N3-, F-, O2-, Ar
Ans: c] P3-,S2- ,Cl-
![Page 4: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/4.jpg)
Q: 3. Fluorine has unexpectedly lower electronic affinity than that of chlorine due to.
![Page 5: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/5.jpg)
a] Big size of fluorine, which results in increase in the electron-electron repulsion 2P subshell
b] Small size of fluorine, which results in increase in the electron-electron repulsion 2p-subshell.
c] Decrease in electron-electron repulsion d] No electron-electron repulsion in 2p-
subshell
Ans: b] Small size of fluorine, which results in increase in the electron-electron repulsion 2p-subshell
![Page 6: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/6.jpg)
Q: 4. The most electronegative element in the periodic table is.
a] Nitrogenb] Oxygenc] Chlorined] Fluorine
Ans: d] Fluorine
![Page 7: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/7.jpg)
Q: 5. Which one of the following has the smallest radius?
a] Cl-
b] S2-
c] K+
d] Ca2+
Ans: d] Ca2+
![Page 8: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/8.jpg)
Q: 6. Atomic size increase as we move down a group because.
a] effective nuclear charge increasesb] Atomic mass increases. c] Additive elements are accommodated in new electron level.d] Atomic number increases.
Ans: c] Additive elements are accommodated in new electron level.
![Page 9: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/9.jpg)
Q: 7. Electrons which are characterised by the outer shell configuration ns1 to ns2 np6 are collectively called.
a) transition elements.b) representative element .c) lanthanides.
d) inner transition elements
Ans: b) representative element .
![Page 10: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/10.jpg)
Q:8. Atomic numbers of V,Cr, Mn and Fe are 23,24,25and 26 respectively, which one of these may have the highest second
ionisation potential
a) Mn b) Fe
c) vd) Cr
Ans:- d) Cr E.c- of Cr – 3d5 ,4s1, as 3d- orbital is
exactly half filled, removal of election is difficult.
![Page 11: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/11.jpg)
Q: 9. Diagonal relationship is shown by
a) all elements with their diagonally opposite elements.
b) all elements of 3rd and 4th periods c) some elements of 2nd and 3rd periods. d) elements of d-block.
Ans: c) some elements of 2nd and 3rd periods.
![Page 12: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/12.jpg)
Q :10. Lithium is not stored in kerosene. It is kept wrapped in paraffin wax because
a) it reacts with kerosene .
b) Lithium floats over kerosene as its density is low c) Lithium is a soft metal. d) Lithium sinks in kerosene
Ans : b) Lithium floats over kerosene as its density is low .
![Page 13: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/13.jpg)
Q : 11. compounds of group 2 elements are less soluble in water than the corresponding alkali metal salts due to
a) their high ionisation energy
b) their high lattice energy c) less basic character d) low electronegativity values.
Ans : b) their high lattice energy
![Page 14: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/14.jpg)
Q :12. The Density of “Ca” is less than that of “Mg” because
a) Nuclear charge of “Ca” is more than “Mg”
b) Size of “Ca” is less than “Mg” c) Vacant 3d orbital of “Ca” d) completely filled 3d orbital’s .
Ans: c) Vacant 3d orbital of “Ca” leads to the much increasing atomic
volume hence density decreases
![Page 15: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/15.jpg)
Q :13. Oxide of an element whose electronic configuration is 1s22s22p63s1 is
a) Basicb) Acidic c) neutral d) Amphoteric
Ans: a) Basic
![Page 16: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/16.jpg)
Q : 14. carbon -60, contains
a) 12 five membered and 20- six membered rings b) 12 five membered and 20- five membered
rings c) Same no of five and six membered rings d) No five and six membered rings
Ans: a) 12 five membered and 20- six memberd rings
![Page 17: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/17.jpg)
Q :15. A radio station broadcasts on a frequency of 1368 KHz, what is the wavelength of
electromagnetic radiation emitted by transmitter
a) 219300m b) 219.3m c) 2.1933md) 0.2193m
Ans: b) 219.3m
![Page 18: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/18.jpg)
C = 3 x 108 m/s λ = ?
= 219.3 m
=
= 3 x 108 1368 x 103
![Page 19: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/19.jpg)
Q :16. Energy of a wave of wavelength 400A0 is
a) 495 x 10-19J b) 4.95 x 10-18Jc) 4.95 x 10-19J d) 495 x 10-18J
Ans: c) 4.95 x 10-19J
![Page 20: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/20.jpg)
λ = 400 A0 = 400 x 10-10 m
E = h = h
= 6.6 x 10-34 x 3 x 108 400 x 10-10
E = 4.95 x 10-19J
![Page 21: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/21.jpg)
Q : 17. What will be de- Broglie wave length of an
electron moving with a velocity of1.2 x10-5m/s
a) 6.068 x 10-9m
b) 3.133 x 10-37 mc) 6.626 x 10-9 md) 6.018 x 10-7 m
Ans: A) 6.068 x 10-9 m
![Page 22: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/22.jpg)
λ = h/mc h = 6.62 x 10-34 js
m = 9.1 x 10-31 Kg c = 1.2 x 105 m/s
λ =
![Page 23: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/23.jpg)
Q:18 . The Quantum numbers for the last electron of an element are given below n = 2, l = 0, m = 0, s = + 1/2 , The atom is
a) Lithium b) Beryllium c) Hydrogen d) Boron
Ans: a) Lithium
![Page 24: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/24.jpg)
Q:19. Which of the following set of Quantum numbers is not possible n l m s a) 3 2 -1 0b) 3 2 -1 + ½ c) 3 2 1 + ½ d) 3 2 1 - ½
Ans: a) n=3 l=2 m=-1 s=0
![Page 25: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/25.jpg)
Q:20. Total number of neutrons in dipositive zinc ion with mass 70 is
a) 34b) 40 c) 36d) 38
Ans b) 40
A = 70, z = 30 n = A-z
= 40
![Page 26: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/26.jpg)
Q:21. Orbital angular momentum of an electron in 2s orbital is
A] + ½ h/2π
B] h/2π
C] √2 h/2π
D] Zero
Ans: d] Zero
Orbital angular momentum = l h/2π
For ‘S’ orbital l=0 0 x h/2π = 0
![Page 27: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/27.jpg)
Q: 22. What is the wave number of radiation emitted when an electron jumps fro n =3 to n= 1 in a hydrogen atom (R=107/m)
a) 1.11 x 108/m b) 8.9 x 108/mc) 8.9 x 106/m d) 8.9 x 107/m
Ans: c) 8.9 x 106/m
![Page 28: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/28.jpg)
Q : 23. The ion that is isoelectronic with CO is
a) b) c)
d) CN‑
Ans : CN-
No of electrons in co = 6+8 = 14
In CN- 6+7+1 = 14
![Page 29: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/29.jpg)
Q: 24. It is impossible to measure simultaneously the position and momentum of a small particle
with absolute accuracy, This is stated by
a) Hund b) pauli c) Heisenberg d) Bohr
Ans : c) Heisenberg
![Page 30: Q: 1.The lowest first ionisation energy would be associated with which of the following structures? a] 1s 2 2s 2 2p 6 3s 1 b] 1s 2 2s 2 2p 5 c] 1s 2 2s.](https://reader036.fdocuments.net/reader036/viewer/2022062511/551c0547550346a34f8b4e1b/html5/thumbnails/30.jpg)
Q : 25. 4s orbital gets filled before 3d because
a) 4s has more energy than 3db) 4s has same energy energy as that 3d c) ( n + 1 ) Value for 4s is more than 3dd) ( n + 1 ) Value for 4s is less than 3d
Ans: d) ( n + 1 ) Value for 4s is less than 3d
![02. ESTRUCTURA ATÓMICA. CLASIFICACIÓN …platea.pntic.mec.es/~jrodri5/2bachquimica/PAU/02_ATOMO_SIST... · ... Li 2+ (Z = 3): 1s ; F (Z = 9): 1s 2 2s 2 2p 5; ... · [Br-] = s ·](https://static.fdocuments.net/doc/165x107/5baa5ede09d3f2cf6d8c4d6e/02-estructura-atomica-clasificacion-jrodri52bachquimicapau02atomosist.jpg)


![Übungen zur Vorlesung Aufbau technischer Werkstoffe · NaCl: Na11: 1s22s22p63s1 [1s22s22p6]++e- Cl 17 : 1s 2 2s 2 2p 6 3s 2 3p 5 +e - [1s 2 2s 2 2p 6 3s 2 3p 6 ] - b) Welchem Bindungstyp](https://static.fdocuments.net/doc/165x107/5dd10b13d6be591ccb63f16d/oebungen-zur-vorlesung-aufbau-technischer-nacl-na11-1s22s22p63s1-1s22s22p6e-.jpg)
![Electron Configuration Na: 1s 2 2s 2 2p 6 3s 1 Na: [Ne] 3s 1.](https://static.fdocuments.net/doc/165x107/56649cdc5503460f949a747c/electron-configuration-na-1s-2-2s-2-2p-6-3s-1-na-ne-3s-1.jpg)


![ELECTRON CONFIGURATIONS · Longhand is 1s 2 2s 2 2p 6 3s 2 3p 5 You can abbreviate the first 10 electrons with a noble gas, Neon. [Ne] replaces 1s 2 2s 2 2p 6 Then continue as you](https://static.fdocuments.net/doc/165x107/5e7709b442f452627a541b17/electron-configurations-longhand-is-1s-2-2s-2-2p-6-3s-2-3p-5-you-can-abbreviate.jpg)

![Il carbonio (Z=6) 2 possibilità: configurazione elettronica del C nello stato fondamentale di tripletto di spin 1s 2s 2p + 2p o 2p - [He] (2s) 2 (2p) 2.](https://static.fdocuments.net/doc/165x107/5542eb50497959361e8bfb78/il-carbonio-z6-2-possibilita-configurazione-elettronica-del-c-nello-stato-fondamentale-di-tripletto-di-spin-1s-2s-2p-2p-o-2p-he-2s-2-2p-2.jpg)
![1 Building Atoms - Period 3 Na Mg Al Si P S Cl Ar 1s 2 2s 2 2p 6 3s 1 [Ne]3s 1 1s 2 2s 2 2p 6 3s 2 [Ne]3s 2 1s 2 2s 2 2p 6 3s 2 3p 1 [Ne]3s 2 3p 1 1s 2.](https://static.fdocuments.net/doc/165x107/56649d445503460f94a20e8c/1-building-atoms-period-3-na-mg-al-si-p-s-cl-ar-1s-2-2s-2-2p-6-3s-1-ne3s.jpg)