cholangiocarcinoma, gallbladder cancer, common bile duct, cystic duct, intrahepatic, perihilar
Primary intrahepatic cholangiocarcinoma with sarcomatous … · 2018-11-26 · Primary intrahepatic...
Transcript of Primary intrahepatic cholangiocarcinoma with sarcomatous … · 2018-11-26 · Primary intrahepatic...

CASE REPORT Open Access
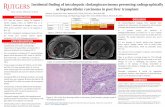
Primary intrahepatic cholangiocarcinomawith sarcomatous stroma: case report andreview of the literatureKyohei Yugawa1,2* , Tomoharu Yoshizumi1, Yohei Mano1, Noboru Harada1, Shinji Itoh1, Toru Ikegami1, Yuji Soejima1,Nobuhiro Fujita3, Kenichi Kohashi2, Shinichi Aishima4, Yoshinao Oda2 and Masaki Mori1
Abstract
Background: Hepatic carcinosarcomas, which include both carcinomatous and sarcomatous elements, are uncommonin adults. Although carcinosarcoma in hepatocellular carcinoma is occasionally reported, carcinosarcoma in intrahepaticcholangiocarcinoma (ICC) is an extremely rare ICC variant. Few such cases have been reported in English and no largestudy of its clinicopathological features exists.
Case presentation: Here, we report a 60-year-old man with an asymptomatic hepatic B infection who developedhepatic carcinosarcoma from an otherwise normal liver. The 6.0-cm tumor was accidentally discovered by PET-CTin a cancer examination. Serum examinations showed no elevation of tumor markers. He underwent left and caudatelobectomy of the liver. The diagnosis of intrahepatic cholangiocarcinoma with sarcomatous stroma was based onthorough pathologic examination and immunohistochemical staining. The tumor exhibited adenocarcinomatous andsarcomatous components; the adenocarcinomatous element was positive for epithelial markers, the sarcomatouselement was positive for mesenchymal markers, but negative for epithelial markers. The patient made an uneventfulrecovery after surgery. At present, 14 months after surgery, he remains well with no evidence of tumor recurrence.
Conclusions: We report an unusual case of hepatic carcinosarcoma (intrahepatic cholangiocarcinoma withsarcomatous stroma) and discuss the etiology and prognosis of this rare disease.
Keywords: Hepatic carcinosarcoma, Intrahepatic cholangiocarcinoma, Etiology, Radiology and pathology
IntroductionHepatic carcinosarcoma (HCS) is a rare tumor, which hasbeen defined by the World Health Organization (WHO)as a malignant tumor containing an intimate mixture ofcarcinomatous (either hepatocellular carcinoma [HCC] orintrahepatic cholangiocarcinoma [ICC]) and sarcomatouselements [1]. The incidence of primary hepatic sarcoma isvery low, but sarcomatous change often occurs in severalepithelial tumors (including HCC) [2, 3]. Although car-cinosarcoma with HCC has occasionally been reported[3–7], few reports of ICC with carcinosarcoma havebeen reported in English. Because of the scarcity of
these reports, preoperative diagnosis of ICC with carci-nosarcoma is challenging; little is known about its eti-ology and prognosis.We herein present a very rare case of primary intrahe-
patic cholangiocarcinoma with sarcomatous stroma,confirmed by pathology following resection, and discussthe etiology and prognosis of its radiological imagingand pathology.
Case presentationA 60-year-old man was admitted to our hospital with aliver tumor, which was discovered during fluorodeoxy-glucose positron emission tomography-computed tom-ography (PET-CT) as a cancer examination. He had ahistory of hepatitis B virus infection (positive for hepa-titis B virus antigen), but was asymptomatic, showed no
* Correspondence: [email protected] of Surgery and Science, Graduate School of Medical Sciences,Kyushu University, Maidashi 3-1-1, Higashi-ku, Fukuoka 812-8582, Japan2Department of Anatomic Pathology, Graduate School of Medical Sciences,Kyushu University, Maidashi 3-1-1, Higashi-ku, Fukuoka 812-8582, JapanFull list of author information is available at the end of the article
© The Author(s). 2018 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, andreproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link tothe Creative Commons license, and indicate if changes were made.
Yugawa et al. Surgical Case Reports (2018) 4:138 https://doi.org/10.1186/s40792-018-0543-z

positive signs when examined, and had not had anymedical interventions.Analysis of serum tumor markers showed no elevated
carbohydrase antigen-19-9 (11.2 U/ml), carbohydraseantigen-125 (18.1 U/ml), or carcinoembryonic antigen(1.0 ng/ml). Other parameter levels were within normalranges. Gastroscopy and colonoscopy also showed nor-mal findings.Plane computed tomography (CT) scan revealed a
well-defined low-density mass, 6.0 cm in diameter, in thecaudate liver (Fig. 1a), which showed two different compo-nents in the enhanced CT scan. Contrast-enhanced CTscan showed the right tumor enhancement during the ar-terial phase and delayed washout in the late phase, butshowed the left component as a hypovascular lesion(Fig. 1b–d). Magnetic response imaging (MRI) showedboth of these components with low intensity on T1-weighted images (Fig. 2a), and right component of iso-highintensity and left component of heterogeneously high onT2-weighted images (Fig. 2b). It also showed higher in-tensity than with normal liver parenchyma ondiffusion-weighted imaging (DWI), with a high b valueof 1000 (Fig. 2c). Apparent diffusion coefficient (ADC)mean values of these two separated components were1.19 × 10− 3 mm2/s (right component) and 1.95 × 10− 3
mm2/s (left component). It was described as ahigh-intensity mass on the ADC map (Fig. 2d).Gadolinium-ethoxybenzyl-diethylene-triaminepentaacetic-acid (Gd-EOB-DTPA)-MRI showed the right tumor as a
hyperintense in the arterial phase (Fig. 2e) and the wholetumor as a hypointense mass in the hepatobiliary phase(Fig. 2f). [18F]-fluorodeoxyglucose positron tomography(FDG-PET) showed accumulation of [18F]-FDG at bothcomponents (Fig. 2g).The preoperative diagnosis, based on the imaging
studies, was an atypical ICC. After the patient under-went left and caudate lobectomy of the liver, macro-pathology of the resected specimen showed that thetumor measured 7.5 cm in the largest dimension. Thecut surface showed two different components, with awell-demarcated, yellowish, and nodular lobulated solidformation in the right, and an elastic soft and cystic for-mation on the left (Fig. 3a).Micropathologically, the right tumor component (indi-
cated as a hypervascular lesion on enhanced CT) showedan adenocarcinomatous element, composed of moderatelyto poorly differentiated adenocarcinoma, arranged in tra-becular and irregular tubular patterns, infiltrated into theliver parenchyma (Fig. 3b). The left component (whichappeared with heterogeneous high intensity on T2WI) wasa sarcomatous element, mainly composed of oval- tospindle-shaped cells with a focal dilated gland ductal struc-ture (Figs. 3c and 4a). These two components were mostlyseparate but with a small intermingled area with well-differ-entiated adenocarcinomatous and sarcomatous elements.There was no evidence of transitional feature between ade-nocarcinomatous and sarcomatous elements. The sur-rounding parenchyma showed no cirrhotic change.
a b
c d
Fig. 1 Contrast-enhanced abdominal computed tomography (CT). Plane CT scan shows a well-defined low-density mass (6.0 cm in diameter) inthe caudate liver (a) Contrast-enhanced CT scan showed right component (arrow) of the tumor enhancement during the arterial phases (b) anddelayed washout in the latter phases (c, d), but left component (arrowhead) as hypovascular lesion (b–d)
Yugawa et al. Surgical Case Reports (2018) 4:138 Page 2 of 9

In immunohistochemical (IHC) tests, the adenocar-cinoma cells were positive for cytokeratin-7 (CK7),cytokeratin-19 (CK19), CD56, and epithelial membraneantigen (EMA), but negative for hepatocellular carcin-oma markers such as Glypican-3 (date not shown).There were no histologic elements suggesting HCC. Thesarcomatous cells were positive for S-100, α-smooth muscleactin (SMA), and CD10, but negative for CK7, CK19,CD56, and EMA (Fig. 4b–g). The Ki67 index was 22% inthe sarcomatous elements (Fig. 4h). These findings led to a
pathological diagnosis of carcinosarcoma (ICC with sar-comatous stroma). The patient recovered uneventfully fromthe surgery, and at present, 14months later, he remainswell with no evidence of tumor recurrence.
ConclusionsPrimary HCS is very rare worldwide, comprising only1.8% to 9.4% of surgical or autopsy HCC cases [3, 8].Few cases have been reported in the English languagejournals and most have been of HCS in HCCs. However,
a b c d
e f g
Fig. 2 Magnetic response imaging (MRI) and [18F]-fluorodeoxyglucose position tomography (FDG-PET). MR images show both components withlow intensity on T1-weighted images (a) and right component (arrow) of iso-high intensity and left component (arrowhead) of heterogeneouslyhigh on T2-weighted images (b). DWI showed higher intensity than normal liver parenchyma with a high b value of 1000 (c). Its ADC value was1.19 × 10− 3 mm2/s (arrow on right) and 1.95 × 10− 3 mm2/s (arrowhead on left) (d). EOB-MRI showed right component (arrow) of the tumor as ahyperintense lesion but left component (arrowhead) as a hypointense lesion during the arterial phases (e) and hypointense mass in thehepatobiliary phase (f). FDG-PET shows accumulation of [18F]-FDG at both components (g)
a b
c
b
c
Fig. 3 Macroscopic and microscopic findings of sarcomatous ICC. a Cut surface shows (right) a well-demarcated, yellowish, nodular lobulatedsolid component and (left) an elastic soft, cystic component. Micropathologically, b the right component was a moderately-to-poorlydifferentiated adenocarcinoma, with a trabecular and irregular tubular pattern, infiltrated into liver parenchyma (hematoxylin and eosin [HE]staining × 100). c The left component was sarcomatous, mainly composed of oval- to spindle-shaped cells with focal dilated gland ductalstructure (H&E × 100)
Yugawa et al. Surgical Case Reports (2018) 4:138 Page 3 of 9

cases of primary HCS in ICCs are even more uncom-mon and ICC with carcinosarcoma has a much worseprognosis than simple ICC [9].In 1989, Craig et al. [10] first reported liver carcinosar-
comas as hepatic tumors with both an HCC and anon-spindle cell sarcoma and excluded non-hepatocyticepithelial elements. According to the WHO definition,HCS is “a malignant tumor containing an intimate mix-ture of carcinomatous (either hepatocellular or cholangio-carcinoma) and sarcomatous elements.” Both the WHOand Craig et al. distinguished HCS from collision tumors,and from carcinomas with foci of spindle-shaped epithelialcells, and included tumors designated as “hepatoblastoma,malignant mixed tumor, spindle cell carcinoma, or sarco-matoid carcinoma” [1]. Still, how to distinguish carcino-sarcoma from sarcomatoid carcinoma is controversial.Rosai [11] suggested that such mixed tumors should be di-agnosed as spindle cell carcinoma or sarcomatoid carcin-oma when the sarcomatous component is predominantlycomposed of spindle cells, but the epithelial cells are stillmorphologically and immunohistochemically identifiable.Wang et al. [12] suggested the absence of significant dif-ferences in survival rates and morphologies of sarcoma-tous components between sarcomatoid carcinoma andcarcinosarcoma, which implies that distinguishing be-tween primary sarcomatoid carcinoma and carcinosar-coma of the liver is clinically unnecessary [12]. Based onour pathological and IHC studies, and according to thedefinitions of WHO and Rosai, we diagnosed this tumoras “hepatic carcinosarcoma.”We searched PubMed to identify the published case
reports of ICC with sarcomatous change in the Englishliterature and used the terms “liver,” “sarcomatous,” “sar-comatoid,” “carcinosarcoma,” and “cholangiocarcinoma.”We reviewed the identified 27 patients, including ourpatient, the characteristics of which we here summarize(Table 1) [13–29]. In radiological images, low-densitymass with enhancement by contrast medium on CT,
hypointensity on T1WI, and hyperintensity on T2WI arereported to be key sarcomatous ICC features [30]. Asshown in Table 1, the identified radiological characteris-tics are similar to those of sarcomatous ICC. As in theprevious reports, the radiological images of the distin-guished sarcomatous component in the present casemight be identical with the dominant sarcomatoid ICC.However, the adenocarcinoma component might differfrom the ordinary ICC; hypervascular ICC is consideredto have less malignant potential than other ICCs [31].Nevertheless, the ADC mean value of two different com-ponents was 1.19 × 10− 3 mm2/s (ICC component) and1.95 × 10− 3 mm2/s (sarcomatous component), respect-ively. Lower mean ADC value is associated with moreaggressive histopathology and poorly differentiated ICCs[32]. The ADC value indicates that the ICC componenthas more malignant potential than the sarcomatouscomponent.In previous reports, pathologists have proposed two
pathogeneses for HCS. One theory, supported by IHC,holds that HCSs develop from multipotent progenitor orstem cells of the liver. This theory indicates dual differ-entiation by an immature malignant cell and shows thatcombination tumors may originate from single toti-potent stem cell, which differentiates in separate epithe-lial and mesenchymal directions [33]. The alternativetheory, which is based on observation of transitionalzones, posits that conventional tumor cells transform ordedifferentiate into sarcomatous components from hepa-tocellular or cholangiocellular carcinoma. Some reportssupport the idea that malignant cells might change intomultipotent immature cells [34, 35].In our case, based on IHC findings for our carcinosar-
coma specimen, we support the theory that the carcino-sarcoma developed from hepatic progenitor cells orstem cells, which differentiates separately into both epi-thelial and mesenchymal elements. These two differentelements were largely separated, although a focal area
a b c
e f
d
g h
Fig. 4 Immunohistochemical staining. Microscopic findings for carcinomatous and sarcomatoid mixed area. a H&E staining revealed that adenocarcinomacells were positive for b CK7, c CK19, and d EMA; and sarcomatous cells were positive for e S-100, f SMA, and g CD10 (a–g: × 100), but negative for CK7,CK19 and EMA. h Ki67 index was 22% in the sarcomatous elements (× 400)
Yugawa et al. Surgical Case Reports (2018) 4:138 Page 4 of 9

Table
1Summarized
data
onpu
blishe
drepo
rtsconcerning
ICCwith
sarcom
atou
schange
Autho
rYear
Age
(years)/Gen
der
Tumor
locatio
nTumor
size
(cm)
PlainCT
Enhancem
entCT
T1WI
T2WI
Sasakiet
al.[13]
1991
79/M
Leftlobe
8ND
ND
ND
ND
Haratakeet
al.[14]
1992
59/M
Leftlobe
Fist-sized
ND
ND
ND
ND
Nakajim
aet
al.[15]
1993
84/F
Hep
atichilum
3.5
ND
ND
ND
ND
43/F
Righ
tlobe
14ND
ND
ND
ND
73/F
Leftlobe
7ND
ND
ND
ND
37/M
Leftlobe
10ND
ND
ND
ND
64/M
Leftlobe
7.5
ND
ND
ND
ND
52/M
Righ
tlobe
7.5
ND
ND
ND
ND
69/M
Leftlobe
10ND
ND
ND
ND
Imazuet
al.[16]
1995
77/M
Segm
ent2
6Low
density
Ring
enhancem
ent
Low
intensity
Low
intensity
Hon
daet
al.[17]
1996
61/F
Righ
tlobe
Num
erou
svario
usly
sized
ND
ND
ND
ND
Matsuoet
al.[18]
1999
77/F
Leftlobe
7.7
Low
density
Ring
enhancem
ent
Iso-
tolow
intensity
Heterog
eneo
ushigh
and
low
intensity
Itamotoet
al.[19]
1999
70/M
Segm
ent5/6
10.7
Low
density
Poor
ND
ND
Shim
adaet
al.[20]
2000
70/M
Segm
ent5
3.4
ND
ND
ND
ND
55/M
Segm
ent7/8
6.7
ND
ND
ND
ND
74/F
Segm
ent8
4.0
ND
ND
ND
ND
64/F
Segm
ent4
8.0
ND
ND
ND
ND
Kaiborietal.[21]
2003
69/F
Lateralseg
men
t20
Low
density
Ring
enhancem
ent
ND
ND
Sato
etal.[22]
2006
87/M
Leftlobe
4.0
ND
ND
ND
ND
Tsou
etal.[23]
2008
69/F
Leftlobe
2.5
Low
density
Ring
enhancem
ent
ND
ND
Malho
traet
al.[24]
2010
60/F
Segm
ent5
20ND
Heterog
eneo
usmass
ND
ND
Inou
eet
al.[25]
2012
61/M
Leftlateralseg
men
t20
Heterog
eneo
usmass
Ring
enhancem
ent
ND
ND
Nakajim
aet
al.[26]
2012
77/F
Righ
tlobe
14Low
density
Heterog
eneo
usen
hancem
ent
Low
intensity
Iso-
tohigh
intensity
Watanabeet
al.[27]
2014
62/M
Hep
atichilum
5.0
Low
density
Ring
enhancem
ent
ND
ND
Kim
etal.[28]
2015
67/F
Leftlateralseg
men
t4.5
Low
density
Heterog
eneo
usen
hancem
ent
ND
ND
Boon
sinsukhet
al.[29]
2018
45/M
Righ
tlobe
7.0
Low
density
Mild
delayeden
hancem
ent
ND
ND
Our
case
2018
60/M
Caudate
lobe
7.5
Low
density
Heterog
eneo
usen
hancem
ent
Low
intensity
Iso-
tohigh
intensity
CKcytokeratin
,CA19-9
carboh
ydrate
antig
en19
-9,C
Tcompu
tedtomog
raph
y,EM
Aep
ithelialm
embran
ean
tigen
,ICC
intrah
epaticcholan
giocarcino
ma,
HCC
hepa
tocellularcarcinom
a,IHCim
mun
ohistochem
ical,
SMAsm
ooth
muscleactin
,Vim
vimen
tin,N
Dno
tde
scrib
ed
Yugawa et al. Surgical Case Reports (2018) 4:138 Page 5 of 9

Table
1Summarized
data
onpu
blishe
drepo
rtsconcerning
ICCwith
sarcom
atou
schange
(Con
tinued)
Autho
rTreatm
ent
Carcino
matou
scompo
nent
Sarcom
atou
scompo
nent
Distribution
Transitio
nal
feature
IHCof
carcinom
atou
scompo
nent
IHCof
sarcom
atou
scompo
nent
Preo
perative
diagno
sis
Sasakiet
al.[13]
Non
eAde
nosquamou
scarcinom
aSpindleand
pleo
morph
iccells
ND
ND
Keratin
+,EMA+,
Vim+
ND
Haratakeet
al.[14]
Non
ePo
orlyaden
ocarcino
ma
Spindlecells
Interm
ingled
ND
EMA+,C
EA+,C
K+Vim+
Liverabscess
Nakajim
aet
al.[15]
Non
eMod
erately
aden
ocarcino
ma
Spindleand
pleo
morph
iccells
Interm
ingled
+Keratin
+,EMA+
Keratin
+,EMA+,
CA19-9+
ND
Righ
tlobe
ctom
yMod
erately
aden
ocarcino
ma
Spindlecells
Interm
ingled
+Keratin
+,EMA+
Keratin
+,EMA+,
Vim+
ND
Anti-cancer
chem
othe
rapy
Mod
erately
aden
ocarcino
ma
Spindleand
pleo
morph
iccells
Interm
ingled
+Keratin
+,EMA+
Non
eND
Non
eMod
erately
aden
ocarcino
ma
Spindleand
pleo
morph
iccells
Interm
ingled
+Keratin
+,EMA+
Keratin
+,EMA+,
Vim+
ND
TAE
Poorlyaden
ocarcino
ma
Spindleand
pleo
morph
iccells
Interm
ingled
+Keratin
+,EMA+
Keratin
+,EMA+
ND
TAE
Poorlyaden
ocarcino
ma
Spindleand
pleo
morph
iccells
Interm
ingled
+Keratin
+,EMA+
Keratin
+,EMA+,
CEA
+ND
Leftlobe
ctom
yPo
orlyaden
ocarcino
ma
Spindlecells
Interm
ingled
+Keratin
+,EMA+
Non
eND
Imazuet
al.[16]
Lateral
segm
entectom
yGland
ular
form
ation
Spindlecells
Interm
ingled
ND
Keratin
+,C
EA+,
Vim+,
Keratin
+,C
EA+,
Vim+,
ICC
Hon
daet
al.[17]
Non
eMod
eratelyto
poorly
aden
ocarcino
ma
Rhabdo
idcells
Separated
(interm
ingled
atthebo
rder)
+Keratin
+Keratin
+,C
EA-,
Vim+
ICCwith
periton
itis
carcinom
atosa
Matsuoet
al.[18]
Leftlobe
ctom
yMod
eratelyto
poorly
aden
ocarcino
ma
Spindlecells
Interm
ingled
+EM
A+,C
K+,C
EA+
Vim+,Epithelial
markers-
Liverabscess
Itamotoet
al.[19]
Righ
tlobe
ctom
yMod
erately
aden
ocarcino
ma
Spindlecells
Interm
ingled
+CA19-9+,EMA+
Keratin
+,Vim
-Keratin
+,EMA+,
Vim-H
Recurren
tHCC
Shim
adaet
al.[20]
Cen
tral
bisegm
entectom
yPo
orlyaden
ocarcino
ma
Spindlecells
Interm
ingled
+EM
A+,Keratin+,
CEA
+,Vim
+EM
A+,Keratin+,
Vim+
ND
Partialh
epatectomy
Mod
eratelyto
poorly
aden
ocarcino
ma
Spindleand
pleo
morph
iccells
Interm
ingled
+EM
A+,Keratin+
EMA+,Keratin+,
Vim+
ND
Righ
tlobe
ctom
yPo
orly
aden
ocarcino
ma
Spindlecells
Interm
ingled
+EM
A+,Keratin+,
CEA
+,Vim
+EM
A+,Keratin+,
CEA
+,Vim
+ND
Lefttriseg
men
tectom
yMod
eratelyto
poorlyaden
ocarcino
ma
Spindlecells
Interm
ingled
+EM
A+,Keratin+,
CEA
+,C
A19-9+,
AFP+,Vim
+
EMA+,Keratin+,
CEA
+,C
A19-9+,
Vim+
ND
Kaiborietal.[21]
Lateral
segm
entectom
yMod
erately
aden
ocarcino
ma
Spindleand
pleo
morph
iccells
Interm
ingled
+ND
Vim+,EMA+,
CK+
Leiomyosarcom
a
Sato
etal.[22]
Non
eMod
erately
aden
ocarcino
ma
Roun
dcells
Interm
ingled
ND
CK7+,C
K19+
,CAM5.2+
,CA19-9+
CK7+,C
K19+
,CAM5.2+
,Vim
+ICC
Yugawa et al. Surgical Case Reports (2018) 4:138 Page 6 of 9

Table
1Summarized
data
onpu
blishe
drepo
rtsconcerning
ICCwith
sarcom
atou
schange
(Con
tinued)
Tsou
etal.[23]
Segm
entectom
yWelltomod
erately
aden
ocarcino
ma
Spindleandpleo
morph
iccells
Interm
ingled
ND
ND
CK7+,Vim
+ND
Malho
traet
al.[24]
Lateral
segm
entectom
yMod
erately
aden
ocarcino
ma
Pleo
morph
icspindle
cells
Interm
ingled
ND
CAM5.2,EM
A+,A
E1/
AE3+,C
K7+,C
K19+
,CEA
+
Vim+,Epithelial
markers-
ND
Inou
eet
al.[25]
Lateral
segm
entectom
yMod
erately
aden
ocarcino
ma
ND
ND
+CK7+,C
K19+
Vim+,Keratin-1+
GIST
Nakajim
aet
al.[26]
Righ
the
patic
triseg
men
tectom
yand
caud
atelobe
ctom
y
Mod
erately
aden
ocarcino
ma
Spindlecells
and
chon
drosarcomatou
schange
Interm
ingled
AE1+
Vim+,Keratin-
CCCor
cystaden
ocarcino
ma
Watanabeet
al.[27]
Extend
edrig
hthe
mihep
atectomy
Mod
eratelyto
poorly
aden
ocarcino
ma
Spindleandpleo
morph
iccells
Interm
ingled
ND
CK+
CK+
,Vim
+ND
Kim
etal.[28]
Leftlobe
ctom
yWelltomod
erately
aden
ocarcino
ma
Pleo
morph
icandspindle
cells
with
osteoclastlike
giantcell
Interm
ingled
ND
CK19+
Vim+
ICC
Boon
sinsukhet
al.[29]
Righ
the
patectom
yMod
erately
aden
ocarcino
ma
Spindlecells
Interm
ingled
ND
Vim+,A
E1/AE3+,
CAM5.2+
,CK7+,
CK19+
ND
ICC
Our
case
Leftlobe
ctom
yand
caud
atelobe
ctom
yMod
eratelyto
poorly
aden
ocarcino
ma
Spindlecells
Separated
(interm
ingled
atthebo
rder)
CK7+,C
K19+
,EMA+
S-100+
,aSM
A+,
CD10+
ICC
Yugawa et al. Surgical Case Reports (2018) 4:138 Page 7 of 9

was intermingled, with small amounts of adenocarcino-matous elements and sarcomatous elements, with noevidence of transitional zones. As shown in Table 1, dis-tribution of these two elements was intermingled andtransitional area was observed in the most cases; how-ever, our histological results were different patterns fromprevious reported cases. Moreover, only the adenocar-cinoma cells were invading the hepatic parenchyma,whereas the sarcomatous element proliferated in thecaudate without invading, except for the intra-inferiorvena cava. In these morphological features, adenocarci-nomatous and sarcomatous elements can have differentproperties. Interestingly, our IHC results revealed thatthe adenocarcinoma elements were positive for epithelialmarkers (CK7, CK19, CD56, and EMA) but negative formesenchymal markers (S-100, alpha-SMA, and CD10),whereas the sarcomatous elements were positive for mes-enchymal markers, but negative for epithelial markers. Toour knowledge, no previous cases of separated ICC carcin-omatous and sarcomatous components shown by radio-logical and IHC findings have been reported.In conclusion, we reported an unusual case of hepatic
carcinosarcoma (ICC with sarcomatous stroma). The re-sults of the present case report supported the etiologicaltheory that sarcomatous elements developed from pro-genitor or stem cells, rather than redifferentiated fromepithelial elements. More epidemiological and patho-logical data will be further required to confirm the eti-ology and prognosis of the rare malignant tumor.
AbbreviationsADC: Apparent diffusion coefficient; CK19: Cytokeratin 19; CK7: Cytokeratin 7;CT: Computed tomography; DWI: Diffuse weighted imaging; EMA: Epithelialmembrane antigen; EOB-MRI: Gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid-enhanced magnetic response imaging; FDG-PET: [18F]-fluorodeoxyglucose position tomography; HCC: Hepatocellular carcinoma;HCS: Hepatic carcinosarcoma; ICC: Intrahepatic cholangiocarcinoma;IHC: Immunohistochemical; MRI: Magnetic response imaging; PET-CT: Fluorodeoxyglucose positron emission tomography- computedtomography; SMA: Smooth muscle actin; WHO: World Health Organization.
AcknowledgementsWe thank Marla Brunker, from Edanz Group (www.edanzediting.com/ac) forediting a draft of this manuscript.
FundingNot applicable.
Availability of data and materialsNot applicable.
Authors’ contributionsKY acquired the data and drafted the manuscript. KY, YM, TT, and TY performedthe surgeries. All other authors attended the patient postoperatively. All authorsread and approved the final manuscript.
Ethics approval and consent to participateNo applicable.
Consent for publicationOral informed consent was obtained from the patient for the publication ofthis case report and accompanying images.
Competing interestsThe authors declare that they have no competing interests.
Publisher’s NoteSpringer Nature remains neutral with regard to jurisdictional claims inpublished maps and institutional affiliations.
Author details1Department of Surgery and Science, Graduate School of Medical Sciences,Kyushu University, Maidashi 3-1-1, Higashi-ku, Fukuoka 812-8582, Japan.2Department of Anatomic Pathology, Graduate School of Medical Sciences,Kyushu University, Maidashi 3-1-1, Higashi-ku, Fukuoka 812-8582, Japan.3Department of Clinical Radiology, Graduate School of Medical Sciences,Kyushu University, Maidashi 3-1-1, Higashi-ku, Fukuoka 812-8582, Japan.4Department of Pathology and Microbiology, Faculty of Medicine, SagaUniversity Hospital, Saga, Japan.
Received: 19 October 2018 Accepted: 12 November 2018
References1. Ishak KG, Anthony PP, Niederau C, et al. Mesenchymal tumours of the liver.
In: Stanley RH, Lauri AA, editors. WHO international histological classificationof tumours pathology & genetics of tumours of the digestive system. Lyon:IARC Press; 2000. p. p198.
2. Matsuoka T, Watanabe H, Enjoji M. Pseudosarcoma and carcinosarcoma ofesophagus. Cancer. 1976;37:1546–55.
3. Kakizoe S, Kojiro M, Nakashima T. Hepatocellular carcinoma withsarcomatous change: clinicopathologic and immunohistochemical studiesof 14 cases. Cancer. 1987;59:310–26.
4. Akasofu M, Kawahara E, Kaji K, Nakanishi I. Sarcomatoid hepatocellularcarcinoma showing rhabdomyoblastic differentiation in the adult cirrhoticliver. Virchows Arch. 1999;434:511–5.
5. Fu Y, Kobayashi S, Kushida Y, et al. Sarcomatoid hepatocellular carcinomawith chondroid variant: case report with immunohistochemical findings.Pathol Int. 2000;50:919–22.
6. Koda M, Maeda Y, Matsunaga Y, Mimura K, Murawaki Y, Horie Y.Hepatocellular carcinoma with sarcomatous change arising afterradiofrequency ablation for well-differentiated hepatocellular carcinoma.Hepatol Res. 2003;27:163–7.
7. Yoshida N, Midorikawa Y, Kajiwara T, et al. Hepatocellular carcinoma withsarcomatoid change without anticancer therapies. Case Rep Gastroenterol.2013;7:169–74.
8. Maeda T, Adachi E, Kajiyama K, Takenaka K, Sugimachi K, Tsuneyoshi M.Spindle cell hepatocellular carcinoma. A clinicopathologic andimmunohistochemical analysis of 15 cases. Cancer. 1996;77:51–7.
9. Okabayashi T, Shima Y, Iwata J, Morita M. Surgical outcomes for 131 cases ofcarcinosarcoma of the hepatobiliary tract. J Gastroenterol. 2014;49:982–91.
10. Craig JR, Peters RL, Edmondson HA. Tumors of the liver and intrahepaticbile duct. In: Hartmann WH, Sobin LH, editors. Atlas of tumor pathology,second series, fascile 26. Washington: Armed Forces Institute of Pathology;1988. p. 179–80.
11. Rosai J. Rosai and Ackerman’s surgical pathology. Edinburgh: Mosby; 2004.12. Wang QB, Cui BK, Weng JM, Wu QL, Lin XJ. Clinicopathological
characteristics and outcome of primary sarcomatoid carcinoma andcarcinosarcoma of the liver. J Gastrointest Surg. 2012;16:1715–26.
13. Sasaki M, Nakanuma Y, Nagai Y, et al. Intrahepatic cholangiocarcinoma withsarcomatous transformation: an autopsy case. J Clin Gastroenterol. 1991;13:220–5.
14. Haratake J, Yamada H, Horie A, et al. Giant cell tumor-likecholangiocarcinoma associated with systemic cholelithiasis. Cancer. 1992;69:2444–8.
15. Nakajima T, Tajima Y, Sugano I, et al. Intrahepatic cholangiocarcinoma withsarcomatous change. Clinicopathologic and immunohistochemicalevaluation of seven cases. Cancer. 1993;72:1872–7.
16. Imazu H, Ochiai M, Funabiki T. Intrahepatic sarcomatouscholangiocarcinoma. J Gastroenterol. 1995;30:677–82.
17. Honda M, Enjoji M, Sakai H, et al. Case report: intrahepaticcholangiocarcinoma with rhabdoid transformation. J Gastroenterol Hepatol.1996;11:771–4.
Yugawa et al. Surgical Case Reports (2018) 4:138 Page 8 of 9

18. Matsuo S, Shinozaki T, Kanematsu T, et al. Intrahepatic cholangiocarcinomawith extensive sarcomatous change: report of a case. Jpn J Surg. 1999;29:560–3.
19. Itamoto T, Asahara T, Katayama K, et al. Double cancer - hepatocellularcarcinoma and intrahepatic cholangiocarcinoma with a spindle-cell variant.J Hepato-Biliary-Pancreat Surg. 1999;6:422–6.
20. Shimada M, Takenaka K, Rikimaru T, et al. Characteristics of sarcomatouscholangiocarcinoma of the liver. Hepatogastroenterology. 2000;47:956–61.
21. Kaibori M, Kawaguchi Y, Yokoigawa N, et al. Intrahepatic sarcomatoidcholangiocarcinoma. J Gastroenterol. 2003;38:1097–101.
22. Sato K, Murai H, Ueda Y, et al. Intrahepatic sarcomatoid cholangiocarcinomaof round cell variant: a case report and immunohistochemical studies.Virchows Arch. 2006;449:585–90.
23. Tsou YK, Wu RC, Hung CF, et al. Intrahepatic sarcomatoidcholangiocarcinoma: clinical analysis of seven cases during a 15-year period.Chang Gung Med J. 2008;31:599–605.
24. Malhotra S, Wood J, Mansy T, et al. Intrahepatic sarcomatoidcholangiocarcinoma. J Oncol. 2010;2010:701476. https://doi.org/10.1155/2010/701476.
25. Inoue Y, Lefor AT, Yasuda Y. Intrahepatic cholangiocarcinoma withsarcomatous changes. Case Rep Gastroenterol. 2012;6:1–4.
26. Nakajima T, Okumura A. A case of huge intrahepatic cholangiocarcinoma. JJpn Soc Gastroenterol. 2012;109:1590–7.
27. Watanabe G, Uchinami H, Yamamoto Y, et al. Prognosis analysis ofsarcomatous intrahepatic cholangiocarcinoma from a review of theliterature. Int J Clin Oncol. 2014;19:490–6.
28. Kim HM, Kim H, Park YN. Sarcomatoid cholangiocarcinoma with osteoclast-like giant cells associated with hepatolithiasis: a case report. Clin MolHepatol. 2015;21(3):309–13.
29. Boonsinsukh T, Viriyaroj V, Trongwongsa T, et al. Intrahepatic sarcomatouscholangiocarcinoma: case report and review of the literature. Case RepSurg. 2018;2018:3862575. https://doi.org/10.1155/2018/3862575.
30. Gu KW, Kim YK, Min JH, Ha SY, Jeong WK. Imaging features of hepaticsarcomatous carcinoma on computed tomography and gadoxetic acid-enhanced magnetic resonance imaging. Abdom Radiol. 2017;42:1424–33.
31. Fujita N, Asayama Y, NIshie A, Honda H. Mass-forming intrahepaticcholangiocarcinoma: enhancement patterns in the arterial phase ofdynamic hepatic CT - correlation with clinicopathological findings. EurRadiol. 2016;27:498–506.
32. Lewis S, Besa C, Wagner M, Taouli B. Prediction of histopathologic findingsof intrahepatic cholangiocarcinoma: qualitative and quantitative assessmentof diffusion-weighted imaging. Eur Radiol. 2018;28:2047–57.
33. Fayyazi A, Nolte W, Oestmann JW, Sattler B, Ramadori G, Radzun HJ.Carcinosarcoma of the liver. Histopathology. 1998;32:385–7.
34. Kubosawa H, Matsuzaki O, Kondo Y, Takao M, Sato N. Carcinosarcoma of theprostate. Acta Pathol Jpn. 1993;43:209–14.
35. Lin YS, Wang TY, Lin JC, Chen MJ, et al. Hepatic carcinosarcoma:clinicopathologic features and a review of the literature. Ann Hepatology.2013;12:495–500.
Yugawa et al. Surgical Case Reports (2018) 4:138 Page 9 of 9