Potassium Permanganate-SodiumSulfite
Transcript of Potassium Permanganate-SodiumSulfite

Applied Chemistry,
Vol. 12, t\o. I, May ?f1뻐, 97-100
Potassium Permanganate-Sodium Sulfite System을 이용한
Sparfloxacin의 흐름주입 화학발광법적 정량
Seikh Mafiz Alam · 이상학f
경북대학교 화학과
Flow-Injection Chemiluminescence Determination ofSparfloxacin Using Potassium Perrnanganate-Sodium Sulfite
System
Seikh Mafiz Alam . Sang Hak Lee tDepartment of Chemistry , Kyungpook National University ‘ Taegu. 702 - 70 1. Korea
Abstract
A rapid and sensitive chemiluminescence method has been described for thedetermination of sparfloxacin by combining the flow injection technique which is based on
its sensitizing effect on the weak chemiluminescence reaction between sodium sulfite and
acidic potassium permanganate. Under the experimental optimum conditions sparfloxacincan be determined over the concentration range of 1.6 xI 0 R to 2.9 x 10 Ii mo l/L with a
correlation coefficient of 0.9988 and a detection limit of 0.7x 10 8 mal/L (3 σ). The
relative standard deviatian (R.S.D.) far 11 repetitive determinations of 1.0 x 10 Ii mal!Lsparflaxacin is 0.9%.
Keywords: Chemiluminescence. sparfloxacin. KMn01 , NalS03
1. INTRODUCTIONSparfloxacin (5-a띠no - 1-cyclopropyl- 7 (α:,,3, 5 dimethyl-l-piperaziny t) -6.8-difluoro
1.4 -dihydro-4-oxo-3-quinoline carboxylic acid) , a broad spectrum antibacterial
fl l.loroquinolone active against some microorganisms induding Gram-positive and
Gram -negative bacteria [J ‘ 2J , is a new member to the class of fluroquinolones
which is used in the treatment of cuttaneous allergy. lung infection and urinary tract
infection [31. The activity against anaerobes and ilψ cobactel때 has been reported in
the literature [,1].
Several techniques have been reported in the literature for the determination of
sparfloxacin such as HPLC [5] , spectrophotometry [6) , liquid chromatography [7]
and fluorimetry [8].
Chemiluminescence (CL) method has been an important detection method for the
pharmaceutical preparations in recent years [9 ,10] because of promising
advantages of low detection limit, wide linear dynamic range and relatively simple
and inexpensive instrumentation. Acidic potassium permanganate has been is one
of the most important oxidants applied in CL reactions and a great number of
investigations have been published [1 1, 12]. This work describes the sensitizing
effect of sparfloxacin on the CL intensity emitted from the reaction of sulfite with
97

98 Seikh Mafiz Alam . 이상학
acidic KMn04. To our best knowledge , this paper describes the first application of
FIA -CL to the determination of sparfloxacin.
2. EXPERIMENTAL
2.1.Instrumentation
A Spex (Edison , NJ , USA) Model FL 111 spectrofluorimeter was used to
accomplish the chemiluminescence measurements. An Ismatec Model 404
peristaltic pump was used to convey the one CL reagent for initiation of the
reaction. During the measurements , the light source of the excitation
monochromator was switched off. Slit width of emission monochromator was fixed
with 0.25 mm. The photomultiplier tube (PMT) used was a Hamamatsu Model R
928 (Hamamatsu , USA) powered at 950 V. Spectra data were collected by Spex
DM 3000 spectroscopy computer.
2.2.Reagents
Individual standard stock solution of sparfloxacin 0 x lO-:l M) was prepared in
deionised water. 0.1 M HCl solution. Stock solution 0 x lO-:l M) of KMn04 was
prepared in 0.1 M sulphuric acid. Aqueous Na2S0:l solution 0 x 10-2 M) was
prepared in distilled water.
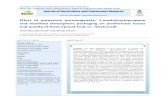
2.3.ProcedureThe basic analysis procedure of the batch type CL consisted (Fig. 1) of addition
of the followingquantities of the equilibrated solutions into the reaction cell: 1.0 ml
KMn04 (3.1 x lO-:l M) solution , 1 ml sparfloxacin 0.0 Xl 0-3 M) solution. The
contents of the reaction cell were allowed to mix for 10 seconds in the cell
compartment prior to injection of 0.5 ml 0.0 x 10-3 M) Na2S03 solution with
peristaltic pump. The analytical signal was taken as the difference in the CL peak
height between a blank and analyte run.
S.....I~
---、~ IDJud8nv야’
C lI.M1er
lI:l‘IDO..
N"2S0~
Fl
p z
T:!; n-••빼
J'1uodlltder
Figure 1. Schematic diagram of the FIA CL manifold employed for the quantitativedetermination of levodopa. PI. P2 ’ Peristaltic pumps; T l. T2: Y- pieces
용용화학, 제 12 권 제 1 호, 2008

FJow- Injection Chemilu미nescence Dctennination of Spa버oxacin Using Potassium P,해nanganate- S떠ium Sulfite System 99
3. Results and discussion
3.1.CL kinetic curve of the system
The CL kinetic curves of KMnO\- NalS03-HlSO~ and sparfloxacin- KMnO~ - Na2S0:1- HlSO •
systems were constructed with the recorder. which are shown in Fig.2
KMn01- Na2S0:1- H2S04 system has an ultra -weak CL. Adding sparfloxacin into the system
largely enhances its CL intensity and the value of enhancement is proportional to the
substances added. By this property. sparfloxacin can be determined sensitively with with
CL method.
1.6x10‘gu 1.“ 10‘홉 1.2x 10‘
훌 1.0x삐x1띠1애o:I 8.0x10’
얻 6.0x10’
들 4.0x10’그 •흩 2.0x10·
를 0.0‘J
b
350 400 450 500 550 800 850
Wavelength , nm
Figure 2.KMn04-NalSO:I-HlS04 (a) and sparfloxacin- KMnOcNa2S03-H2S04 (b)
CL spectra of
3.2.Selection of potassium permanganate concentrationThe effect of KMn04concentration on the CL reaction was examined in the range of
1.0 X 10-3 to 2.5 x 10- r. M. It has been observed from the results (Fig 3) that the CL signal
rapidly increased with increasing in KMn04 concentration up to 3.1 X 10-3 M. Larger
concentrations resulted in a decrease of emission intensity. Therefore. 0.5 x 10-‘’ mollLKMn04 was chosen for subsequent experiments.
2xl0'---‘
‘•,
애
뼈
띠m
애
띠
띠
2
’’’’’9
·au.강
·I·를
-·uC@u·g-E
그=EEu
8x l0' 0.0 0.2 0.4 0.6 0.8Concentration , mM
1.0
Figure 3. The effect of the corκentration of KMn0 1.
Applied Chemistry, Vol. 12, No. I , a:뼈

100 Seikh Mafiz 매am . 이상학
3.3.Selection of sulfite concentrationNo CL signal has been observed in the absence of sodium sulfite. It played an important
role in the reaction system. The variation of the CL emission with sulfite concentration in
the 0.01 to 3.0 xl 0 4 M range was studied. The chemiluminescence intensity increased
with the increase of the concentration of NazS03 when it was lower than 1 x 10-3 mo1/L.
Thus. 1 x 10년 mo1/L mo1/L was chosen as optimum.
3 .4 .Calibration
Under the optimal experimental conditions established above. the calibration graph of the
concentration of gibberellic acid versus peak height was linear in the range of 1.6 x 10 - 8 to
2.9 X10 “ mo1/L. The detection limit was found to be 0.7 x 10 8 mo1/L.
Acknowledgment
Acknowledgement is made to the Korea Research Foundation (KRF-2004-005-C00009)
for the support of this research.
References
1. S. Nakamura , A. Minami , K. Nakata , N. Kurobe , K. Kouno , Y. Sakaguchi , S.
Kashimoto , H. Yoshida , T. Kojima , T. Ohue , K. Fujimoto , M. Nakamura , M. Hashimoto , M.
Shimizu, Antimicrob. Agents Chemother. 33 (989) 1167
2. K.L. Goa , H.M. Bryson , A. Markham , Drugs 53 (997) 700
3. 1. Martindale , The Extra Pharmacopoeia , 29th edn. , The Pharmaceutical Press, London ,
1989.
4. K. Borner, E. Borner , H. Lode , 1. Chromatogr. Biomed. App l. 579 (992) 285-289
5. Borner , K., Borner , E.. and Lode , H. 1. Chromatogr. Biomed. App l. 579 (1 992)285
6. EI-Enany , N. 2004. JL Farmaco , 59 (2004) 63
7. H.R.N.Marona , E.E.S. Schapoval. 20(999) 413
8. L.Wang. Ana l. Sci. , 20(2004) 1237
9. Y.F. Mestre , L.L. Zamora , 1.m . Calatayud °Luminescence 16 ( 2001 ) 213 。
10. A °Townshend , W °Ruengsitagoon , C °Thongpoon , S. Liawruangrath °Ana l. Chim.
Acta 5 4 1 ( 2005 ) 10 5
11. N °Anastos , N oW °Barnet , S.W Lewis , N °Gathergood , Scammells P oJ 0 , D.N.
Sims ° Talanta 6 7 ( 2005 ) 354
12. Y.D. Liang ,J.F. Song Ana l. Bioanal. Chern. 380 (2004) 918
용용화학, 제 12 권 제 1 호, 2008