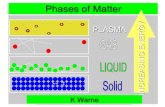
Phases of Matter Chp 13 and 14. Phases of Matter Solid – molecules are held tightly together by...
-
Upload
dwight-carpenter -
Category
Documents
-
view
214 -
download
1
Transcript of Phases of Matter Chp 13 and 14. Phases of Matter Solid – molecules are held tightly together by...

Phases of MatterPhases of Matter
Chp 13 and 14Chp 13 and 14

Phases of MatterPhases of Matter Solid – molecules are held tightly together by Solid – molecules are held tightly together by
intermolecular forces, molecules move slowlyintermolecular forces, molecules move slowly Liquid – some intermolecular forces still exist, Liquid – some intermolecular forces still exist,
but they are becoming weaker as molecules but they are becoming weaker as molecules speed upspeed up
Gas – all intermolecular forces have been Gas – all intermolecular forces have been broken, molecules move very quickly and broken, molecules move very quickly and rarely interactrarely interact
It take ENERGY to break those intermolecular It take ENERGY to break those intermolecular forces and cause a phase change!!!forces and cause a phase change!!!

Phase ChangesPhase Changes
Evaporation – liquid to gas, is a cooling process Evaporation – liquid to gas, is a cooling process since hottest molecules escape (why we sweat)since hottest molecules escape (why we sweat)– Boiling – evaporation throughout a substance, it can be Boiling – evaporation throughout a substance, it can be
decreased as air pressure decreasesdecreased as air pressure decreases Condensation – gas to liquid, is a warming Condensation – gas to liquid, is a warming
process since hot gas molecules bring their process since hot gas molecules bring their energy with themenergy with them
Melting – solid to liquidMelting – solid to liquid Freezing – liquid to solidFreezing – liquid to solid Sublimation – solid to gas, skipping liquid phaseSublimation – solid to gas, skipping liquid phase
– This occurs especially at low pressures since the boiling This occurs especially at low pressures since the boiling point becomes lowered so muchpoint becomes lowered so much

Intermolecular forcesIntermolecular forces
Exist between moleculesExist between molecules 3 types:3 types:
– Dipole-dipole: occurs between polar moleculesDipole-dipole: occurs between polar molecules– Hydrogen bond: special dipole that occurs when Hydrogen bond: special dipole that occurs when
hydrogen is involved (stronger than regular dipole-dipole hydrogen is involved (stronger than regular dipole-dipole interactions)interactions)
– London dispersion force: occurs for noble gases and London dispersion force: occurs for noble gases and nonpolar molecules, very weak and doesn’t last longnonpolar molecules, very weak and doesn’t last long
The stronger the intermolecular forces involved, The stronger the intermolecular forces involved, the higher the boiling point since more energy is the higher the boiling point since more energy is needed to break those interactions.needed to break those interactions.

Heating/Cooling CurvesHeating/Cooling Curves Graph that represents at what temperature phase Graph that represents at what temperature phase
changes occur and how heat and temperature are changes occur and how heat and temperature are relatedrelated
Note, the temperature doesn’t change during a Note, the temperature doesn’t change during a phase change (boiling water stays at 100phase change (boiling water stays at 100ooC until is C until is becomes a gas)becomes a gas)– Heat of fusion – energy needed to turn a solid into a Heat of fusion – energy needed to turn a solid into a
liquid (or released when a liquid turns into a solid)liquid (or released when a liquid turns into a solid) 6.02 kJ/mol for water6.02 kJ/mol for water
– Heat of vaporization – energy needed to turn a liquid Heat of vaporization – energy needed to turn a liquid into a gas (or released when a gas turns into a liquid)into a gas (or released when a gas turns into a liquid) 40.6 kJ/mol for water40.6 kJ/mol for water
– HOV is always higher than HOF because it takes more HOV is always higher than HOF because it takes more energy to completely break the intermolecular forcesenergy to completely break the intermolecular forces

Using HOV and HOFUsing HOV and HOF
Ex. How much energy is required to melt Ex. How much energy is required to melt 8.5 g of ice at 08.5 g of ice at 0ooC?C?
8.5 g8.5 g 1 mol1 mol 6.02 kJ6.02 kJ = = 2.8 kJ2.8 kJ
18 g18 g 1 mol1 mol

Combining temperature and phase Combining temperature and phase changeschanges
Ex. How much energy is required to heat 25 g of Ex. How much energy is required to heat 25 g of water from 25water from 25ooC to 100C to 100ooC as steam?C as steam?– 2 parts: heat 752 parts: heat 75ooC, then hov to get steamC, then hov to get steam– Part 1Part 1
Q = smQ = smTT
Q = 4.184 (25)(75) = 7800 J or 7.8 kJQ = 4.184 (25)(75) = 7800 J or 7.8 kJ– Part 2Part 2
25 g25 g 1 mol1 mol 40.6 kJ40.6 kJ = 57 kJ= 57 kJ
18 g18 g 1 mol1 mol– Now, we add since both must occur Now, we add since both must occur
7.8 kJ + 57 kJ = 64.8 kJ7.8 kJ + 57 kJ = 64.8 kJ

PressurePressure
All gases, including the atmosphere, exert a All gases, including the atmosphere, exert a pressurepressure– Result of gravity pulling on the gas’s massResult of gravity pulling on the gas’s mass– 760 mm Hg at sea level on Earth760 mm Hg at sea level on Earth
Has a variety of unitsHas a variety of units– 1 standard atmosphere = 1 atm = 760 mm Hg = 1 standard atmosphere = 1 atm = 760 mm Hg =
760 torr = 101,325 Pa = 14.69 psi760 torr = 101,325 Pa = 14.69 psi We can convert between units using a T-We can convert between units using a T-
chartchart– We typically use pascals in scienceWe typically use pascals in science

Unit conversionUnit conversion
Ex. What is 7.3 atm in mm Hg? Ex. What is 7.3 atm in mm Hg?
7.3 atm7.3 atm 760 mm Hg760 mm Hg = 5548 mm Hg= 5548 mm Hg1 atm1 atm
Ex. What is 7.3 mm Hg in Pascals?Ex. What is 7.3 mm Hg in Pascals?
7.3 mm Hg7.3 mm Hg 101325 Pa101325 Pa == 973.2 Pa973.2 Pa760 mm Hg760 mm Hg

Boyle’s LawBoyle’s Law
As the volume of a gas is decreased, it’s pressure As the volume of a gas is decreased, it’s pressure increasesincreases
PP11VV11 = P = P22VV22
Ex. If a 1.5 L sample of freon gas at 56 torr is Ex. If a 1.5 L sample of freon gas at 56 torr is increased to a pressure of 150 torr, what is the increased to a pressure of 150 torr, what is the new volume of the gas?new volume of the gas?56 (1.5) = 150 (V)56 (1.5) = 150 (V)V = 0.56 LV = 0.56 L*Any units are fine, as long as they are the same *Any units are fine, as long as they are the same on both sides of the equation.on both sides of the equation.

Charles’ LawCharles’ Law
As the temperature of a gas increases, it’s volume As the temperature of a gas increases, it’s volume increases (temp must be in K)increases (temp must be in K)
At -273At -273ooC, all gases occupy a volume of 0, which C, all gases occupy a volume of 0, which is impossible, so that is coldest temp possible is impossible, so that is coldest temp possible (absolute zero)(absolute zero)
VV11 = V = V22
TT11 T T22
Ex. If a 2 L gas sample at 298 K is cooled to 278 Ex. If a 2 L gas sample at 298 K is cooled to 278 K, what is it’s new volume?K, what is it’s new volume?2 = V2 = V22
298298 278 278VV22 = 1.9 L = 1.9 L

Combined Gas LawCombined Gas Law
Stick charles’ law and boyle’s law togetherStick charles’ law and boyle’s law together Pressure can be in any unit, as long as it’s Pressure can be in any unit, as long as it’s
the same on both sides, but temperature the same on both sides, but temperature must be in kelvinmust be in kelvin
PP11VV11 = P = P22VV22
TT11 T T22

Avogadro’s lawAvogadro’s law
As the number of moles of gas increases, so As the number of moles of gas increases, so does the volumedoes the volume
VV11 = V = V22
nn11 n n22
If 0.5 mol of oxygen occupy 12.2 L, what If 0.5 mol of oxygen occupy 12.2 L, what volume would 0.33 mol of oxygen occupy?volume would 0.33 mol of oxygen occupy?
12.2 = V12.2 = V22
0.5 0.330.5 0.33
VV22 = 8.1 L = 8.1 L

Ideal Gas LawIdeal Gas Law
Combines all gas laws into oneCombines all gas laws into one PV = nRTPV = nRT
R = 0.08206 Latm/molKR = 0.08206 Latm/molK*so, temp must be in kelvin, volume must be *so, temp must be in kelvin, volume must be in liters and pressure must be in atmin liters and pressure must be in atm
Ex. A 8.56 L sample of hydrogen gas at 0Ex. A 8.56 L sample of hydrogen gas at 0ooC C has a pressure of 1.5 atm. How many has a pressure of 1.5 atm. How many moles are present in the sample?moles are present in the sample?1.5 (8.56) = n (0.08206) (273)1.5 (8.56) = n (0.08206) (273)
n = 0.57 molsn = 0.57 mols

Some TermsSome Terms
STP – standard temperature at pressureSTP – standard temperature at pressure– 1 atm and 01 atm and 0ooCC
Molar volume – the volume that one mole of Molar volume – the volume that one mole of any gas takes up at STPany gas takes up at STP– 22.4 L at STP22.4 L at STP
Kinetic molecular theory – predicts why Kinetic molecular theory – predicts why gases have the properties that they dogases have the properties that they do– As temp increases, particles move faster and As temp increases, particles move faster and
collide with the container more often and collide with the container more often and interact with each other lessinteract with each other less

Dalton’s Law of Partial PressureDalton’s Law of Partial Pressure
When gases are mixed, their pressures add When gases are mixed, their pressures add together to create a new pressure in the together to create a new pressure in the containercontainer– Occurs because gases move so fast, what the Occurs because gases move so fast, what the
particle is doesn’t matter as much as how many particle is doesn’t matter as much as how many there arethere are
PPtotaltotal = P = P11 + P + P22 … … PPtotaltotal = n = ntotaltotal (RT) (RT)
VV

An exampleAn example
If 12 g of oxygen and 46 g of He are pumped into a If 12 g of oxygen and 46 g of He are pumped into a 5.0 L container at 255.0 L container at 25ooC, what will the total pressure in C, what will the total pressure in the container be?the container be?– First g convert to moles using molar massFirst g convert to moles using molar mass
12 g 1 mol = 0.375 mol 46 g 1 mol = 11.5 mol12 g 1 mol = 0.375 mol 46 g 1 mol = 11.5 mol 32 g 4 g32 g 4 g
– Next Next ooC becomes K by adding 273C becomes K by adding 27325 + 273 = 298 K25 + 273 = 298 K
– Then plug into formulaThen plug into formulaPPtotal total = (.375 + 11.5) (0.08206)(298)= (.375 + 11.5) (0.08206)(298)
55PPtotaltotal = 58.1 atm = 58.1 atm