Quark-Matter 2008 20 th International Conference on Nucleus Nucleus Collisions
Phases of Matter and Heat Transfer. Matter Video on the discovery of the nucleus.
-
Upload
magdalen-terry -
Category
Documents
-
view
215 -
download
0
Transcript of Phases of Matter and Heat Transfer. Matter Video on the discovery of the nucleus.

Phases of Matter and Heat Transfer

Matter
• Video on the discovery of the nucleus

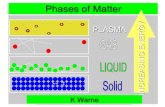
Phases of Matter
• There are 5 phases of matter
• Phase changes are controlled by the energy of the material
• Can anyone name the 5 phases?

Phases of Matter
• There are 5 phases of matter
• Phase changes are controlled by the energy of the material
• Can anyone name the 5 phases?

Phases of Matter
• Video on plasma and BE condensate

Temperature
• Temperature – A quantity that tells you how hot or cold a material is compared to a standard.
• We usually measure temperature in Fahrenheit (F), and Celsius (C). For our class we will use Kelvin (K)

Temperature
Kelvin = Celsius + 273
Celsius = 9/5 (Fahrenheit – 32)


Heat Transfer
• Heat – The energy that transfers from one object to another because of a temperature difference.
• Heat flows from a higher temperature substance, to a lower temperature substance

Heat Transfer
• We measure heat in the unit known as calories
• A calorie is defined as the amount of heat to change 1 gram of water 1 C°
• We say “cal” for short. 1000 calories is a kilocalorie

Heat Transfer
• Materials have a quantity known as specific heat capacity.
• Specific heat capacity is how much heat a material can hold per mass and degree Celsius.
• We use the variable “c” to define specific heat.
• Units are calories/(grams °C)

Heat Transfer
• The higher the specific heat capacity, the more heat a material can hold.
• Material with lower specific heat capacities get hotter quicker, like Iron or sand.
• Water has a high specific heat capacity = 1cal/g°C

Heat Transfer
• The quantity of heat, which will be defined with the variable Q, can be found with the following equation
Q = mcΔT
• Heat = mass x specific heat capacity x Temperature change (Celsius)

Heat Transfer
• You can calculate the heat required to change the phase of a material from one phase to another
Q = mLHeat = mass x latent heat
• The units of latent heat are calories/gram. Every phase change has a specific latent heat. Heat of fusion involves interchanging between a solid and liquid, and heat of vaporization involves changing between a liquid and a gas.

Heat Transfer
• Try these problems