Experimental endotoxemia induces leukocyte adherence and plasma extravasation within the rat
Partial Liquid Ventilation Reduces Pulmonary Neutrophil Accumulation in an Experimental Model of...
-
Upload
manuel-felipe-cruz-morales -
Category
Documents
-
view
221 -
download
0
Transcript of Partial Liquid Ventilation Reduces Pulmonary Neutrophil Accumulation in an Experimental Model of...
-
8/13/2019 Partial Liquid Ventilation Reduces Pulmonary Neutrophil Accumulation in an Experimental Model of Systemic Endot
1/13
23/08/13 Ovid: Partial liquid ventilation reduces pulmonary neutrophil accumulation in an experimental model of systemic endotoxemia and acute lung injury.
ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi 1/13
[Laboratory Investigations]
Critical Care Medicine
Issue: Volume 26(10), October 1998, pp 1707-1715
Copyright: Williams & Wilkins 1998. All Rights Reserved.
Publication Type: [Laboratory Investigations]
ISSN: 0090-3493
Accession: 00003246-199810000-00026
Partial liquid ventilation reduces pulmonary neutrophil accumulation in an experimental model of systemic
endotoxemia and acute lung injury
Rotta, Alexandre T. MD; Steinhorn, David M. MD
Author Information
From the Division of Pediatric Critical Care Medicine at The Children's Hospital of Buffalo and State University of New York at Buffalo, Buffalo, NY.
Supported, in part, by a grant from the Alliance Pharmaceutical Corporation and Hoechst Marion Roussel, and by The American Lung Association-NY Affiliate.
Presented, in part, at the 26th Educational and Scientific Symposium of the Society of Critical Care Medicine, San Diego, CA, February 1997.
Address requests for reprints to: Alexandre T. Rotta, MD, Division of Pediatric Critical Care Medicine, The Children's Hospital of Buffalo, 219 Bryant Street, Buffalo,
NY, 14222-2006.
Abstract
Objective: To determine whether pulmonary neutrophil sequestration and lung injury are affected by partial
liquid ventilation with perfluorocarbon in a model of acute lung injury (ALI).
Design: A prospective, controlled, in vivo animal laboratory study.
Setting: An animal research facility of a health sciences university.
Subjects: Forty-one New Zealand White rabbits.
Interventions: Mature New Zealand White rabbits were anesthetized and instrumented with a tracheostomy
and vascular catheters. Animals were assigned to receive partial liquid ventilation (PLV, n = 15) with perflubron (18
mL/kg via endotracheal tube), conventional mechanical ventilation (CMV, n = 15) or high-frequency oscillatory
ventilation (HFOV, n = 5). Animals were ventilated, using an FIO2of 1.0, and ventilatory settings were required to
achieve a normal PaCO2. Animals were then given 0.9 mg/kg of Escherichia coli endotoxin intravenously over 30
mins. Partial liquid ventilation, conventional mechanical ventilation, or h igh-frequency osc illatory ventilation was
continued for an additional 4 hrs before the animals were killed. A group of animals not challenged with
endotoxin underwent conventional ventilation for 4.5 hrs, serving as the control group (control, n = 6). Lungs
were removed and samples were frozen at -70[degree sign]C. Representative samples were stained for histology. A
visual count of neutrophils per high-power field (hpf) was performed in five randomly selected fields per sample in
a blinded fashion by light microscopy. Lung samples were homogenized in triplicate in phosphate buffer,
ultrasonified, freeze-thawed, and clarified by centrifugation. Supernatants were analyzed for myeloperoxidase
(MPO) activity by spectrophotometry with o-dianisidine dihydrochloride and hydrogen peroxide at 460 nm.
Measurements and Main Results: Histologic analysis of lung tissue obtained from control animals showed
normal lung architecture. Specimens from the PLV and HFOV groups showed a marked decrease in alveolar
proteinaceous fluid, pulmonary vascular congestion, edema, necrotic cell debris, and gross inflammatory
infiltration when compared with the CMV group. Light microscopy of lung samples of animals supported with PLV
and HFOV had significantly lower neutrophil counts when compared with CMV (PLV, 4 +/- 0.3 neutrophils/hpf;
HFOV, 4 +/- 0.5 neutrophils/hpf; CMV, 10 +/- 0.9 neutrophils/hpf; p < .01). In addition, MPO ac tivity from lung
extracts of PLV and HFOV animals was significantly lower than that o f CMV animals (PLV, 61 +/- 13.3 units of MPO
activity/lung/kg; HFOV, 43.3 +/- 6.8 units o f MPO activity/lung/kg; CMV, 140 +/- 28.5 units o f MPO activity/lung/kg;
p < .01). MPO activity from lungs o f uninjured con trol animals was significantly lower than that o f animals in the
PLV, HFOV, and CMV groups (control, 2.2 +/- 2 units of MPO activity/lung/kg; p < .001).
Conclusions: Partial liquid ventilation decreases pulmonary neutrophil accumulation, as shown by decreased
neutrophil counts and MPO activity, in an experimental animal model of ALI induced by systemic endotoxemia.
The attenuation in pulmonary leukostasis in animals treated with PLV is equivalent to that obtained by a
ventilation strategy that targets lung recruitment, such as HFOV. (Crit Care Med 1998; 26:1707-1715)
Key Words: perfluorocarbon; endotoxin; myeloperoxidase; liquid ventilation; acute respiratory distress
syndrome; respiratory failure; inflammation; neutrophil; pulmonary emergencies; critical illness
Acute respiratory distress syndrome (ARDS) is marked by profound impairment in pulmonary mechanics and
function. It is associated with a diffuse inflammatory reaction which may lead to secondary injury [1]. An early
event in the development of ARDS in experimental animal models [2,3]and humans [4,5]is the accumulation and
activation of neutrophils in the lung. Evidence of the neutrophil's role in the evolution of ARDS is provided by
reports that granulocyte depletion before an acute lung insult attenuates or prevents the development of
pulmonary dysfunction [6,7], although ARDS is also known to occur in the setting of severe neutropenia without
pulmonary neutrophil infiltration [8]. Ventilation strategies have been shown to play an important role in the
development and progression of ARDS [9,10]. Ventilation strategies that permit inhomogeneous lung inflation are
associated with progressive atelectasis, high inflation pressures with patchy overdistention of lung units, global
http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?- -
8/13/2019 Partial Liquid Ventilation Reduces Pulmonary Neutrophil Accumulation in an Experimental Model of Systemic Endot
2/13
23/08/13 Ovid: Partial l iquid ventilation reduces pulmonary neutrophil accumulation in an experimental model of systemic endotoxemia and acute lung injury.
ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi 2/13
decrease in lung compliance, and progressive structural injury [10,11]. Conversely, strategies that target early
establishment of a more homogeneous alveolar inflation, such as high-frequency oscillatory ventilation (HFOV), are
associated with better outcomes [10,12]. Sigiura et al. [13]demonstrated that HFOV minimizes neutrophil influx
and activation as well as attenuates lung injury in a surfactant deficient model when compared with conventional
mechanical ventilation.
Partial liquid ventilation with perfluorocarbons has been studied as a support modality in a variety of
experimental models of respiratory failure and acute lung injury (ALI). Gross inspection of lung specimens
supported with partial liquid ventilation in models of ALI has shown decreased atelectasis, hemorrhage, and
necrosis in comparison with similarly injured gas ventilated controls [14]. Under light microscopy, injured lungs
treated with partial liquid ventilation appear more homogeneously inflated with less evidence of inflammation and
tissue damage than spec imens from gas ventilated animals [14,15]. The precise mechanisms by which partial liquid
ventilation reduces the histologic evidence of lung injury are still unknown. However, we have previously shown
that partial liquid ventilation with perfluorocarbons decreases free radical release by alveolar macrophages [16]
and attenuates oxidative damage to lung tissue [17]. In view of neutrophil's important role in the evolution of
tissue damage following a pulmonary insult, we hypothesized that partial liquid ventilation might reduce neutrophil
accumulation in the lung during the early phase of ALI. To investigate this hypothesis, we undertook a controlled
laboratory investigation using a systemic, endotoxemia-induced model of acute alveolitis.
MATERIALS AND METHODS
Animals and Instrumentation. Forty-one New Zealand White rabbits, weighing 1.5 to 3.5 kg, were anesthetized
with ketamine (25 mg/kg im) and xylazine (4 mg/kg im). The animals were preoxygenated du ring spontaneous
breathing with 100% oxygen by nose cone. Muscle paralysis was induced by intravenous administration of
pancuronium bromide (0.2 mg/kg) and maintained with 0.1-mg/kg doses as needed to control movement.Anesthesia was maintained with a continuous intravenous infusion of ketamine (10 mg/kg/hr) and xylazine (4
mg/kg/hr).
The anterior neck was dissected carefully and a tracheostomy was performed. A cuffed endotracheal tube
(3.0 to 3.5 mm inner diameter) (Sheridan Catheter, Argyle, NY) was placed in the trachea and secured in position
with umbilical tape. A vascular cannula was inserted in the common carotid artery and a double-lumen catheter
was advanced into the superior vena cava through the jugular vein. Central venous and arterial blood pressures
were monitored by attaching the vascular c atheters to standard pressure transducers, connec ted to a monitor
(Horizon 2000, Mennen Medical, Clarence, NY). The temperature was monitored continuously, using an
esophageal probe, and normothermia was maintained with electric warming pads. Maintenance fluids were
provided by a continuous infusion of 0.9% saline solution containing 5% dextrose. The animals were assigned
sequentially in alternating fashion to receive conventional mechanical ventilation (CMV, n = 15), partial liquid
ventilation (PLV, n = 15), high-frequency oscillatory ventilation (HFOV, n = 5), or to a control group (control, n =
6). The animals in the CMV, PLV, and control groups were ventilated in the supine position with a ventilator (Servo
900C, Siemens-Elema, Solna, Sweden) in the volume control mode, using an effective tidal volume of 8 to 10 mL/kg,
FIO2of 1.0, positive end-expiratory pressure of 5 cm H2O, and a respiratory rate of 25 to 35 breaths/min, as
needed to maintain normocarbia (PaCO235 to 45 torr [4.7 to 6.0 kPa]).
After a stabilization per iod of 30 mins, the animals assigned to the PLV group had partial liquid ventilation
initiated by instilling perflubron (18 mL/kg, LiquiVent[registered sign], Alliance Pharmaceutical Corporation, San
Diego, CA) into the trachea over a 15-min period via a side port connector attached to the endotracheal tube.
Supplemental perflubron (2 to 4 mL/kg) was administered every hour in the same fashion to compensate for
evaporative losses, unless a liquid meniscus was visible in the endotracheal tube during a brief period of ventilator
disconnection. Animals assigned to the HFOV group were ventilated in the supine position with an oscillatory
ventilator (3100A, SensorMedics, Yorba Linda, CA) with an FIO 2of 1.0, mean airway pressure of 12 to 13.5 cm H2O,
frequency of 10 Hz, inspiratory time of 33%, and amplitude of 18 to 22 cm H2O to maintain normocarbia.
Animals were cared for in accordance with the guidelines published by the National Institutes of Health [18].
This study was approved by the Institutional Animal Care and Use Committee of the State University of New York
at Buffalo.
Induction of Lung Injury. Thirty minutes after initiating mechanical ventilation, i.e., conventional mechanical
ventilation, partial liquid ventilation, or h igh-frequenc y osc illatory ventilation, 0.9 mg/kg of Escherichia c oli
endotoxin (type F583, Sigma Chemical, St. Louis, MO) was administered over a 30-min period by intravenous
infusion through the central venous catheter. The animals in the control group did not receive intravenous E. coli
endotoxin but were otherwise treated identically to animals in the CMV group. Mean arterial blood pressure was
maintained at >65 mm Hg, as needed, by a continuous infusion of 10 to 20 [micro sign]g/kg/min of dopamine and
administration of 6% hetastarch in 0.9% sodium chloride (Hespan[registered sign], McGaw, Irvine, CA). Arterial
blood gas analysis was performed hourly, using an ABL-3 blood gas analyzer (Radiometer, Copenhagen, Denmark).
Conventional, partial liquid, or high-frequency oscillatory ventilation was continued for an additional 4 hrs beforethe animals were killed.
Tissue Extraction. The rabbits were killed with an intravenous dose of pentobarbital (100 mg/kg). The thorax
was opened carefully to observe for signs of a pneumothorax, to confirm proper catheter placement, and to
harvest tissue. The lungs were removed en bloc from the thoracic cavity, rinsed externally with sterile normal
saline, blotted dry, and weighed separately. A representative section of the left upper lobe was excised in full
http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?- -
8/13/2019 Partial Liquid Ventilation Reduces Pulmonary Neutrophil Accumulation in an Experimental Model of Systemic Endot
3/13
23/08/13 Ovid: Partial liquid ventilation reduces pulmonary neutrophil accumulation in an experimental model of systemic endotoxemia and acute lung injury.
ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi 3/13
thickness (axial plane) and preserved in formalin for histologic examination. The right lung was frozen for
subsequent evaluation while the remaining left lung was divided into three portions and snap frozen at -70[degree
sign]C for batch analysis [19]. Samples from three different regions of the left lung were analyzed to minimize
sampling error related to inhomogeneous distribution of neutrophils throughout the injured lung. The lung
samples were homogenized in 0.5% hexadecyltrimethyl-amonium bromide (HTAB) (Sigma Chemical) in 50 mM of
phosphate buffer, with a pH of 6.0, in a ratio of 5 mL HTAB solution/g tissue, using a homogenizer (2000 Variable
Speed Tissue Homogenizer, Omni International, Waterbury, CT). The homogenates were sonicated in an ice bath
with an ultrasonic dismembrator (300, Dynatech Laboratories, Chantilly, VA) for 10 secs at 40% power. Specimens
were freeze-thawed three times (liquid nitrogen and 37[degree sign]C), after which sonication was repeated as
above. The suspensions were clarified by centrifugation at 20,000 rpm for 30 mins, and the supernatants were
reserved for analysis.
Myeloperoxidase (MPO) Assay. MPO activity was assayed spectrophotometrically, using the method of Bradley
et al [20]. In brief, 0.1 mL of the supernatant was mixed with 2.9 mL of 50-mM potassium phosphate buffer, pH of
6.0, containing 0.167 mg/mL of o-dianisidine dihydro chloride (Sigma Chemical) and 0.0005% hydrogen peroxide
(Sigma Chemical). The change in absorbance at 460 nm [18]was measured, using a spectrophotometer (U-2000,
Hitachi Instruments, Webster, NY). To minimize the effect of possible regional variations in neutrophil infiltration
throughout the injured lung, three separate regions were analyzed. MPO activity was then derived from the
observed change in absorbance per minute. MPO activity was expressed as total MPO activity/lung/kg body
weight and represents both lungs taken together. This measure was undertaken to avoid the weight artifact
produced by the presence of the heavy perflubron in the lungs of animals in the partial liquid ventilation group.
Histologic Analysis. Tissue samples preserved in formalin were fixed and embedded in paraffin before cutting
and staining with hematoxylin and eosin. Slides were then studied under light microscopy. To minimize possible
regional variations in neutrophil distribution which could lead to sampling bias, the visual count of neutrophils perhigh-power field (hpf) (500x) was performed in five randomly selected fields per sample in a blinded fashion by the
principal investigator (A.T.R.). A mean number of neutrophils/hpf for each sample was then obtained.
A lung injury score was used to quantify changes in lung architecture visible by light microscopy [21]. The
degree of microscopic injury was scored based on the following variables: alveolar and interstitial inflammation,
alveolar and interstitial hemorrhage, edema, atelectasis, and necrosis. The severity of injury was graded as follows
for each of the seven variables: no injury = 0; injury to 25% of the field = 1; injury to 50% of the field = 2; injury to
75% of the field = 3; and diffuse injury = 4. The highest possible score was 28 and the lowest was 0. In order to
minimize regional variations in lung histology, two different areas from each specimen were examined under light
microscopy.
Statistical Analysis. Results are expressed as mean +/- SEM, unless otherwise noted. Data were analyzed using
SigmaStat for Windows, Ve rsion 2.0 (1995, Jandel, Chicago, IL) on a personal computer. A Kruskal-Wallis one-wayanalysis of variance was used for comparison of multiple groups for each single variable. Post hoc analysis was
performed using the Dunn's method for all pairwise multiple comparison procedures. The strengths of the
relationships between neutrophil counts/hpf and M PO activity, and between M PO activity and lung injury sc ores
were estimated, using linear regression analysis based on data from all four experimental groups. Statistical
significance was observed at p < .05.
RESULTS
Four rabbits died before completion of the experimental protocol and were excluded from the final data
analysis. In the CMV group, one rabbit died with a tension pneumothorax and the other developed profound
hypotension and acidemia immediately after infusion of the E. coli endotoxin. In the PLV group, one animal
developed a fatal tachyarrhythmia shortly after instrumentation and the other animal had refractory hypotension
and acidemia early in the course of the experiment. Thirty-seven animals completed the experimental protocol,
leaving 13 in the CMV and PLV groups, 5 in the HFOV group, and 6 in the control group. The baseline
characteristics and values for the rabbits assigned to each group are shown in Table 1. As expected, the baseline
PaO2of animals in the liquid ventilation group was significantly lower than that of gas ventilated controls. This
finding is thought to be due to the obligatory oxygen gradient across the liquid-alveolar interface, as a
consequence of the relative rate limitation for the diffusion of oxygen through the thin liquid layer lining the
alveolus during inspiration, as oxygen is taken up by the capillary blood [22].
http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cTT1&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cTT1&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://-/?-http://-/?-http://-/?-http://-/?-http://-/?- -
8/13/2019 Partial Liquid Ventilation Reduces Pulmonary Neutrophil Accumulation in an Experimental Model of Systemic Endot
4/13
23/08/13 Ovid: Partial l iquid ventilation reduces pulmonary neutrophil accumulation in an experimental model of systemic endotoxemia and acute lung injury.
ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi 4/13
Table 1. Baseline characteristics of rabbits assigned to control, partial liquid ventilation (PLV), conventional
mechanical ventilation (CMV), and high-frequency oscillatory ventilation (HFOV) groups (mean +/- SEM)
Gross visual inspection of the lungs of animals in the control group revealed normal anatomic features. Lungs
from animals in the CMV group had a congested appearance alternated with dark areas of patchy hemorrhage
throughout the external surface, with greater c oncentration over the dependent areas. The lungs supported
with partial liquid ventilation grossly exhibited a translucent pink appearance, with fewer hemorrhagic areas. The
lungs from animals in the HFOV group had few hemorrhagic areas, mostly over the dependent portions. In all the
experimental groups, the right and left lungs appeared similar by gross, visual examination. When representative
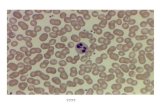
samples of lung tissue were analyzed under light microscopy, the control group exhibited normal histology (Figure
1), whereas the CMV group showed a markedly abnormal aeration pattern and evidence of intense inflammatory
response (Figure 2). There were extensive areas of atelectasis, as well as areas of severe injury, with alveoli filled
by proteinaceous material, necrotic debris, erythrocytes, and inflammatory cells. Thickened and deformed
alveolar walls also exhibited abnormal accumulation of erythrocytes and granulocytes. In contrast, lung tissue
from the PLV (Figure 3) and HFOV groups exhibited a more homogeneous expansion, with thin walled alveoli and
little evidence of lung injury. Congestion and inflammation were minimal, although a few areas had small amounts
of hyaline material within the alveoli. The mean number of neutrophils/hpf for each group is demonstrated in
Figure 4. Animals in the control group had fewer pulmonary neutrophils than did animals in the CMV, PLV, and
HFOV groups (p < .01). Animals in the PLV and HFOV groups had a significantly lower pulmonary neutrophil
count/hpf than animals in the CMV group (p < .01). The lung injury scores for each experimental group are shown
in Table 2. These data demonstrate a correspondingly lower injury score in those animals with fewer
neutrophils/hpf. The MPO activity for each experimental group is demonstrated in Figure 5. MPO activity was
significantly lower in lung samples from the control group when compared with lung samples from the CMV, PLV,
and HFOV groups (p < .01). Animals from the PLV and HFOV groups had intermediate MPO activity that was
significantly lower than that of animals in the CMV group (p = .01). The number of neutrophils/hpf was significantly
correlated with MPO activity (r2= .18, p = .01), and MPO activity was significantly correlated with lung injury
scores (r2= .46, p < .001) (Figure 6).
Figure 1. Photomicrographs of lung tissue obtained after 4.5 hrs of conventional mechanical ventilation (control
group) from rabbits not injured with an intravenous infusion of Escherichia coli endotoxin. Hematoxylin and eosin
stain, x100 (A) and x500 (B).
http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cTT1&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF1&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cTT1&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1 -
8/13/2019 Partial Liquid Ventilation Reduces Pulmonary Neutrophil Accumulation in an Experimental Model of Systemic Endot
5/13
http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF3&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1 -
8/13/2019 Partial Liquid Ventilation Reduces Pulmonary Neutrophil Accumulation in an Experimental Model of Systemic Endot
6/13
23/08/13 Ovid: Partial l iquid ventilation reduces pulmonary neutrophil accumulation in an experimental model of systemic endotoxemia and acute lung injury.
ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi 6/13
Figure 3. Photomicrographs of lung tissue obtained after 4.5 hrs of partial liquid ventilation from rabbits injured
with an intravenous infusion of Escherichia coli endotoxin. Hematoxylin and eosin stain, x100 (A) and x500 (B).
Figure 4. Neutrophil counts per high-power field (hpf) in lung specimens of rabbits from the control, conventional
mechanical ventilation (CMV), partial liquid ventilation (PLV), and high-frequency oscillatory ventilation (HFOV)
groups. Mean +/- SEM values. *p < .01 vs. control; sup # p < .01 vs. CMV.
Table 2. Lung injury scores (mean +/- SD)
http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF3&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cTT2&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF4&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF3&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1 -
8/13/2019 Partial Liquid Ventilation Reduces Pulmonary Neutrophil Accumulation in an Experimental Model of Systemic Endot
7/13
23/08/13 Ovid: Partial liquid ventilation reduces pulmonary neutrophil accumulation in an experimental model of systemic endotoxemia and acute lung injury.
ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi 7/13
Figure 5. Pulmonary myeloperoxidase activity of rabbits from the control (triangles), conventional mechanical
ventilation (CMV; squares), partial liquid ventilation (PLV; circles) and high-frequency oscillatory ventilation (HFOV;
diamonds) groups. Myeloperoxidase activity is expressed as activity/lung/kg body weight. Bars represent mean +/-
SEM. *p < .01 vs. contro l; sup # p < .01 vs. CMV.
http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF6&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF6&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF5&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1 -
8/13/2019 Partial Liquid Ventilation Reduces Pulmonary Neutrophil Accumulation in an Experimental Model of Systemic Endot
8/13
23/08/13 Ovid: Partial l iquid ventilation reduces pulmonary neutrophil accumulation in an experimental model of systemic endotoxemia and acute lung injury.
ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi 8/13
Figure 6. Scatter plots of correlations between myeloperoxidase (MPO) activity and lung injury scores (r 2= .46, p
< .001) (top) and neutrophil count per high-power field (hpf) and myeloperoxidase (MPO) activity (r 2= .18, p = .01)
(bottom). MPO activity is expressed as activity/lung/kg body weight. The regression lines (solid) and 95%
confidence intervals (dashed) are shown.
DISCUSSION
This study demonstrates that partial liquid ventilation significantly reduces neutrophil accumulation in the
lungs of animals exposed to a well-defined pulmonary insult. It represents the first published study of an objective
reduction in early pulmonary leukostasis following an acute injury treated with partial liquid ventilation.
The technique of perfluorocarbon-associated gas exchange, now commonly known as partial liquid
ventilation, was originally described by Fuhrman et al. [23]as a form of respiratory support in the normal piglet
model. Since then, partial liquid ventilation has been investigated as an adjunctive therapy in various experimental
models of acute lung injury, including oleic acid injury [24], gastric acid aspiration [14], meconium aspiration [25],
and neonatal respiratory distress syndrome [26]. In addition, limited c linical trials have demonstrated its safety and
efficacy in human disease [27,28]. A number of these studies [14,15,24]have hinted at a decrease in the degree of
injury observed during gross and microscopic examination of lung specimens from animals treated with either
partial [14,24]or total liquid ventilation [15]when compared with controls. The basis for this attenuation in lung
injury is not yet fully understood. Some investigators [15,29]have suggested that a "lavage" or dilutional effect
during liquid ventilation may remove inflammatory debris. Others [14]have proposed mechanisms involving the
mechanical stenting of the alveoli by the immiscible dense liquid perfluorocarbon in the alveolar spaces,
maintaining a more "open" lung for ventilation, as advocated by Amato and others [30]. The later conc ept of
reduced inflammation by maintaining a more "open" lung is further supported by the HFOV data, a modality
thought to maintain the lung in a more recruited state [13]. A further possible mechanism is that perflubron has
an anti-inflammatory effect. Virmani et al. [31]showed a decrease in superoxide release by circulating human
neutrophils exposed to a perfluorochemical emulsion. We [16]previously demonstrated a decrease in free radical
release by alveolar macrophages exposed in vitro to perfluorocarbons. We [17]have also shown that partial liquid
ventilation with perfluorocarbons reduces oxidative damage to lung tissue, as reflected by a decrease in tissue
content of thiobarbituric acid reactive substances (a measure of lipid oxidative damage) and carbonylated
proteins (a measure of protein oxidative damage).
Neutrophil accumulation within the pulmonary microvasculature has been implicated in the pathogenesis of
acute lung injury [2-5]. Sequestration of granulocytes in the lungs is thought to be an important early event in
ARDS as well as in the acute lung injury associated with sepsis and multiple organ system dysfunction. Human [4,5]
and animal models [2,3]have shown that the diffuse inflammatory picture seen in the lung tissue during ARDS is
generally preceded by accumulation and activation of neutrophils within that organ. During ARDS, pulmonary
neutrophils exhibit a state of functional activation as indicated by increased chemiluminescence activity [32],
increased superoxide generation [33], and altered chemotaxis [34]. The functional and metabolic activation of
neutrophils seen in patients with clinical ARDS is similar to that demonstrated in animal models of ARDS caused by
endotoxin infusion [33]. The importance of the neutrophil in the evolution of ARDS is further demonstrated by the
ability of granulocyte depletion to prevent the development of acute lung injury [6,7], although, as previously
noted, ARDS has also been well documented in patients with severe neutropenia [8], suggesting that neutrophil
sequestration is not the only factor implicated in this complex process. Based on these observations, the current
findings provide a unifying hypothesis which suggests that the reduced neutrophil sequestration in the lung and
attenuated activation of phagocytes may be contributing to the reduction in pulmonary tissue damage seen
during partial liquid ventilation.
The argument that the decrease in neutrophil count in histologic lung specimens following partial liquid
ventilation is solely due to a mechanical artifact must be carefully considered. One could postulate that the fluid-
filled lung might have a lower neutrophil count/hpf in comparison with a gas ventilated lung solely due to the fact
that the dense perfluorocarbon is capable of recruiting and maintaining patency of alveolar spaces that would
http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF6&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF6&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1 -
8/13/2019 Partial Liquid Ventilation Reduces Pulmonary Neutrophil Accumulation in an Experimental Model of Systemic Endot
9/13
23/08/13 Ovid: Partial l iquid ventilation reduces pulmonary neutrophil accumulation in an experimental model of systemic endotoxemia and acute lung injury.
ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi 9/13
otherwise have collapsed, thus decreasing the amount of actual tissue observed per field. This scenario does not
explain the attenuation in the overall inflammatory response (alveolar proteinaceous fluid, pulmonary vascular
congestion, and necrotic cell debris) of the lung tissue following partial liquid ventilation observed by us and
others [14,15,24]. Furthermore, the decrease in MPO activity in the lungs of animals supported with partial liquid
ventilation, compared with animals supported with conventional mechanical ventilation, provides objective
evidence that the reduction in pulmonary neutrophil infiltration is not merely a histologic artifact. MPO activity is
an accepted technique for measuring pulmonary leukostasis and has been shown to correlate linearly to the
absolute number of granulocytes found in a tissue sample [19,20]. Using a less common injury, cobra venom factor
challenge, to promote acute transient pulmonary sequestration of neutrophils, Bradley and colleagues [35]
presented preliminary data that showed a reduction in MPO activity in lungs rescued with partial liquid ventilation
in comparison with gas ventilated controls; these data thus corroborated our findings. The animals studied by
Bradley et al. [35]did not receive partial liquid ventilation until after being challenged with cobra venom factor,
suggesting that our findings are not solely dependent on the fact that the animals were pretreated before
endotoxin administration.
Comparing MPO activity per mass of lung tissue between gas ventilated and partial liquid ventilated groups
poses a significant problem, as an equal amount of lung tissue in the PLV group weighs more than that in the gas
ventilated group due to the presence of perfluorocarbon (density = 1.8 g/mL) in the air spaces. Therefore, simply
reporting MPO activity per mass of wet lung tissue would have underestimated the amount of MPO actually found
in that tissue in liquid ventilated lungs. Indexing MPO activity per mass of dry tissue or lung protein could
eliminate this problem; however, this procedure could also theoretically underestimate the MPO activity in
animals with significant capillary leak and third spacing of fluid in the pulmonary interstitium and alveolar spaces.
Therefore, we chose to express our results in each animal as units of MPO activity/lung/kg in an attempt to
estimate the total pulmonary MPO per animal. Although this method represents an extrapolation of MPO activity
in the whole lung, derived by the regional MPO activity in different areas of that organ, we believe it to be a
more reliable method for expressing these data in situations where variable capillary leak and edema are present,
when comparing gas and liquid ventilated lungs.
Sigiura et al. [13]demonstrated that the influx and activation of pulmonary neutrophils can be influenced by
different ventilator strategies. A possible implication of their study [13]is that the degree of neutrophil
infiltration, activation, and subsequent lung injury, could be attenuated by a ventilatory strategy that minimizes
cyclic alveolar distention and collapse in the injured or surfactant deficient lung. This scenario would be more
likely to occur during HFOV compared with conventional intermittent mechanical ventilation. Similarly, the
prevention of atelectasis seems to play an important role in this process. Thus, the proposition that mechanical
ventilation is not only a support modality, but also has the potential to influence inflammation, lung injury, and
the disease course follows logically from those data [13].
ARDS occ urs in a sequential fashion involving the complex interac tion of inflammatory mediators, inflammatory
cells, and injury to the target organ. While our model of endotoxin-induced lung injury produced a reliable andsignificant lesion in all rabbits, other investigators have proposed that a second stimulus is required to generate
neutrophil-mediated lung injury [3,36]. Henson et al. [3]reported that the neutrophil sequestration seen
following an endotoxin challenge did not cause lung injury or permeability changes, unless a second stimulus,
such as hypoxemia, was also present. Furthermore, hypoxemia is thought to induce pulmonary capillary
permeability changes, probably by local accumulation and activation neutrophils [3]. Prevention of atelectasis, as
suggested by Sigiura et al. [13], can alter neutrophil influx patterns. Partial liquid ventilation is thought to be a
ventilatory strategy that promotes the recruitment of alveolar units and the reduction of atelectasis [23]. Our
results suggest that the alteration in pulmonary leukostasis seen during liquid ventilation is comparable with that
of a ventilatory strategy that targets lung recruitment such as HFOV. The presence of oxygenated perflubron in
the airways may not only prevent the collapse of diseased alveoli but may also serve as a reservoir for oxygen
molecules to minimize local hypoxia assoc iated with atelec tasis. Thus, partial liquid ventilation may reduce
pulmonary neutrophil infiltration and subsequent injury by several mechanisms in concert.
The delivery of a fixed inspiratory tidal volume to lungs with significant atelectatic areas and heterogeneousdisease is capable of causing overdistention of the less involved regions, leading to the process of volutrauma
[30,37]. The high shear stresses within the alveoli may promote increased capillary permeability, protein leak, and
secondary surfactant deficiency. PLV has been shown to improve lung compliance in surfactant deficient models,
partially by its ability to recruit and maintain functional residual capacity [26]. This effect is probably achieved by
reducing surface tension in the alveolus and through the distending pressure exerted by the dense perflubron
itself. Partial liquid ventilation has also been shown to maintain surfactant syntheses [38]. The maintenance of
surfactant production by partial liquid ventilation in disease states associated with low compliance and
atelectatic lung segments could theoretically play a role in decreasing pulmonary neutrophil accumulation and
progression of lung injury by stabilizing the functional residual capacity.
This study provides new data demonstrating that partial liquid ventilation attenuates pulmonary neutrophil
influx during endotoxemia, a condition frequently leading to the development of ALI and ARDS in the clinical
setting. However, the current short-term experimental design does not allow for one to make extrapolations
attempting to correlate the observed attenuation in pulmonary leukostasis with clinical outcome. The attenuation
in pulmonary inflammatory response seen during partial liquid ventilation is likely to be multifactorial and appears
to be of similar magnitude to that observed with other ventilatory strategies that promote lung recruitment and
avoid patchy overdistention, e.g., HFOV. We speculate that partial liquid ventilation, instituted early in the course
of lung injury, could potentially blunt or delay the evolution of the pulmonary tissue injury that leads to ARDS.
This ventilatory strategy may not only support the patient and injured lungs but may also play a role in modifying
the disease process by maintaining the functional residual capacity, recruiting affected alveoli early on,
preventing surfactant inactivation, and suppressing neutrophil accumulation and activation.
http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?-http://-/?- -
8/13/2019 Partial Liquid Ventilation Reduces Pulmonary Neutrophil Accumulation in an Experimental Model of Systemic Endot
10/13
23/08/13 Ovid: Partial l iquid ventilation reduces pulmonary neutrophil accumulation in an experimental model of systemic endotoxemia and acute lung injury.
ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi 10/13
ACKNOWLEDGMENTS
The authors thank Mr. Paul Frisicaro, Mr . Mark Dowhy, and Mr. M ark Rath, RRT, for their expert tec hnical
assistance in carrying out the intact animal studies.
REFERENCES
1. Rinaldo JE, Christman JW: Mechanisms and mediators of the adult respiratory distress syndrome. Clin Chest Med
1990; 11:621-632 Bibliographic Links [Context Link]
2. Goldblum SE, Reed WP, Sopher RL, et al: Pneumococcus-induced granulocytopenia and pulmonary leukostasis in
rabbits. J Lab Clin Med 1981; 97:278-290 Bibliographic Links [Context Link]
3. Henson PM, Larsen GL, Webster RO, et al: Pulmonary microvascular alterations and injury induced by
complement fragments: Synergistic effect of complement activation, neutrophil sequestration and prostaglandins.
Ann NY Acad Sci 1982; 384:287-300 Bibliographic Links [Context Link]
4. Reed WP, Chick TW, Jutilia K, et al: Pulmonary leukostasis in fatal human pneumococcal bacteremia without
pneumonia. Am Rev Respir Dis 1984; 130:1184-1187 Bibliographic Links [Context Link]
5. Weiland JE, Davis WB, Holter JF, et al: Lung neutrophils in the adult respiratory distress syndrome. Am Rev
Respir Dis 1986; 133:218-225 [Context Link]
6. Heflin AC Jr, Brigham KL: Prevention by granulocyte depletion of increased vascular permeability of sheep lung
following endotoxemia. J Clin Invest 1981; 68:1253-1260 Bibliographic Links [Context Link]
7. Kawano T, Mori S, Cybulsky M, et al: Effect of granulocyte depletion in a ventilated surfactant-depleted lung. J
Appl Physiol 1987; 62:27-33 Bibliographic Links [Context Link]
8. Ognibene FP, Martin SE, Parker MM, et al: Adult respiratory distress syndrome in patients with severe
neutropenia. N Engl J Med 1986; 315:547-551 Bibliographic Links [Context Link]
9. Hamilton PP, Onayemi A, Smyth JA, et al: Comparison of conventional and high-frequency ventilation:
Oxygenation and lung pathology. J Appl Physiol 1983; 55:131-138 Bibliographic Links [Context Link]
10. McCulloch PR, Forkert PG, Froese AB: Lung volume maintenance prevents injury during high-frequency
osc illatory ventilation in surfactant-deficient rabbits. Am Rev Resp ir Dis 1988; 137:1185-1192 Bibliographic Links
[Context Link]
11. Sandhar BK, Nibblett DJ, Argiras EP, et al: Effects of positive end-expiratory pressure on hyaline membrane
formation in a rabbit model of the neonatal respiratory distress syndrome. Intensive Care Med 1988; 14:538-546Bibliographic Links [Context Link]
12. Meredith KS, deLemos RA, Coalson JJ, et al: Role of lung injury in the pathogenesis of hyaline membrane
disease in premature baboons. J Appl Physiol 1989; 66:2150-2158 [Context Link]
13. Sigiura M, McCulloch PR, Wren S, et al: Ventilator pattern influences neutrophils influx and activation in
atelectasis-prone rabbit lung. J App l Physiol 1994; 77:1355-1365 [Context Link]
14. Nesti FD, Fuhrman BP, Steinhorn DM, et al: Perfluorocarbon-associated gas exchange in gastric aspiration. Crit
Care M ed 1994; 22:1445-1452 Bibliographic Links [Context Link]
15. Hirschl RB, Tolley R, Parent A, et al: Evaluation of gas exchange, pulmonary compliance, and lung injury during
total and partial liquid ventilation in the acute respiratory distress syndrome. Crit Care Med 1996; 24:1001-1008
Ovid Full Text Bibliographic Links [Context Link]
16. Smith TM, Steinhorn DM, Thusu K, et al: A liquid perfluorochemical decreases in vitro production of reactive
-
http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003246_1995_23_1533_smith_perfluorochemical_%7c00003246-199810000-00026%23xpointer%28id%28R16-26%29%29%7c60%7c%7covftdb%7c00003246-199509000-00014&P=66&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003246_1995_23_1533_smith_perfluorochemical_%7c00003246-199810000-00026%23xpointer%28id%28R16-26%29%29%7c10%7c%7covftdb%7c00003246-199509000-00014&P=66&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003246_1996_24_1001_hirschl_ventilation_%7c00003246-199810000-00026%23xpointer%28id%28R15-26%29%29%7c60%7c%7covftdb%7c00003246-199606000-00021&P=65&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003246_1996_24_1001_hirschl_ventilation_%7c00003246-199810000-00026%23xpointer%28id%28R15-26%29%29%7c10%7c%7covftdb%7c00003246-199606000-00021&P=65&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003246_1994_22_1445_nesti_perfluorocarbon_%7c00003246-199810000-00026%23xpointer%28id%28R14-26%29%29%7c60%7c%7covftdb%7c00003246-199409000-00015&P=64&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://-/?-http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00004464_1988_14_538_sandhar_respiratory_%7c00003246-199810000-00026%23xpointer%28id%28R11-26%29%29%7c60%7c%7covftdb%7c&P=61&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00000488_1988_137_1185_mcculloch_maintenance_%7c00003246-199810000-00026%23xpointer%28id%28R10-26%29%29%7c60%7c%7covftdb%7c&P=60&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00004560_1983_55_131_hamilton_conventional_%7c00003246-199810000-00026%23xpointer%28id%28R9-26%29%29%7c60%7c%7covftdb%7c&P=59&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00006024_1986_315_547_ognibene_respiratory_%7c00003246-199810000-00026%23xpointer%28id%28R8-26%29%29%7c60%7c%7covftdb%7c&P=58&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00004560_1987_62_27_kawano_granulocyte_%7c00003246-199810000-00026%23xpointer%28id%28R7-26%29%29%7c60%7c%7covftdb%7c&P=57&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00004686_1981_68_1253_heflin_permeability_%7c00003246-199810000-00026%23xpointer%28id%28R6-26%29%29%7c60%7c%7covftdb%7c&P=56&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00000488_1984_130_1184_reed_pneumococcal_%7c00003246-199810000-00026%23xpointer%28id%28R4-26%29%29%7c60%7c%7covftdb%7c&P=54&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00000620_1982_384_287_henson_prostaglandins_%7c00003246-199810000-00026%23xpointer%28id%28R3-26%29%29%7c60%7c%7covftdb%7c&P=53&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00004965_1981_97_278_goldblum_granulocytopenia_%7c00003246-199810000-00026%23xpointer%28id%28R2-26%29%29%7c60%7c%7covftdb%7c&P=52&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003126_1990_11_621_rinaldo_respiratory_%7c00003246-199810000-00026%23xpointer%28id%28R1-26%29%29%7c60%7c%7covftdb%7c&P=51&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026 -
8/13/2019 Partial Liquid Ventilation Reduces Pulmonary Neutrophil Accumulation in an Experimental Model of Systemic Endot
11/13
23/08/13 Ovid: Partial liquid ventilation reduces pulmonary neutrophil accumulation in an experimental model of systemic endotoxemia and acute lung injury.
ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi 11/13
. -
[Context Link]
17. Steinhorn DM, Papo MC, Fuhrman BP, et al: Oxidative damage is reduced during partial liquid ventilation with
Perflubron. Crit Care M ed 1996; 24:A148 [Context Link]
18. Anonymous: NIH guide for the care and use of laboratory animals. Institute of Laboratory Animal Resources
(Ed). Washington, DC, National Academy Press, 1996, pp 1-125 [Context Link]
19. Goldblum SE, Wu KM, Jay M: Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J ApplPhysiol 1985; 59:1978-1985 Bibliographic Links [Context Link]
20. Bradley PP, Priebat DA, Christensen RD, et al: Measurement of cutaneous inflammation: Estimation of
neutrophil content with an enzyme marker. J Invest Dermatol 1982; 78:206-209 Full Text Bibliographic Links
[Context Link]
21. Mrozek JD, Smith KM, BingDR, et al: Exogenous surfactant and partial liquid ventilation: Physiologic and
pathologic effects. Am J Respir Crit Care M ed, 1997; 156:1058-1065 [Context Link]
22. Hernan LJ, Penfil S, Fuhrman BP, et al: Functional FIO2(fFIO (2)) predicts alveolar PO2(PAO2) during
perfluorocarbon-associated gas exchange (PAGE). Crit Care Med 1996; 24:A149 Bibliographic Links [Context Link]
23. Fuhrman BP, Paczan PR, DeFrancisis M: Perfluorocarbon-associated gas exchange. Crit Care Med 1991; 19:712-
722 Bibliographic Links [Context Link]
24. Papo MC, Paczan PR, Fuhrman BP, et al: Perfluorocarbon-associated gas exchange improves oxygenation, lung
mechanics, and survival in a model of adult respiratory d istress syndrome. Crit Care M ed 1996; 24:466-474 Ovid
Full Text Bibliographic Links [Context Link]
25. Foust R, Tran NN, Cox C, et al: Liquid assisted ventilation: An alternative ventilatory strategy for acute
meconium aspiration injury. Pediatr Pulmonol 1996; 21:316-322 Bibliographic Links [Context Link]
26. Leach CL, Fuhrman BP, Morin FC III, et al: Perfluorocarbon-associated gas exchange (partial liquid ventilation)
in respiratory distress syndrome: A prospective, randomized, controlled study. Crit Care Med 1993; 21:1270-1278
Bibliographic Links [Context Link]
27. Leach CL, Greenspan JS, Rubenstein D, et al: Partial liquid ventilation with perflubron in premature infants
with severe respiratory distress syndrome. N Engl J Med 1996; 335:761-767 Bibliographic Links [Context Link]
28. Hirchl RB, Pranikoff T, Gauger P, et al: Liquid ventilation in adults, children and full-term neonates. Lancet
1995; 346:1201-1202 [Context Link]
29. Wolfson M R, Greenspan JS, Deoras KS, et al: Comparison o f gas and liquid ventilation: Clinical, physiological,
and h istological cor relates. J Appl Physiol 1992; 72:1024-1031 Bibliographic Links [Context Link]
30. Amato MB, Barbas CS, Medeiros DM, et al: Beneficial effects of the "open lung approach" with low distending
pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. Am J
Respir Crit Care Med 1995; 152:1835-1846 Bibliographic Links [Context Link]
31. Virmani R, Fink LM, Gunter K, et al: Effect of perfluorochemical blood substitutes on human neutrophil
function . Transfusion 1984; 24:343-347 Bibliographic Links [Context Link]
32. Zimmerman GA, Renzetti AD, Hill HR: Functional and metabolic activity of granulocytes from patients with adult
respiratory distress syndrome. Am Rev Respir Dis 1983; 127:290-300 Bibliographic Links [Context Link]
33. Holman RG, Maier RV: Superoxide production by neutrophils in a model of adult respiratory distress syndrome.
Arch Surg 1988; 123:1491-1495 Bibliographic Links [Context Link]
34. Fowler AA, Fisher BJ, Centor RM, et al: Development of the adult respiratory distress syndrome: Progressive
alteration of neutrophil chemotactic and secretory processes. Am J Pathol 1984; 116:427-435 Bibliographic Links
http://-/?-http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00000457_1984_116_427_fowler_development_%7c00003246-199810000-00026%23xpointer%28id%28R34-26%29%29%7c60%7c%7covftdb%7c&P=84&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00000853_1988_123_1491_holman_neutrophils_%7c00003246-199810000-00026%23xpointer%28id%28R33-26%29%29%7c60%7c%7covftdb%7c&P=83&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00000488_1983_127_290_zimmerman_granulocytes_%7c00003246-199810000-00026%23xpointer%28id%28R32-26%29%29%7c60%7c%7covftdb%7c&P=82&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00007885_1984_24_343_virmani_perfluorochemical_%7c00003246-199810000-00026%23xpointer%28id%28R31-26%29%29%7c60%7c%7covftdb%7c&P=81&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00019521_1995_152_1835_amato_respiratory_%7c00003246-199810000-00026%23xpointer%28id%28R30-26%29%29%7c60%7c%7covftdb%7c&P=80&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00004560_1992_72_1024_wolfson_physiological_%7c00003246-199810000-00026%23xpointer%28id%28R29-26%29%29%7c60%7c%7covftdb%7c&P=79&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00006024_1996_335_761_leach_ventilation_%7c00003246-199810000-00026%23xpointer%28id%28R27-26%29%29%7c60%7c%7covftdb%7c00006024-199609120-00001&P=77&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003246_1993_21_1270_leach_perfluorocarbon_%7c00003246-199810000-00026%23xpointer%28id%28R26-26%29%29%7c60%7c%7covftdb%7c00003246-199309000-00008&P=76&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00006448_1996_21_316_foust_ventilation_%7c00003246-199810000-00026%23xpointer%28id%28R25-26%29%29%7c60%7c%7covftdb%7c&P=75&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003246_1996_24_466_papo_perfluorocarbon_%7c00003246-199810000-00026%23xpointer%28id%28R24-26%29%29%7c60%7c%7covftdb%7c00003246-199603000-00017&P=74&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003246_1996_24_466_papo_perfluorocarbon_%7c00003246-199810000-00026%23xpointer%28id%28R24-26%29%29%7c10%7c%7covftdb%7c00003246-199603000-00017&P=74&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003246_1991_19_712_fuhrman_perfluorocarbon_%7c00003246-199810000-00026%23xpointer%28id%28R23-26%29%29%7c60%7c%7covftdb%7c00003246-199105000-00019&P=73&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003246_1996_24_a149_hernan_perfluorocarbon_%7c00003246-199810000-00026%23xpointer%28id%28R22-26%29%29%7c60%7c%7covftdb%7c&P=72&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00004897_1982_78_206_bradley_inflammation_%7c00003246-199810000-00026%23xpointer%28id%28R20-26%29%29%7c60%7c%7covftdb%7c&P=70&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00004897_1982_78_206_bradley_inflammation_%7c00003246-199810000-00026%23xpointer%28id%28R20-26%29%29%7c20%7c%7covftdb%7c&P=70&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00004560_1985_59_1978_goldblum_myeloperoxidase_%7c00003246-199810000-00026%23xpointer%28id%28R19-26%29%29%7c60%7c%7covftdb%7c&P=69&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://-/?-http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003246_1995_23_1533_smith_perfluorochemical_%7c00003246-199810000-00026%23xpointer%28id%28R16-26%29%29%7c60%7c%7covftdb%7c00003246-199509000-00014&P=66&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003246_1995_23_1533_smith_perfluorochemical_%7c00003246-199810000-00026%23xpointer%28id%28R16-26%29%29%7c10%7c%7covftdb%7c00003246-199509000-00014&P=66&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026 -
8/13/2019 Partial Liquid Ventilation Reduces Pulmonary Neutrophil Accumulation in an Experimental Model of Systemic Endot
12/13
23/08/13 Ovid: Partial l iquid ventilation reduces pulmonary neutrophil accumulation in an experimental model of systemic endotoxemia and acute lung injury.
ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi 12/13
Select All Export Selected to PowerPoint
ontext n
35. Bradley JD, Spooner ML, Rusheen PD, et al: Intratracheal perflubron (PFB) liquid or vapor reduces lung
myeloperoxidase (MPO) levels following cobra venom factor (CVF) challenge in rats. FASEB J 1996; 626:A108
Bibliographic Links [Context Link]
36. Nahum A, Chamberlin AW, Sznajder JI: Differential activation o f mixed venous and arterial neutrophils in
patients with sepsis and acute lung injury. Am Rev Respir Dis 1991; 143:1083-1087 Bibliographic Links [Context
Link]
37. Tsuno K, Prato P, Kolobow T: Acute lung injury from mechanical ventilation at moderately high airway
pressures. J Appl Physiol 1990; 69:956-961 Bibliographic Links [Context Link]
38. Steinhorn DM, Leach CL, Fuhrman BP, et al: Partial liquid ventilation enhances surfactant phospholipid
production. Crit Care M ed 1996; 24:1252-1256 Ovid Full Text Bibliographic Links [Context Link]
IMAGE GALLERY
Table 1
Figure 1 Figure 2
Figure 3
Figure 4
Table 2
http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF5&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF5&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cTT2&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF4&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF3&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF2&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF1&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cTT1&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003246_1996_24_1252_steinhorn_phospholipid_%7c00003246-199810000-00026%23xpointer%28id%28R38-26%29%29%7c60%7c%7covftdb%7c00003246-199607000-00031&P=88&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003246_1996_24_1252_steinhorn_phospholipid_%7c00003246-199810000-00026%23xpointer%28id%28R38-26%29%29%7c10%7c%7covftdb%7c00003246-199607000-00031&P=88&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00004560_1990_69_956_tsuno_ventilation_%7c00003246-199810000-00026%23xpointer%28id%28R37-26%29%29%7c60%7c%7covftdb%7c&P=87&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00000488_1991_143_1083_nahum_differential_%7c00003246-199810000-00026%23xpointer%28id%28R36-26%29%29%7c60%7c%7covftdb%7c&P=86&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?Link+Set+Ref=00003246-199810000-00026|00003860_1996_626_a108_bradley_myeloperoxidase_%7c00003246-199810000-00026%23xpointer%28id%28R35-26%29%29%7c60%7c%7covftdb%7c&P=85&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&Full+Text=L%7cS.sh.22.44%7c0%7c00003246-199810000-00026&image_gallery_select=selectall&resultset=S.sh.22%7c1 -
8/13/2019 Partial Liquid Ventilation Reduces Pulmonary Neutrophil Accumulation in an Experimental Model of Systemic Endot
13/13
23/08/13 Ovid: Partial l iquid ventilation reduces pulmonary neutrophil accumulation in an experimental model of systemic endotoxemia and acute lung injury.
Figure 5
Figure 6
Back to Top
Copyright (c) 2000-2013 Ovid Technologies, Inc.
Terms of Use Support & Training About Us Contact Us
Version: OvidSP_UI03.09.01.101, Sou rce ID 58904
http://www.ovid.com/site/support/techSupportForm.jsp?top=34&mid=35http://www.ovid.com/site/about/index.jsp?top=42http://www.ovid.com/site/resources/index_support.jsphttp://www.ovid.com/site/about/terms.jsphttp://www.ovid.com/http://-/?-http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF6&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1http://ovidsp.tx.ovid.com.zdl.zu.edu.eg:81/sp-3.9.1a/ovidweb.cgi?&S=DLOFFPKJAPDDIOLFNCNKMEOBJMOFAA00&View+Image=00003246-199810000-00026%7cFF5&D=ovft&width=700&height=400&WebLinkReturn=Full+Text%3dL%7cS.sh.22.44%7c0%7c00003246-199810000-00026&resultset=S.sh.22%7c1