Outline p - Awillian's Weblog · Gas Chromatography Introduction • Mobile Phase is a carrier gas....
Transcript of Outline p - Awillian's Weblog · Gas Chromatography Introduction • Mobile Phase is a carrier gas....
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
1
Outline
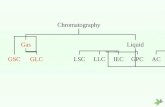
Separation Techniques‘Instrumentation for Gas Chromatography’
• Instrumental Configuration
• Carrier Gas
• Injection Port, Syringes andAutosamplers, Loops
• Packed and Capillary Columns
• Detectors
• Data Collection
Vaq
p
KD
D
www.chem.unsw.edu.au/UGNotes/Guilhaus/
q
Vorg
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
2
Gas Chromatography Introduction
• Mobile Phase is a carrier gas.
• Stationary Phase is a solid orliquid (supported on a surface).
• Stationary phase is located in a(usually) long and narrow column .
• Sample introduced near beginningof column where it is vaporised.
• Detector senses compoundseluting from the column.
• GC is most important analyticalmethod for volatile compounds.
GC Measures Flavours Preservatives Additives Drugs Contaminants,e.g., from packaging, pesticides and herbicides
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
3
Instrument Layout
analystmobilephase supply separation in
heated column
sample loadingdetection
data analysis
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
4
Carrier Gas
• A pure gas usually suppliedfrom a pressurised cylinder
• Gas must be inert with respectto sample.
• Gas must not be retainedsignificantly by stationary phase.
• Prefer less expensive gases.
• Typical gases are He, H2 and N2
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
5
Carrier Gas
?• Which gas is best?• Clue: analysis speed andresolution
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
6
Carrier Gas
• He and H2 give the bestresolution at higher flow rates.
• Higher flow rates mean fasteranalysis times.
• H2 is a fire/explosion risk
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
7
Injectors
• Syringes (liquids 1-5 mL; gases10-100 mL).
• Gas sampling valves
• Autosamplers
• Injection port has complex gasflows to make injection ‘sharp’and reproducible.
• The injection port (injector) isalways in a heated zoned of theGC and it is usually kept hotterthan the GC column
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
8
Syringes
• Syringes require good operatortechnique to be reproducible
• Autosamplers are roboticinjectors - very reproducible - upto 100 samples.
100 mL gas syringe
5 mL liquid syringe
1 mL liquid syringe
autosampler
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
9
Gas Sampling Loops
Step 1Flush loop withnew sample
Step 2Rotate valve and insertloop into mobile phase
For sampling gases
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
10
Columns
• Packed and Capillary columns are used
• Capillary columns most widely used
• Columns kept in a heated oven -temperature needed to keep compoundsin vapour form.
• Column temperature affects partitionequilibrium and diffusion - greatly affectsspeed of analysis.
• Temperature is very carefully controlled
• Isothermal and temperature programsare used
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
11
Packed Columns
• Usually made from 3-6 mm diametertubing of about 1 to 3 m length
• Packed columns are inexpensive tomake, have high capacity butrelatively low resolution
• Require flow rates of 20-50 mL/min
Tube - metal, glassor teflon
Inert - finely dividedparticles act as support forliquid stationary phase orelse are porous and serve asadsorption stationary phases
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
12
Capillary (Open Tubular) Columns
• Long very narrow fused silica tubes typically0.3 mm diameter and 10-50 m long
• Stationary phase coated on the inside wall ofthe column in one of three ways
– Wall coated (WCOT)
– Support coated (SCOT)
– Porous layer (PLOT)
WCOT SCOT PLOT
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
13
Capillary (Open Tubular) Columns
• Capillary columns requirelower flow rates (1-10mL/min)
• Low sample capacity usuallynecessitates a split injection(only small part of injectedliquid reaches column - rest isvented.
• Capillary columns are usuallypurchased ($500-$1000 ea)and are used for severalhundred analyses,
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
14
Capillary Columns
• Higher resolution
• Shorter analysis times
• Greater sensitivity
• Less sample capacity
Compared to Packed Columns OT columns have:
Perfume Oil
Packed2mm x 1.5m
WCOT0.25 x 30m
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
15
Detectors
• Detectors sense the elution of compounds (other than thecarrier gas).
• They create an electrical signal that is recorded versus time tocreate the chromatogram.
• Usually the area under a peak increases in proportion to theamount of a particular substance eluting.
• Main detectors are:
– Flame Ionisation (FID)
– Thermal Conductivity (TCD)
– Electron Capture (ECD)
– Flame Photometric (FPD)
– Mass Spectrometer (MS)
GC Detectors arevery sensitive
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
16
FID
• Eluate mixed with air and H2
• Mixture ignites in flame
• Ions form in flame (about 1CHO+ for 105 reduced Catoms
• Flame becomes slightlyconductive
• Linear response to mass ofreduced carbon atoms ineluate (7-orders)
• Can detect 10-13 g/s
• Insensitive to inorganics
FID is an excellentgeneral detector fororganic compounds
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
17
TCD
• Less sensitive than FID
• Measures thermal conductivity ofcarrier stream (by resistance changeof heated wire).
• H2 and He have high TC and are usedas carrier gas
• Linear to concentration of analytesover 5 orders.
• Sensitivity about 10-8 g analyte / mL ofcarrier
• Can detect many inorganics
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
18
Other Detectors
• Extremely sensitive to compoundscontaining F and Cl groups
• Highly selective for certain pesticideresidues found in food.
Electron Capture Detector:
• H2/O2 flame excites S and P to emitcharacteristic wavelengths.
• A selective detector for S and Pcontaining compounds
• Useful in food analysis
Flame Photometric Detector:
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
19
GC-Mass Spectrometry
• The mass spectrometer obtains acharacteristic fingerprint (a massspectrum) of compounds as they elutefrom the column.
• This is an extremely powerfulcombination that is highly sensitive andselective.
• You will learn much more about thistechnique in CHEM3801.
The ultimate detector for a GC:
m/z
IonAbund.
CH3SCH2CH2CHNH2COOCH2CH3Methionine ethyl ester MW 177
GCMS
Micromass
-
CHEM2801 Analytical & Physical Chemistry for Food Science - Separations -3
© M
. Guilhaus U
NS
W 1999 - A
ll rights reserved.
20
Data Collection
• Most GC systems are now sold with electronic integrators or PCs withsoftware to control the GC and analyse the data.
• Area of peaks for unknowns and standards are compared in thesoftware and concentrations are reported.
• Software can control the whole analysis including the autosampler,calibration, analysis and final reporting.
Shimadzu