Organic - University of Western Ontario · PDF fileFeatures of Organic Compounds ......
Transcript of Organic - University of Western Ontario · PDF fileFeatures of Organic Compounds ......

– 379 –
ORGANIC CHEMISTRY[MH5; Chapter 22, Tutorial Notes]
• The common feature of all organic compounds is that they containthe element carbon together with only a few other elements,principally hydrogen, oxygen and nitrogen.
• The number of known organic compounds already exceeds tenmillion, a number vastly larger than that of all other elements takentogether (with the exception of hydrogen), and the possible numberis virtually limitless.
• For this reason one modern definition of organic chemistry is thechemistry of carbon compounds.
Features of Organic Compounds• Organic compounds are molecular, rather than ionic.
• Each carbon atom always forms a total of 4 covalent bonds.
• Carbon atoms may be bonded to each other, or to other non metalatoms; commonly hydrogen, a halogen, oxygen or nitrogen.

– 380 –
SHAPES OF ORGANIC MOLECULES• The arrangement of atoms bonded to a carbon atom follows VSEPR
theory.......
CH4: four single bonds tetrahedral sp3hybrids
C2H4: two single bonds trig. planar sp2 hybridsone double bond
CO2: two double bonds linear sp hybrids
C2H2: one single bond, linear sp hybrids one triple bond

– 381 –
• Carbon occurs as three allotropes, with three differentstructures.
• The incredible hardness of diamond results from a three-dimensional network of C—C bonds; it is in fact a single giantmolecule; each carbon is bonded to four other carbons.
• In contrast, graphite is built up from two-dimensional sheets of sp2
hybridized C atoms which can easily slip and slide over one another.
• The third allotrope of carbon exists as a network of 60 carbonatoms arranged in a sphere; this form is commonly known as a “buckyball”.
• Organic molecules exhibit isomerism,both structural isomerism andstereoisomerism.
• This means two distinct and different compounds can have the samemolecular formula.

– 382 –
Structural Classification of Organic Compounds• Most organic compounds fall into a small number of groups.• Within each of these groups, all compounds have similar chemical
and physical properties and can be synthesized by similar reactions. • So....... we can concentrate on learning the characteristics of these
groups, or families without discussing individual compounds in detail.
• These "groups" or homologous series can be classified according tosimilarities in structure in two basic ways.
• The first is the Skeletal classification.• The backbone of all organic compounds (except for those having
only one C atom) is a skeleton of C atoms linked to each other inchains or rings.
• These C chains or rings are quite stable; which means that theysurvive unchanged throughout most chemical reactions.
• The majority of the remaining bonds to Carbon are satisfied by Hatoms.
• Compounds that contain only C and H, the hydrocarbons, areregarded as the parent structures.
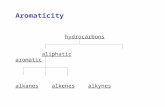
Hydrocarbons
aliphatic aromatic
acyclic cyclic
alkanes alkenes alkynes alkanes alkenes alkynes
• The second classification is that of Functional Groups.• All other compounds may be derived from the parent structures by
replacement of one or more H atoms by other atoms or "groups ofatoms".
• It is the nature of these other atoms which determines thecharacteristic chemical properties or functionality of thecompounds.
THE SKELETAL CLASSIFICATION

– 383 –
• We begin with the hydrocarbons, compounds containing only C and Hatoms.
• The first subdivision is the aliphatic hydrocarbons, which may bedivided into acyclic compounds (having no rings of C atoms) andcyclic compounds (having rings of C atoms).
Acyclic alkanes [MH5; 22.1]• The distinctive feature of the acyclic category is that the C atoms
are linked in chains only. • This basic skeletal structure may be characterized further,
according to the presence or absence of either branches in thechain or of multiple bonds linking the C atoms.
• A continuous sequence of C atoms in an unbranched chain has oftenbeen called a straight chain; not really correct in view of the 109.5Ebond angles!!
• When all of the C atoms are linked by single bonds only, thecompound is said to be saturated, and is called an alkane.
• The first eight members of the acyclic alkane family are:

– 384 –
• A particularly important characteristic of any such homologousseries of compounds is that it can be represented by a generalmolecular formula.
• The general formula for acyclic aliphatic hydrocarbons is CnH2n+2(where n = 1,2,3.... the number of Carbon atoms).
• Let’s take a look at butane, C4H10:
• We see that TWO different arrangements of the atoms arepossible, one with an unbranched chain, and the other with abranched chain.
• Both have molecular formula C4H10 but different molecularstructures; they are isomers, and the general phenomenon istermed isomerism.
• Isomers are categorized as either structural isomers orstereoisomers.

– 385 –
ISOMERISM IN ALKANESStructural Isomerism• When the differences between isomers arise from a difference in
which atoms are bonded to which other atoms, the isomers aretermed structural isomers.
• If one looks at compounds of C and H, it becomes apparent thatthree of the simplest compounds, CH4, C2H6 and C3H8, can have onlyone bonding arrangement.
• Each C atom forms 4 bonds; each H atom 1 bond.

– 386 –
• For C4H10, we saw that two non-equivalent bonding arrangements arepossible, depending upon how the C atoms are linked.
A: CH3CH2CH2CH3..... m.p. !138E b.p. 1E
density 0.579 g mLG1
B: CH3CH(CH3)2 ..... m.p. !159E b.p. !12E density 0.549 g mLG1
A B
• Each isomer has its own unique physical and chemical properties,which can differ greatly.
• For the two isomers of C4H10, the only way that isomer A couldpossibly be converted into isomer B would be if two single bondswere broken, CH3 and H were to exchange positions, and two newbonds were to form. (This does not happen under normalconditions.)
• Because the energy required to break many single bonds is about300!400 kJ molG1 at ordinary temperatures, structural isomers arenormally stable and distinct chemical species.
• When you are drawing structural isomers you must remember thatone or more bonds must be broken to change one bondingarrangement into another.
• This is characteristic of all structural isomers.

– 387 –
Drawing Structural Isomers
C6H14
C4H9CR

– 388 –
• The number of structural isomers possible for large molecules canbe enormous, as the following data show:
CH4 none C6H14 5C2H6 none C7H16 9C3H8 none C8H18 18C4H10 2 C10H22 75C5H12 3 C20H42 366,319
Cyclic or Cyclo Alkanes, CnH2n• It is also possible for an unbranched chain of Carbon atoms to form
a ring through loss of two H atoms, and formation of a C - C sigma(δ) bond.
EXAMPLE: Hexane is C6H14:
It can form a ring:
• As a result, Cyclohexane, C6H12 has two less H atoms than C6H14. • The smallest number of carbon atoms that can form a ring is three,
which results in cyclopropane, C3H6.

– 389 –
1
24
5
3
6
7
8
ORGANIC CHEMISTRY SHORTHAND• The most common method for depicting rings is by simple polygons,
in which each corner is a C atom.• Hydrogen atoms are not shown; the number of H’s at each corner C
is the difference between 4 and the number of line-bonds shown. • Also, each line bond is assumed to terminate in a C atom.
• This is illustrated in the following example where the number of Hatoms at each C (identified by number) is indicated:
There are at: C1 0 H atomsC2 1 H atomsC3,C4,C5 2 H atomsC6, C7, C8 3 H atoms

– 390 –
CH2 CH2
CH2 CH2C4H8
or CH3 or
C6H12
Linked Ring Decalin Fused Rings
Norborane Bridged Ring System
• Each type of ring system is classified also according to the numberof rings (mono- vs poly-cyclic) and, more importantly, the degree ofunsaturation (discussed later).
• Polycyclic molecules can differ in the structural relationshipbetween rings.
• The rings may be separate and independent and simply linkedtogether or they may share one or more atoms.
• Thus fused ring systems share two adjacent atoms (e.g. decalin) andbridged ring systems share at least three atoms (e.g. norbornane).

– 391 –
Bond Rotation and Conformers• In addition to structural isomers, differing short-lived
arrangements of atoms in a molecule, termed conformations, canresult by rapid rotation of atoms, or groups of atoms, around singlebonds.
• When two atoms are connected by a single bond, the atoms are freeto spin about the bond axis.
• For simple molecules such as HCR, this rotation of the two atomswith respect to the bond axis has no effect on the moleculargeometry, and has no structural consequences.
• For a bond to persist, overlap between orbitals must be maintained. Rotation about the H —CR bond ( a σ-bond ) does NOT break theoverlap.
• For molecules containing more than two atoms, however, therotation about a single bond axis may change the moleculargeometry.
• When a rotation around a single bond does result in a change in themolecular geometry, the structures which can be drawn are calledconformations, and the molecules are conformers.

– 392 –
CC
H H
H
HH
HH
H HH
HH
CC C C
H H
HHH
H
• The concept of a preferred conformation is simply illustrated usingethane, C2H6:
A B C
• Figure A shows ethane as a flat molecule (which we know is not so!!).
• Figure B shows ethane in 3-D (recall the wedges indicate the bondcoming forward and the dotted line indicate the bond going away).
• Figure C shows a different conformation of ethane; one “end” ofthe molecule has rotated, so the positions of the Hydrogen atomswith respect to each other have changed.
• There is another notation system known as Newman Projections;these show more effectively the radial distribution of the atomsattached to two adjacent atom centres.

– 393 –
H
H
H
H
H
HH
H
H
H
H
H
Eclipsed Staggered
• The Newman diagrams for ethane show the spatial orientationsobserved when looking along the C—C bond axis - where the centralpoint and its three spokes represent carbon atom 1 (in front), andthe circle represents carbon atom 2 (behind).
• For a simple symmetrical molecule such as ethane there are twoextreme conformations obtained by twisting rotation about theC—C single bond.
• In the eclipsed conformation, each H atom bonded to C atom 1 isdirectly aligned with ("eclipses") one bonded to C atom 2.
• In the staggered conformation (obtained by rotation of the rearCH3 group through 60o), each H atom on carbon 1 is spacedequidistantly between two H atoms on carbon 2.

– 394 –
H
C l
H
H
C l
H
C l
H
H
H
C l
HC l
H
H
H
C l
H
• All other conformations are intermediate in geometry and energybetween these two extremes.
• A staggered conformation is of slightly lower energy than theeclipsed, so at any given instant, the majority of molecules will befound to be roughly staggered.
• Because of their rapid interconversion at room temperature,conformations cannot normally be separated or isolated and aretherefore not isomers.
• Consider the molecule dichloroethane, CR!CH2CH2!CR, and some ofits conformations
• These are different molecular geometries, but because they resultfrom rotation about the C—C single/sigma bond, they areconformers, not isomers.
• They cannot be separated as distinct molecules with uniquechemical and physical properties.
• They are simply two different conformations of the same molecule.

– 395 –
Conformations of Cyclic Compounds• There many compounds, both naturally-occurring and synthetic,
which contain rings of atoms. • The cyclic structure limits the rotation about the bonds linking the
ring atoms, and has extremely important consequences for theconformations and hence the shapes of a molecule.
• The most frequently occurring ring structures contain five or sixatoms bonded together to form a ring.
• The reason for this becomes apparent when one considers thenormal bond angle anticipated for polyvalent atoms on the basis ofVSEPR rules.
• Atoms like carbon prefer to place their four bonding shell electronpairs at the corners of a tetrahedron, thus creating bond angles of109.5o.
• If we think about the internal angles for a series of regular, planarpolygons, it is clear that only in the case of the five - memberedring will the angle be close to the ideal tetrahedral angle.
• This is illustrated by the following diagrams, thedeviation from ideal bond angles is shown below:
49.5E 19.5E 1.5E 10.5E

– 396 –
• In order to avoid these unfavourable bonding angles, many ringslarger than 3-membered are not planar, and, more significantly, ifthe ring contains six C atoms, the tetrahedral bond angle can beexactly achieved by adoption of a non-planar conformation.
Cyclohexane, C6H12• This molecule is unique in that it can adopt two basic conformations
in which all bond angles are perfectly tetrahedral, referred to asthe "chair" and "boat" conformations.
• Even more importantly, in one of these two conformations, thechair, all bonds to any two adjacent carbon atoms are perfectlystaggered !
• This is shown in the Newman projection of the chair conformer,structure A:
A B
• While at room temperature there is relatively easy and rapid inter-conversion between these two extreme conformations, the chair isslightly lower in energy than the boat, so at any given instant themajority of molecules will be found in the chair conformation.

– 397 –
• If we look at the geometry of 6-membered ring systems moreclosely, two important characteristics of the chair becomeevident.......
• Despite its non-planar nature, the ring approximates a planarsystem having 'top' and 'bottom' sides with one of the two H atomson each C atom on top and the other on the bottom. (Structure B).
• Of the twelve H atoms, six lie close to the average plane of thering, and are described as equatorial, while the other six lie aboveand below the plane, and are called axial hydrogen atoms.

– 398 –
ALKENES AND ALKYNES [MH5; 22.2]• When one or more multiple bonds (C=C or C/C) are present, the
molecule is said to be unsaturated because such compounds alwayscontain fewer H atoms than the saturated analogs, the alkanes.
• The presence of a double bond (C = C) means the compound is analkene; general formula CnH2n.
• If the molecule contains a double bond, it will have very differentchemical properties than those of the corresponding alkane.
• The double bond is classified as a functional group; in addition tothe skeletal classification.
• If a triple bond (C/C) is present, the compound is an alkyne; ofgeneral formula CnH2n-2.
• You may have noticed that for each multiple bond introduced, two Hatoms are lost from the fully saturated alkane parent....
• For example, the two C atom compounds:Ethane
Ethene (ethylene)
Ethyne (acetylene)
are considered to have zero, one and two degrees of unsaturation,in that ethane is fully saturated, ethene is short 2 H atoms, whileethyne is missing 4 H atoms.

– 399 –
• 2 H atoms = one degree of unsaturation.• How else can a compound “lose” 2 H atoms??• When it forms a ring; as we saw with cyclohexane.
• Look at a compound of molecular formula C8H14......• This molecule is short of two pairs of H atoms from the
corresponding alkane (C8H18).
• It could contain either a triple bond (2-octyne), two double bonds(1,3-octa-diene), a ring and a double bond (cyclooctene), or even tworings.
CH3(CH2)4C/CCH3
2-octyne 1,3-octadiene
cyclooctene bicyclo octane
• Shown in the figures above are some of the methods used to depict

– 400 –
the structural formula of a compound. • In the condensed structural formula, the H atoms (or other
atoms/groups of atoms) are written on the right hand side of the C to which they are attached (e.g. CH3CH2CH2CH3).
• You may also see:
(CH3)2CHCH2CH3 or CH3(CH2)4CH3
• How do we count the number of rings ?
• Consider decalin....... there are two obvious six-membered rings,fused together, but is there also a third, formed by ignoring thefused bond?
• Is this molecule tricyclic ? • The answer is no - it's bicyclic. • This is indicated by the fact that only two bonds in the structure
need to be broken to make it acyclic.

-401-
O S NH
O O
Decalin
• The skeletal classification may be extended by the introduction of
hetero-atoms, usually N, O and S, as depicted below for a number ofheterocyclic species
STEREOISOMERISM [MH5; 22.5]• Structural isomers are those which arise from the differing
bonding connections among atoms in a molecule. • Stereo isomers are isomers in which the bonding connections
remain unchanged, but the spatial arrangements of specific groupsrelative to one another differ.
• One type of stereoisomerism is know as geometric isomerism.
Geometric Isomerism in Alkenes• The most common structural feature which gives rise to geometric
isomers in carbon compounds is the carbon/carbon double bond. • For the molecule 1,2 - dichloroethene, CRCH=CHCR, two bonding
arrangements exist, one in which both chlorine atoms are on thesame side of the double bond, and one in which they are on oppositesides.

-402-
Cis Trans

-403-
• There is no rotation at room temperature about a double bond, soconversion of cis to trans would require that bonds be broken andreformed.
• Because there is no rotation, the isomers do not interconvert and amixture of them can be separated because of their differingphysical and chemical properties.
• Therefore, they are not conformations, but are actually differentcompounds.
• Geometric isomers exist only when the two carbon atoms of thedouble bond each bear two different groups.
• For example, the molecule 1,1 - dichloroethene, CH2= CCR2 (astructural isomer of 1,2-dichloroethene) does not have geometricisomers...
1,1-dichloroethene
• Although many compounds containing double bonds have geometricisomers, it is incorrect to assume that all do.

-404-
• As a further example, the formula CH3CH=CHCH3 has geometricisomers, but (CH3)2C=CHCH3 does not.......
CH3CH=CHCH3
geometric isomers
(CH3)2C=CHCH3
no geometric isomers
• For each double bond in a molecule, a maximum of two geometricisomers is possible.

-405-
• So for compounds containing two double bonds, a maximum of fourgeometric isomers can result; for the formulaCH3CH=CH-CH=CHCH2CH3..............
• All four of these isomers have different chemical and physicalproperties, and to convert any one into one of the others requiresthe breaking and reforming of one or more bonds.

-406-
H
F
F
H
F
F
Geometric Isomerism in Cyclic molecules• The presence of a ring of atoms in a molecule can also give rise to
geometric isomers. • If one of the H atoms in cyclopentane, A, is replaced by another
atom/group (e.g. CH3 or F), there is no difference between it beingon the top, B or the bottom side C. Structures B and C areidentical.
A B C D
• If the second H atom on that same Carbon atom is replaced by a Fatom in D, there is again no stereoisomerism possible.

-407-
F F F
F
• But if the second F atom is bonded to a different Carbon atom,then the possibility of geometric isomerism arises; the second Fatom can be on the same side of the ring, E, or on the opposite side,structure F, from the first.
E F
E: cis!1,2!difluorocyclopentane F: trans!1,2!difluorocyclopentane
• Structures E and F are obviously different; they are two geometricisomers each with its own characteristic properties.
• Note also that E and F are both structural isomers of D, becausethe connectivity of the atoms is different in D, though E is not astructural isomer of F.
• As is the case with double-bond geometric isomers, the essentialrequirement for geometric isomerism in cyclic molecules is thatthere be two different groups on each of two different ringatoms.

-408-
Optical Isomers• Optical isomers are another example of stereoisomerism.• Optical isomers occur when a carbon atom in a molecule is bonded to
four different atoms or groups.• This type of bonding arrangement always results in two different
forms of the molecule, and the forms are mirror images of eachother.
• The carbon atom in such molecules is called the chiral centre ( orsometimes the stereocentre) ; and these molecules are said to bechiral.
• The two different forms of the molecule are called enantiomers.• Chiral molecules have no plane of symmetry; molecules that do have
a plane of symmetry therefore cannot be chiral.
• A molecule may have more than one chiral centre, in this case, therewill be more than one pair of enantiomers.
• Stereo isomers which are not mirror images of each other arecalled diastereomers.

-409-
• Because molecules that exist as pairs of enantiomers have the sametetrahedral structure and bonded groups, they exhibit nearlyidentical chemical properties.
• However, they may behave differently when they react with otherchiral molecules....
• Most biochemical reactions involve chiral molecules (some withseveral chiral centres) and the “fit” between molecules is crucial.
• So, if chiral molecules exist as pairs of enantiomers, how do we tellthem apart ?
• A characteristic of chiral molecules is their ability to rotate a planeof polarized light; one isomer will rotate the light clockwise (to theright) and the other isomer will rotate the light counter clockwise(or to the left).
• The direction of rotation must be determined experimentally.• We often label these molecules “R” (right handed) and “S” (left
handed); this designation has to do with the orientation of thevarious groups bonded to the chiral carbon.
EXAMPLES:
• In the course of a chemical reaction which produces molecules witha chiral centre, often a 50:50 of the two isomers is formed.
• This is called a racemic mixture; it has no effect on plane polarizedlight.

-410-
OH
CH3
OH
CH3
OH
CH3 CH3
H3C
CH2OH
HH
OHH
OH H
OHOH
HO
Finding chiral carbons (or the chiral centres) in a molecule:
OH H H l l l
H3C - CH2 - C - CH = CH2 H3C - C - CH2 - C - CH2CH3
l l l CH2CR OH CH2CH3

-411-
O H
C OO C H 3
C 6 H 6 benzene C 12 H 10 biphenyl
Oil of wintergreen naphthalene
Aromatic Hydrocarbons or Arenes • When rings are highly unsaturated their chemical behaviour is
different from the corresponding saturated compounds, so theirchemistry is usually treated separately.
• Since most of the examples first identified had distinctive smellsor aromas, they became known as aromatic compounds.
• The simplest (and probably most discussed!!) arene, benzene, C6H6
was discovered in 1825 by Michael Faraday. • All aromatic compounds are cyclic by definition.
• Their most distinctive feature is that the structure cannot beadequately represented by a single Lewis structure.
• Thus benzene (C6H6) is depicted by the two contributing structures:
• Benzene is considered the parent aromatic hydrocarbon, with fourdegrees of unsaturation (three double bonds and one ring).
• The benzene ring is an exceptionally stable structure, even when itis a part of a larger compound.
FUNCTIONAL GROUP CLASSIFICATION

-412-
• Now that we know all (!!??) about the structural skeletons oforganic molecules, we will look at what happens when one or more Hatoms of a hydrocarbon is replaced by other atoms or groups ofatoms.
• These other atoms/groups - the functional groups - are whatdetermine both the chemical and the physical properties of the"families" of compounds.
• A tabular summary of some common functional groups arrangedaccording to the heteroatoms involved follows on page 415 - 416.
• The first families listed are the hydrocarbons. • The presence of a C = C functional group or a C/C functional group
in the unsaturated hydrocarbons gives the molecule characteristicchemical properties.
• Also shown is a short-hand device employed by organic chemists inwriting structural formulas.
• Because the focus of attention is usually the functional group, thehydrocarbon skeleton to which it is attached is frequentlyabbreviated to the symbol, R (for residue, or the rest of themolecule).
• The families containing O are very important. • Those containing a saturated O group (i.e. singly bonded, R—O—X)
may be considered as though it came from water by successivereplacement of the H atoms by hydrocarbon groups (R = alkyl group,e.g. CH3, CH3CH2, etc.).
• This analogy is particularly apt in the case of alcohols which exhibitmany chemical and physical properties similar to water.
• An alcohol, R - OH, can be represented by the general molecularformula, CnH2n+2O (insertion of the O atom has no effect on thenumber of H atoms present).

-413-
C O
• Water, H - O - H and an alcohol, R - O - H
• Ethers have the general formula: R - O - R'
• The remaining families of O-containing compounds arecharacterized by the presence of a carbonyl group, where two Hatoms attached to a C atom of a hydrocarbon have been replaced byan O atom.
• The chemical properties of the carbonyl group are very dependenton the nature of the other groups attached to the carbonyl carbon.
• Therefore, the carbonyl group appears in many different types ofcompounds.
• Unless a molecule has only one C atom (the carbonyl carbon), one ofthe two atoms attached to the C atom of the carbonyl group isalways a C atom (of an alkyl or aryl group).

-414-
C O
C O
C O
• If the second atom is a C the compound is a ketone.
• If the second atom is a H atom, the compound is an aldehyde.
• Due to the C=O bond these compounds are said to be unsaturated. • So, the general formula for acyclic aliphatic aldehydes and ketones
is CnH2nO.
• The other families form a sub-set in that the second atom attachedto the carbonyl C atoms is a heteroatom (i.e. N, O, X).
• When that second atom is the O atom of a hydroxy group, the ombination is called a carboxyl group.
• Because of their acidity, this family of organic compounds is knownas the carboxylic acids.

-415-
• The other members of this sub-set (carboxylate salts and esters)are referred to as acid derivatives because they each can bederived from carboxylic acids.
• The last two families listed in the table contain N atoms. • The amines are derivatives of ammonia where the H atoms have
been replaced successively by C atoms of (alkyl or aryl groups),• Amines exhibit chemical properties similar to ammonia; in particular
they are weak bases.• The amides contain both O and N, with a carbonyl group bonded to
an amino group. • This linkage is of great importance in polyamide polymers such as
nylon and forms the peptide bond that links two amino acids innaturally occurring proteins.

-416-
CC
CC
CCor Alkanes:
Alkenes C Cor
Alkynes:
:
C Cor
Arenes: Alcohols: R OH
R'Ethers: R
O
Aldehydes:
R
O
H R
O
CH
or
The Functional Group Classification

-417-
Ketones:
R
O
R' RC
O
R'
or
Carboxylic Acids:
R
O
OH RC
O
OH
or
Esters:R
O
OR'
R
O
OR'Cor
Amines: R NH2 R NH R' R
R"
R'Nor;
or C
O
R
O
R
Amides:
N
H
R' R'
H
N

-418-
• You can see that in several of the functional groups there areoxygen atoms, some of which are double bonded, or a nitrogen atom.
• Determination of degrees of unsaturation is trickier when there areheteroatoms in the formula of a molecule......
• Oxygen - inclusion of singly bonded O has no effect on the numberof H atoms when compared with the corresponding hydrocarbon.
• In calculations of degrees of unsaturation, one simply ignores Oatoms, if the molecule correctly corresponds to the fully saturatedhydrocarbon.
EXAMPLE: C5H12O
• If the molecule does not correspond to the fully saturatedhydrocarbon, we use the same method to determine degrees ofunsaturation, then we deal with the O.
EXAMPLE: C4H8O

-419-
• Halogen atoms, F, CR, Br, and I : When a halogen atom is presentit is considered to have replaced a H atom, and therefore thenumber of halogen atoms and H atoms must be added to arrive atthe 'base hydrocarbon'.
EXAMPLE: C4H5Br3
• Nitrogen - An amine (R3N, R2NH, RNH2), has one more hydrogenatom than the corresponding hydrocarbon: CH3NH2 vs CH4.
EXAMPLE: C5H9N

-420-
REACTIVITY of ORGANIC COMPOUNDS• Most reactions of organic compounds involve a second reactant,
called a reagent. • For a reaction to take place usually two species must collide and do
so in such a way that one or more covalent bonds are broken and/orone or more bonds are made.
• These reactions occur typically through a number of separate anddiscrete steps.
• Reaction mechanism is the term applied to the description of thedetailed course of the overall reaction - any mechanism mustexplain and account for all observable, experimental facts.
• These include such things as reaction conditions (heat, light,catalysts), formation of intermediates or of by-products throughside reactions, and ultimately, why these changes occur.
• When a covalent bond breaks it can do so in two ways which differin the fate of the shared electron pair...........
(1) HOMOLYTIC BOND BREAKINGA—B ! A• + B• (radicals)e.g. the free radical chlorination of methane
(2) HETEROLYTIC BOND BREAKINGA—B ! A+ + :B G (ions)e.g. a substitution reaction
• Heterolytic bond breaking is the more common of the two andusually involves a polar covalent bond between two atoms ofdifferent electronegativities.
• The more electronegative atom always acquires the electron pair.

-421-
ELECTRONEGATIVITIES OF SOME ELEMENTS
H C N O F2.1 2.5 3.0 3.5 4.0
Si P S CR 1.8 2.1 2.5 3.0
• Many organic reactions take place via initial ionization to give areactive, ionic intermediate.
• When the positive or negative charge is located on a C atom, theintermediate is called a carbocation or a carbanion, respectively.
l — C — CR º CR + l
CARBOCATION
l
— C— H + :B º HB + l
CARBANION• Many of the reactions involve a conversion of one functional group
into another while the C skeleton remains intact and unchanged. • Most of the reactions we will consider in the sections which follow
can be classified in one of three categories:
C
C:

-422-
H BrBr2
FeBr3
Substitution• An atom or group of atoms is substituted for another atom or group
of atoms attached to a C atom, without any change in the number ofdouble or triple bonds if any are present.
CH3CH2OH + HBr ! CH3CH2Br + H2O
• Above, a bromo "group", Br, replaces a hydroxy group, OH, or a Hatom (below).
Addition• Atoms or groups of atoms are added to the compound without any
loss of atoms from it. • There is an increase in the number of atoms attached to at least
one C atom:
Pd catalystCH3CH 4 CH2 + H2 ! CH3CH2CH3
• Here 2 H atoms are added, one to each of the two C atoms of the C = C group.

-423-
Elimination• Atoms or groups of atoms are eliminated, or removed from the
compound without any atoms being added to it. • There is a decrease in the number of atoms attached to at least
one C atom, with a corresponding increase in the number of doubleor triple bonds.
H2SO4 catalystCH3CH2CH2—OH ! CH3CH 4 CH2 + H2O
• Here water is eliminated; a hydrogen atom is removed from one Catom and a hydroxy group from another.
REACTIONS OF THE FUNCTIONAL GROUPS
Acyclic and cyclic alkanes• The outstanding feature of saturated hydrocarbons is their general
lack of reactivity. • This is due to the relatively strong, yet non-polar, C—C and C—H
bonds. • In the absence of any reactive site (or functional group) alkanes do
not react with common acids and bases, or oxidizing and reducingagents.
• Because of this inertness, alkanes can often be used as solvents forreactions of other substances.
• However, under certain conditions alkanes will react with O2, CR2, orBr2.

-424-
Oxygen• The most important use of alkanes is as fuels. • When initiated (e.g. with a spark), alkanes burn in an excess of oxygen
according to the combustion equations discussed in earlier parts ofthe course...
C8H18 (R ) + 25/2 O2 (g) ! 8 CO2 (g) + 9 H2O (R )
• Although an initial input of energy is required, once initiated thereaction proceeds spontaneously and exothermically.
• These combustion reactions are the basis for the use ofhydrocarbons for heat ( natural gas and fuel oil) and for power (gasoline).
• Virtually all organic compounds are combustible in air, so this is not acharacteristic reaction of only alkanes.
Chlorine• When illuminated with ultraviolet light, or heated to 300!400EC, a
mixture of methane and chlorine gases reacts vigorously to formchloromethane and hydrogen chloride:
• This is a substitution reaction, in which a H atom has been replacedby a CR atom.

-425-
• The CH3CR formed can then undergo a second substitution reaction toform dichloromethane, CH2CR2, which in turn can form CHCR3
(chloroform), and yet again to yield carbon tetrachloride, CCR4....
• It is very difficult to limit chlorination of methane to themonosubstituted product.
• Mixing one mole of CR2 with one mole of CH4 might be expected togive one mole of CH3CR and one mole of hydrogen chloride, but this isnot the case.
• At the start of the reaction there is only methane for chlorine toreact with, as is desired, but as the reaction progresses and CH3CR is formed in increasing amounts, there is an increasing chance ofchlorine reacting with CH3CR instead of with the diminishing amountof methane.
• Even using a large excess of methane gives a mixture of products.• Fortunately methane and chloromethane have greatly differing boiling
points (!161 and !24EC, respectively) and can be separated bydistillation.

-426-
Alkenes and alkynes• In contrast to alkanes, unsaturated aliphatic hydrocarbons react
readily with the halogens, acidic reagents and a variety of oxidizingand reducing agents.
• Reaction is characterized by addition of reagent X—Y to the doubleor triple bond.......
• This reaction usually occurs by means of a multi step mechanism.......

-427-
• Once addition to an alkene has occurred the product is saturated, sofurther addition is not possible.
• The most common alkene addition reactions of practical importanceare outlined below.
• Alkynes react in the same way; often the only essential difference isthat further addition can occur since after one addition to an alkyne,the product is an alkene........
H2 CH3C/CH !
Pd
Hydrogen, H2
• The addition of hydrogen converts an alkene to an alkane. • This saturation or hydrogenation of the double bond is an important
reaction both in the research laboratory and in commercialapplications.
• Although this addition reaction is strongly exothermic, it is very slowif no catalyst is used.
Pd catalystCH3CH 4 CH2 + H2 !
• The common catalysts are finely divided metals such as palladium,nickel or platinum.
• The heterogeneous catalyst lowers the activation energy barrier toreaction by adsorbing both reactants on its surface, which facilitatesboth the bond-breaking and the bond-making steps.

-428-
Halogens, X2
• In contrast to hydrogen, both CR2 and Br2 react rapidly with alkenesin the absence of a catalyst.
CH3CH 4 CHCH3 + CR2 !
CH3CH 4 CH2 + Br2 !
• The reaction with Br2 is a useful qualitative test for the presence ofa carbon-carbon double or triple bond.
• Solutions of Br2 in most solvents are coloured red-brown; alkenes anddibromoalkanes are typically colourless.
• The rapid disappearance of the reddish colour of the Br2 solution is acharacteristic reaction of an alkene or alkyne, and provides a simplevisual method of detection.
Water, H2O• Water adds to alkenes in the presence of an acid (catalyst) to form
alcohols: H2SO4 catalyst
CH3CH 4 CHCH3 + H - OH !

-429-
• If the alkene is unsymmetrical (the two C atoms of the double bonddo not bear the same groups), addition can lead to two structurallyisomeric products.
H2SO4 catalystCH3CH 4 CH2 + HOH !
• Both products are formed but, in most instances, one tends to bestrongly favoured over the other.
Hydrogen Halide, HX• Hydrogen halides (HCR, HBr, HI) add to alkenes to give alkyl halides:
CH3CH 4 CHCH3 + H—X !
• Just as in addition of water, addition of hydrogen halide to anunsymmetrical alkene gives a mixture of two products, although onceagain, the formation of one particular isomer tends to predominate.
CH3CH 4 CH2 + HCR !

-430-
Br
Br
Br
Br
Br
Br
Arenes, or Aromatic compounds
• Unlike the addition reaction of alkenes and alkynes, benzene andother aromatic compounds undergo substitution reactions in which aH atom is replaced by a variety of atoms or groups of atoms
Cl, Br NO2 OH alkyl alkenyl
• Substitution is limited to one H atom replaced per carbon atom, butthere are three possible sites where a second substituent could go.....
• The six carbon aromatic ring DOES NOT react !!!
Br NO2 OH CH3 CH CH2

-431-
Alcohols• The system which has been developed for classifying alcohols
specifies the location of the hydroxy group —OH on the C skeleton.
• An alcohol is designated as primary, secondary, or tertiary, whenthe C atom bonded to the OH is attached to one, two or threeother C atoms
H R’ R’ l l l
R - C - OH R - C - OH R - C - OH l l l
H H R”
primary secondary tertiary
• A familiar primary alcohol is Ethyl alcohol, or ethanol, C2H5OH.• This is the one that you drink! (Methanol, CH3OH is the one you do
NOT drink!)
Acid/Base Properties of Alcohols• Like water, alcohols are very weak acids, but, depending on their
structure, usually somewhat weaker still. • A solution of an alcohol in water is neutral.....
HOH º H+ + OH G Ka = 1.8 × 10G16 ROH º H+ + RO G Ka = 10G16 to 10G19
• As water does, alcohols react with the alkali metals (eg. Li, Na, K)to liberate H2 gas.......

-432-
HOH + Na ! Na+ OHG + ½ H2 (g)
ROH + Na ! Na+ ORG + ½ H2 (g)
• The resulting salt, a metal alkoxide, is a very strong base whichhydrolyses extensively in water:
RO G + H2O ! ROH + OH G
• Water is not only a very weak acid but also a very weak base. • In the presence of a strong acid it accepts a proton to form the
hydronium ion, H3O+:
H2O + HCR ! H3O+ + CR G
• Similarly, alcohols behave as bases and can react with strong acidsto form oxonium ions, the equivalent of the hydronium ion:
ROH + H2SO4 ! ROH2+ + HSO4G
• This behavior can be attributed to their related structures -methanol, CH3OH , is simply water with a H atom replaced by amethyl group.
• The —OH functional group, with its lone pairs of electrons, isunchanged.

-433-
Oxidation of Alcohols• Oxidation of alcohols is the first organic oxidation reaction we will
study.• While we know that oxidation means “loss of electrons”, in organic
chemistry it means other things too......• Oxidation also means: loss of 2 H’s or gain of O.• Primary and secondary alcohols oxidize to form carbonyl compounds:
H l [ O ] \
— C —OH ! C = O + H2O l /
• The [O] refers to an oxidizing agent; the most commonly usedoxidants are chromic oxide, CrO3 and dichromate salts Na2Cr2O7 inacidic solution.
• In this reaction is 2 H atoms are eliminated.• This requires that the hydroxy-bearing C atom have at least one H
atom. • The ease of reaction and the nature of the product formed is
dependent on the type of alcohol.• Primary alcohols oxidize extremely readily and the initially formed
product is an aldehyde. • However, aldehydes are very easily oxidized.....
O O ll ll
CH3CH2OH ! CH3 C—H ! CH3 C—OHEthanol Acetaldehyde Acetic acid

-434-
• Unless the oxidation is carried out under carefully controlledconditions, the product isolated from the oxidation of a primaryalcohol is the corresponding carboxylic acid.
• The fact that the alcohol and the aldehyde are being oxidized hereis most easily recognized by drawing analogy to the one-C series
CH3OH ! CH2O ! HCO2Hmethanol formaldehyde formic acid
• The oxidation numbers of C go from !2 through 0 to +2 in thisseries.
• How do we determine the Oxidation Number of Carbon in an organiccompound?
• For each bond between Carbon and an atom less electronegativethan Carbon (usually Hydrogen), assign a -1.
• For each bond between Carbon and an atom of equalelectronegativity (another Carbon or Sulfur), assign a 0.
• For each bond between Carbon and an atom of greaterelectronegativity (Oxygen, Nitrogen or Halogens), assign a +1.
• Add all these numbers together, being sure to keep the signsstraight.
• The result is the Oxidation Number of Carbon.

-435-
EXAMPLE:CH3OH
CH2O
HCOOH

-436-
• Oxidation of a secondary alcohol gives a ketone:
CH3CH — CH3 ! CH3—C—CH3 acetone l ll
OH O
• Ketones do not usually undergo further oxidation.
• Under the same conditions, where primary and secondary alcoholsreact easily, tertiary alcohols, which lack a H atom at the C atombearing the OH group, are not oxidized at all:
CH3 l
CH3— C—OH ! NO REACTION l
CH3
• As no reaction occurs, the red-orange colour of the acidic CrO3 solution remains.
• In contrast, oxidation of 1E and 2E alcohols by CrO3 results in achange in colour from red-orange to the green of Cr3+(aq).
• This difference is the basis of a qualitative test to distinguishprimary and secondary alcohols from tertiary alcohols, and was alsoused in the “breathalyzer” test for inebriated drivers.

-437-
Reduction of Aldehydes and Ketones • It should come as no surprise that the oxidation reactions
described above can be reversed with a suitable reducing agent,converting aldehydes and ketones into alcohols.
• Sodium borohydride, NaBH4 and lithium aluminum hydride, LiAlH4
are commonly used in the laboratory, but H2 gas (with a catalyst) isused for industrial scale reactions.
• If oxidation reactions lose 2 H atoms, then reduction reactionsmust gain 2 H atoms!!
Ethers• The general formula of an ether is R—O—R’ and they are usually
named by stating the nature of R and R’
symmetrical unsymmetricalCH3 — O — CH3 C2H5 — O — CH3
dimethyl ether ethyl methyl ether
• Ethers are weakly polar, with a solubility in water comparable tothat of alcohols.
• Symmetrical ethers are important industrial solvents.• Ethers are synthesized by dehydration of alcohols:
conc. H2SO42 C2H5OH ! C2H5— O — C2H5 + H2O
heat to 140EC

-438-
• Ethers are quite non-reactive; they do not undergo reduction,elimination, oxidation, or reaction with bases, so they are popular inthe laboratory as solvents.
• Because they do not have the — OH functional group, ethersmolecules do not hydrogen bond to each other; this results in quitelow boiling points. (For diethyl ether, the b.p. is 35o C)
Carboxylic Acids and Their Derivatives • A carboxylic acid is a combination of a carbonyl group and a hydroxy
group:
• Because of the unsaturated and dipolar character of thiscombination, acids undergo a variety of reactions, nearly all ofwhich are reactions of the — OH group made possible by thepresence of the carbonyl group.

-439-
Acid/Base Properties of Carboxylic Acids [MH5; 13.4]• The most distinctive chemical property of these compounds is their
acidity. • All are weak acids undergoing partial ionization in water to give
weakly acidic solutions:
R — COOH º H+ + R — COO G Ka . 10G5
• For most of these acids, the magnitude of the acid ionizationconstant, Ka, is in the vicinity of 10G5.
• Why are the carboxylic acids so much stronger acids than water oralcohols - compounds that also contain an —OH group ?
CH3CH2— O — H º H+ + CH3CH2O G Ka = 10G16
ethyl alcohol ethoxide ion
• The difference is attributed to stabilization of the conjugate baseof the acid by resonance through the carbonyl group.
• In the ethoxide ion the lone pairs of electrons are localized on asingle O atom, whereas in the acetate ion electrons are delocalizedequally over both O atoms..........

-440-
• As there are two contributing structures; each O atom has a formalcharge of !½.
• Since this delocalized structure is of lower energy than a localizedone, equilibrium for the formation of the carboxylate lies fartherto the right than that for the formation of an alkoxide ion.
• Titration of an aqueous solution of the acid with one equivalent ofbase gives a solution of the salt...
and the pure carboxylate salt can be isolated in crystalline form byevaporation of the water.
• Carboxylic acids are strong enough acids to liberate CO2 gas frombicarbonate salts (e.g. NaHCO3).

-441-
Formation of Carboxylic Acid Derivatives• Carboxylic acids can be converted into a number of structurally
related derivatives, compounds in which the —OH group is replacedby other groups.
• One important derivative is the ester.
Esters• When a carboxylic acid and an alcohol are heated in the presence of
an acid catalyst (usually conc. HCR or conc. H2SO4) an equilibrium isestablished with an ester and water:
O O ll ll
CH3 C—OH + HO - CH3 º CH3 - C—O—CH3 + H2Oacetic acid methanol methyl acetate
• The yield of the ester can be maximized by continuous removal ofthe water as it is formed; this is an example of an eliminationreaction.
• The most important reaction of esters, and of all other acidderivatives, is hydrolysis to the corresponding carboxylic acid,which is simply the reverse of ester formation:

-442-
• Volatile esters generally have pleasant odours, and are ofimportance in the perfume industry and as artificial flavourings.
• The last two families of compounds to be considered in this briefsummary, amines and amides, contain a N atom.
Amines• Amines are derivatives of ammonia and are classified as primary,
secondary or tertiary according to the number of C atoms attachedto the nitrogen atom...........
H - N - H R - N - H R - N - R’ R - N - R’ l l l l H H H R” ammonia primary secondary tertiary
• Note that the terms primary, secondary and tertiary are applied toamines in a different way than they are applied to alcohols.
• As is the case for ethers and ketones, the R groups may be thesame or different, and the nitrogen atom may be part of a ring ofatoms.

-443-
Basicity of Amines [MH5; 13.5]• Like ammonia, amines are weak bases and their aqueous solutions
are basic:
NH3 + H2O º NH4+ + OHG Kb = 1.8 × 10G5
CH3NH2 + H2O º CH3NH3
+ + OHG Kb = 5.0 × 10G4
• As bases, amines react quantitatively with acids to formsubstituted ammonium salts:
CH3NH2 + HCR ! CH3NH3+ CR G
methylamine methylammonium chloride
(CH3)3N: + CH3COOH ! (CH3)3NH+ CH3COOGtrimethylamine trimethylammonium acetate
• For most aliphatic amines the magnitude of the base ionizationconstant, Kb, is about 10G4, close to that of ammonia, 1.8 x 10G5
• In contrast to esters, amines usually smell awful !• Methylamine has a sharp odour like that of ammonia, and
trimethylamine smells like dead fish.

-444-
Amides• Amides are compounds formed from the reaction of a carboxylic
acid with an amine.• In these compounds, the —OH of the carboxylic acid is replaced by
—NH2 or —NHR or —NRR'.• So, an amide contains a carbonyl group linked to an amino group....
• Amides are generally prepared by a two step process:
1)

-445-
2)
• Amides are hydrolysed when heated with aqueous acids or aqueousbases, reforming the carboxylic acid and the amine.
• Depending upon the pH of the reaction, either the acid or the amineis obtained as a salt.
• The amide linkage, sometimes called the peptide bond, isparticularly important in some synthetic polymers and proteins.
• The analgesic acetaminophen is an amide.

-446-
ORGANIC POLYMERS [MH5;22.6]• Polymers are giant molecules (from the Greek, 'many parts' poly
meros ) made by joining many small molecules often referred to asmonomers.
• Polymer molecules can have molecular weights ranging fromthousands to millions.
• Polymers may be naturally occurring, such as proteins,polysaccharides and nucleic acids.
• Synthetic, or man made polymers include many plastics, polyesters,polyamides and composites.
• Polymers are often classed according to their method ofsynthesis......
• Addition polymers such as polyethylene, polystyrene (PS)andpolyvinylchloride (PVC) are made by adding together the simplealkenes: CH2=CH2 , C6H5CH=CH2 and CH2=CHCR respectively.

-447-
• Heating ethylene to 100-250EC at 1000-3000 atmospheres in thepresence of a catalyst gives polymers with molar masses of severalmillion.
• A polymer with molar mass of one million would contain almost36,000 ethylene molecules!!!!
• Depending upon the reaction conditions, different molecularstructures result, with different properties.
• Polymer chains can be linear (HDPE),
branched (LDPE),

-448-
C C
or cross-linked (CLPE)
• HDPE is dense,hard and strong, due to the close packing formed by the long, linearchains.
• LDPE is soft and flexible; it has a lower density due to thebranching of the polymer chains.
• CLPE is rigid and inflexible; the cross linking adds to the rigidity ofthe polymer.
• Teflon is polytetrafluoroethylene:
• Styrofoam (food and beverage containers) is made by coolingfoamed, molten polystyrene

-449-
CC
O
OH
HO
O
HOCH2CH2OH+
C C
OOH
O OCH2CH2OH
+ H2O
OCH2CH2
O
O
CC
OCH2CH2OO
O O
CC
H
OH
• Condensation polymers such as polyesters and polyamides are madeby elimination reactions.
• The reaction between terephthalic acid and ethylene glycol givesthe polymer PETE, polyethylene terephthalate, by elimination of awater molecule.
• Soft drinks are sold in PETE bottles.• PETE is an example of a polyester..........when recycled, 5 large pop
bottles will make a T- shirt!!

-450-
H
N
H
N
O
O
+N
H
HN
H
H
OHHO
O
O
H
HO
O
O
N
H
N
H O
O
OH
H
H
N
H
N
• Nylon is a polyamide prepared by elimination of H2O in the reactionof adipic acid and hexamethylenediamine:
• You will note that the linkage in nylon is another example of anamide linkage.

-451-
Natural Polymers: Polypeptides and Proteins• Proteins contain an amide linkage formed by elimination of a water
molecule in a condensation reaction between two amino acids.• Amino acids (with one exception) are chiral molecules......
• When two amino acids form an amide linkage, it is known as apeptide bond; the molecule formed is known as a dipeptide.
• When the resultant molecule becomes really large (consisting ofhundreds or thousands of amino acids), the polymer becomes knownas a protein.
• The properties of a protein depend on the sequence of amino acidsthat make up the backbone of the molecule.
• Enzymes (biological catalysts) are polypeptide molecules and may berendered useless if even one amino acid in the polymer chain ismissing or out of place.