NeuroFlexor brochure 090312 - Aggero MedTech ·...
Transcript of NeuroFlexor brochure 090312 - Aggero MedTech ·...
NeuroFlexorTM Brochure Aggero Medtech AB 9 March 2012
1
Moving spasticity measurement forward
Background and rationale for NeuroFlexor
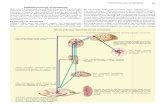
Spasticity is a type of muscle stiffness often occurring after brain injury related to for
example stroke, trauma, multiple sclerosis and cerebral palsy. Spasticity limits movement
and functional activities (dressing, feeding walking, etc, can cause pain and reduce quality of
life. Many therapies and pharmaceutical interventions are used to reduce spasticity such as
physiotherapy, orthoses and injection of botulinum toxin into muscle). However, treatments
are costly and often inefficient. One problem is that the treatments are not properly
targeted to the underlying causes of spasticity. Spasticity is composed of both muscle
stiffness and neural over activity (increased stretch reflex). Today in the clinic spasticity is
measured manually using a subjective scale. Treatment today cannot be properly targeted
since the clinical measures used do not allow for separate measurement of these spasticity
components. Another problem with subjective scales for measurement of spasticity is the
NeuroFlexorTM Brochure Aggero Medtech AB 9 March 2012
2
lack of accuracy and reliability. Spasticity measurement is therefore a major problem in
neurology and rehabilitation today.
Aggero Medtech AB is a Swedish medical technology company that develops, manufactures
and markets innovative diagnostic and training instruments for research and clinical work.
Our ambition is to provide a full range of clinically validated and easy-‐to use tools for
quantitative measurement and training in the field of neurorehabilitation. At present, we
offer a new solution to accurate clinical measurement of spasticity (NeuroFlexor). The
NeuroFlexor device offers the unique possibility to objectively measure the different
components of spasticity in a highly reliable manner. The device measures the resistance
when the limb is moved passively (subject is relaxed). Using a validated biomechanical
model the neural (reflex) and muscle (stiffness) components of spasticity are estimated. The
NeuroFlexor measurement gives information on the individual’s unique spasticity profile
and is thus useful for optimizing treatment. The method is reliable making it suitable for
patient follow-‐ups and evaluation of treatments.
NeuroFlexor: Main features and advantages
Innovative combination of technology and biomechanical know-‐how
The NeuroFlexor is composed of a servo motor and force sensor coupled to a portable
computer. Passive limb displacements are produced at controlled velocities. Resistance is
measured accurately using a force sensor. The resulting resistance trace is then analyzed
according to a validated biomechanical model. The biomechanical model describes how
various mechanical components in the stretched muscle and the resulting neural activation
by the stretch reflex contribute to the resistance produced during stretching. This new
model for estimation of spasticity components has been validated and the findings have
been published in the journal Neurorehabilitation Neural Repair (2011).
Measurements are objective and reliable
The NeuroFlexor provides objective data from a force sensor. This eliminates the subjective
step of rating how the spasticity feels (the most common clinical way to measure spasticity
NeuroFlexorTM Brochure Aggero Medtech AB 9 March 2012
3
today). A recent study also shows that the NeuroFlexor measurements are highly reliable,
i.e., measurements performed by different clinicians or on different times are very similar
(see NeuroFlexor: Scientific results and clinical benefits).
NeuroFlexor is easy to use in the clinic
NeuroFlexor has been developed to be suitable for clinical use. The device is compact and
easy to use being suitable for bedside measurements as well as research protocols.
Controlling the device by use of the developed interface on the computer is simple and
interactive. Illustration of measurement results on the computer screen is informative for
both clinician and patient.
NeuroFlexor user interface is
designed to be easy to use for the
clinician. Movement settings can be
saved and reloaded. This means that
similar measurements can be
performed on different days
increasing reliability of
measurements.
NeuroFlexorTM Brochure Aggero Medtech AB 9 March 2012
4
NeuroFlexor results are detailed and informative. Above is an example of results in a a stroke patient with
spasticity. Bright red curves on top represent force traces produced with a fast velocity (240°/s) stretch. Bright
red curves below represent force traces produced with a slow velocity (5°/s) stretch. Dark red curves show
force profile without hand (run empty). The software estimates the Neural, Elastic and Viscosity components
contributing to the resistance according to a validated biomechanical model.
NeuroFlexor provides useful data for research
The NeuroFlexor method offers many advantages for clinical research on spasticity.
NeuroFlexor is the only device available today which allows measurement of both neural
and mechanical contributions to passive movement resistance in spastic muscles. With
NeuroFlexor spasticity patient-‐specific spasticity profiles can be obtained allowing a more
specific approach when evaluating effect of anti-‐spasticity treatments. Good reliability of
measurements also makes NeuroFlexor suitable for detection of treatment induced
benefits.
NeuroFlexorTM Brochure Aggero Medtech AB 9 March 2012
5
NeuroFlexor and patient satisfaction
Patients are very content to have their spastic muscle measured using the NeuroFlexor.
Patients express that they are very happy that there now is a method that allows the
clinicians to measure their spasticity correctly and accurately. Patients are also very
interested in seeing their own spasticity profile (reflex and mechanical contributions).
Differences occurring after anti-‐spasticity treatment can be visualized and are of major
interest to patients.
NeuroFlexor: Scientific results and clinical benefits Validation of model
In a study published in Neurorehabilitation Neural Repair (2011) 31 chronic hemiparetic
stroke patients with hand spasticity were measured using the same biomechanical model as
implemented in NeuroFlexor. The findings support the validity of the biomechanical model.
This study scientifically shows that muscle and neural spasticity components can be
measured using the NeuroFlexor method.
Spasticity components before and during
ischemic nerve block in five stroke patients.
The neural component (NC) was abolished or
reduced drastically after the ischemic nerve
block (T0=before nerve block; T25=with
effective nerve block). The mechanical
components (elasticity, E and viscosity, V)
were not affected by the nerve block.
Reliability
Preliminary findings from an ongoing study in 32 chronic stroke patients shows that
measurements with NeuroFlexor have high intra-‐ and interrater reliability.
NeuroFlexorTM Brochure Aggero Medtech AB 9 March 2012
6
The results suggest that measurements with NeuroFlexor have good reliability. This is
important for the clinician wishing to follow-‐up how the spasticity profile changes in
response to treatment.
Reliability of neural component measurements. On left, comparison of two separate measurements by one
rater. On right, comparison of measurements from two raters.
NeuroFlexor: scientific publications, presentations and awards
Publications
Validation of a new biomechanical model to measure muscle tone in spastic muscles.
Lindberg P, Gäverth J, Islam M, Fagergren A, Borg J, Forssberg H. Neurorehabilitation Neural
Repair. 2011;25(7):617-‐625.
Conference presentations
Validity and reliability of a biomechanical method for quantification of "spasticity" in chronic
stroke patients. Gäverth J, Islam M, Fagergren A, Sandgren M, Borg J, Eliasson A-‐C, Forssberg
H, Lindberg P. Scientific poster presented at World Phyical Therapy Conference, Amsterdam,
June 2011
Reliability of a new biomechanical method for the estimation of neural and non-‐neural
contributions to spasticity. Gäverth J, Sandgren M, Lindberg P, Eliasson AC. Scientific poster
presented at European Neurological Society Conference, Lisbon, Portugal, May 2011
NeuroFlexorTM Brochure Aggero Medtech AB 9 March 2012
7
Validation of a biomechanical model for quantification of “spasticity” in chronic stroke
patients. Gäverth J, Lindberg P, Islam M, Fagergren A, Borg J, Forssberg H. Scientific poster
presented at World Neurorehabilitation Conference, Vienna, Austria, March 2010
Prizes and awards
The NeuroFlexor invention was awarded the regional Stockholm 2009 SKAPA-‐Prize. The
SKAPA Prize is awarded to inventors in memory of Alfred Nobel. The award is designed to
incentivise the commercialisation of technological innovation and creativity.
Scientific Poster prize, World Neurorehabilitation Conference, Vienna, Austria (2010)
NeuroFlexorTM Brochure Aggero Medtech AB 9 March 2012
8
Partners
The development of NeuroFlexor has been supported by Swedish universities, research and
innovation agencies.
Contact details
Aggero Medtech AB
MT, Danderyds Sjukhus
182 88 Danderyd
Stockholm, Sweden.
www.aggeromedtech.com
Email: [email protected]















![Manual Pratico Rede Cegonha [444 090312 SES MT]](https://static.fdocuments.net/doc/165x107/54df415b4a7959df518b4f09/manual-pratico-rede-cegonha-444-090312-ses-mt.jpg)