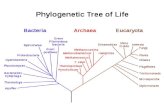
MAJOR CLADES IN SOLANUMBASED ON ndhF SEQUENCE DATA
Transcript of MAJOR CLADES IN SOLANUMBASED ON ndhF SEQUENCE DATA

MAJOR CLADES IN SOLANUM BASED ON ndhF SEQUENCE DATA
Lynn Bohs
ABSTRACT. Analysis of sequence data from the chloroplast gene ndhF identifies at least 12 major well-supported clades within the genus Solanum. These are brieflydescribed, given informal clade names, and compared with the groups recognized by previous Solanum workers. Non-molecular synapomorphies are proposed for many of the clades. Continued use of informal taxonomic designations is advocated for new infrageneric groups within Solanum.
Key words: ndhF, phylogeny, Solanaceae, Solanum.
3

28
A FESTSCHRIFT FOR WILLIAM G. D’ARCY
Solanum L., with approximately 1400species, is the largest and most diversegenus in the Solanaceae. Solanum is distinguished from most of the other
genera in the tribe Solaneae by its poricidalanther dehiscence, a character present in nearlyall Solanum species and shared only with therelated genus Lycianthes. Although some previous authors considered Lycianthes to be partof Solanum, recent molecular studies have con-firmed the distinction between the two genera (Bohs & Olmstead, 1997; Olmstead & Palmer, 1997; Olmstead et al., 1999).Morphologically, Lycianthes is differentiated fromSolanum by differences in calyx structure (D’Arcy,1986).
Although poricidal anther dehiscence is a rela-tively striking synapomorphy that allowsSolanum to be recognized as a genus, its divisioninto infrageneric subunits is less clear. Early work-ers attempted to divide Solanum into two largegroups based on spininess, anther morphology,or hair type. Linnaeus, for instance, dividedSolanum into two groups, Spinosa and Inermia,based on the presence or absence of spines(Linnaeus, 1753). Dunal, in his early treatments(Dunal, 1813, 1816), maintained this distinction ashis categories Aculeata and Inermia, but in hisSolanum treatment for DeCandolle’s Prodromus
(Dunal, 1852) he established two major infra-generic divisions (“sections”) based on anthershape as well as presence or absence of spines. Hisgroup Pachystemonum encompassed species thatlack spines and have relatively short, broadanthers with large terminal pores which oftenenlarge into longitudinal slits, whereasLeptostemonum included prickly species with rel-atively narrow, distally tapered anthers with smallterminal pores that do not elongate with age.Bitter (1919) also recognized two major infra-generic groups, the subgenera Eusolanum andLeptostemonum, based on the same characters asDunal (1852). Seithe (1962), in contrast, dividedSolanum into two groups based not on spininessor anther morphology, but rather on hair type.She recognized two categories in Solanum at therank of “Chorus subgenerum,” distinguished bythe presence of unbranched or dendriticallybranched hairs (Chorus subgenerum Solanum)versus stellate hairs (Chorus subgenerumStellatipilum). Danert (1970) integrated charac-ters of branching patterns and shoot morphologywith previous systems, and, along with Gilli(1970), summarized the infrageneric groups rec-ognized by Bitter and Seithe.
These works provided the elements of D’Arcy’s(1972) classification scheme and conspectus,which is the most widely used system today.
1. Solanum subg. Archaesolanum Marzell
ca. 8 species, Australian region
2. Solanum subg. Bassovia (Aubl.) Bitter
ca. 15 species, New World
3. Solanum subg. Leptostemonum (Dunal) Bitter
ca. 250–450 species, worldwide
4. Solanum subg. Lyciosolanum Bitter
1 species, South Africa
5. Solanum subg. Minon Raf. [subg. Brevantherum(Seithe) D’Arcy, in D’Arcy (1972)]
ca. 70 species, New World
6. Solanum subg. Potatoe (G. Don) D’Arcy
ca. 300 species, worldwide
7. Solanum subg. Solanum
200 species, worldwide
TABLE 1.Solanum subgenera according to D’Arcy (1972, 1991).

MAJOR CLADES IN SOLANUM BASED ON ndhF SEQUENCE DATA
D’Arcy’s scheme recognizes seven subgenera inSolanum (Table 1; D’Arcy, 1972, 1991). Theserange in size from the monotypic subgenusLyciosolanum to the subgenera Solanum,Leptostemonum, and Potatoe, each of which con-tains hundreds of species. In his 1972 paper,D’Arcy lectotypified all subgeneric names andprovided a provisional conspectus of Solanum. Inthis conspectus, Solanum subgenera, sections,and series are listed along with their respectivetype species, but all the component species ofeach infrageneric group are not listed, nor arethe characters given that circumscribe each of thegroups. D’Arcy (1991) made minor modificationsto this system. Whalen (1984) provided a detailedconspectus of Solanum subg. Leptostemonum(the spiny solanums). Subsequently, both Nee(1999) and Child and Lester (2001) provided infra-generic schemes for Solanum. Nee (1999) listedthe species that belong to each of his taxonomiccategories, but his system includes only NewWorld taxa. Child and Lester (2001), like D’Arcy(1972), listed only the type species for each oftheir infrageneric groups. Hunziker (2001) modi-fied D’Arcy’s (1972) system and provided descrip-tions and commentary for each recognized sec-tion. All of these classifications relied completelyon morphological data and, except for Whalen(1984), none utilized techniques of cladistic analysis.
The advent of molecular data has revolutionizedthe field of plant systematics and has led to newinsights into phylogenetic relationships at all tax-onomic levels. In the Solanaceae, Olmstead andcolleagues have used restriction site andsequence data to examine phylogenetic relation-ships across the entire family (Olmstead & Palmer,1992; Olmstead et al., 1999). Molecular studiesabove the sectional level in Solanum include theworks of Spooner et al. (1993), Olmstead andPalmer (1997), and Bohs and Olmstead (1997,1999, 2001). These studies provide informationon major clades within Solanum, but none havesampled from all the subgenera recognized bymorphological systematists such as Bitter, Seithe,Danert, and D’Arcy.
This paper presents results of a molecular phylo-genetic study designed to identify major clades
within Solanum using sampling from a broadspectrum of Solanum subgroups. Results are pre-sented from an analysis of sequence data fromthe chloroplast gene ndhF. Sampling includesmembers of all seven of D’Arcy’s subgenera andover 40 of the 62 sections listed in D’Arcy (1991).All the sections listed in D’Arcy’s (1972) conspec-tus as well as many sections described after 1972are discussed in context of the major ndhF clades.Major lineages are described with informal cladenames and their component sectional groups arelisted. Possible non-molecular synapomorphiesare suggested for most of the identified clades.These characters have been taken from the gen-eral references listed above and from the person-al observations of the author. Although they mayprovide general guidelines for the recognition ofclades, this is not intended to be a substitute forthorough morphological analyses, as many of thesuggested characters are variable within cladesand may be found in more than one clade. A fewoverall recommendations are made for taxonom-ic rearrangements within the genus Solanum.Results of analyses using data from nuclear genessuch as ITS and waxy (Bohs, in prep.) and fromcombined chloroplast and nuclear sequence datasets will be presented in a future publication.
MATERIALS AND METHODSSampling comprised 120 species of Solanaceae,including five outgroup genera from the tribeSolaneae. Outgroup taxa were chosen on thebasis of previously published results of Olmsteadet al. (1999) and Bohs and Olmstead (2001).Solanum taxa sampled included representativesof all seven of D’Arcy’s subgenera and a numberof sections or species groups thought to representdistinctive clades based on morphology.Collection and voucher information is given inTable 2.
DNA was extracted from fresh or silica-driedleaves or, in rare cases, from herbarium speci-mens, using either the modified CTAB procedureof Doyle and Doyle (1987) or a microextractionprotocol that used QiaQuick columns and buffer(Qiagen, Inc.) in place of the isopropanol precipi-tation step in the CTAB procedure. Samples
29

Cap
sicu
m b
acca
tum
L. v
ar. p
end
ulu
m(W
illd
.) E
shb
aug
h2
Bo
livia
Esh
bau
gh
158
4(M
U)
U08
916
Cap
sicu
m c
hac
oen
se H
un
z.2
Bo
livia
Esh
bau
gh
158
6A(M
U)
AF5
0080
9
Jalt
om
ata
pro
cum
ben
s (C
av.)
J. L
. Gen
try3
Mex
ico
Dav
is 1
189A
U47
429
Jalt
om
ata
sin
uo
sa (
Mie
rs)
Mio
ne1
Bo
livia
Nee
et
al. 5
1830
(NY
)A
F500
835
Lyci
anth
es h
eter
ocl
ita
(Sen
dtn
.) B
itte
r1C
ost
a R
ica
Bo
hs
2376
(U
T)U
7275
6
Lyci
anth
es r
anto
nn
ei (
Car
rièr
e) B
itte
r2B
IRM
S.0
928
RG
O S
-96
(WTU
)A
F500
840
Phys
alis
alk
eken
giL
.2D
’Arc
y co
llect
ion
D’A
rcy
1770
7(M
O)
U08
927
Sola
nu
m a
bu
tilo
ides
(Gri
seb
.) B
itte
r &
Lill
o2
Min
on
Bre
van
ther
um
BIR
M S
.065
5R
GO
S-7
3 (W
TU)
U47
415
Sola
nu
m a
ccre
scen
s St
and
l. &
C. V
. Mo
rto
n1
Lep
tost
emo
nu
mEr
yth
rotr
ich
um
eC
ost
a R
ica
Bo
hs
2556
(U
T)A
F500
795
Sola
nu
m a
dh
aere
ns
Ro
em. &
Sch
ult
.1Le
pto
stem
on
um
Mic
raca
nth
aC
ost
a R
ica
Bo
hs
2473
(U
T)A
F224
061
Sola
nu
m a
dsc
end
ens
Sen
dtn
.1So
lan
um
Go
nat
otr
ich
um
Bo
livia
Bo
hs
& N
ee 2
738
(UT)
AF5
0079
6
Sola
nu
m a
eth
iop
icu
m L
.2Le
pto
stem
on
um
Olig
anth
esB
IRM
S.0
344
RG
O S
-74
(WTU
)A
F500
797
Sola
nu
m a
gg
reg
atu
m J
acq
.2Ly
cio
sola
nu
mLy
cio
sola
nu
mSo
uth
Afr
ica
RG
O 9
9-25
(W
TU)
AF5
0079
8
Sola
nu
m a
liger
um
Sch
ltd
l.1M
ino
nH
olo
ph
ylla
Bo
livia
Nee
et
al. 5
1822
(NY
)A
F500
799
Sola
nu
m a
llop
hyl
lum
(Mie
rs)
Stan
dl.1
No
nef
Allo
ph
yllu
mf
Pan
ama
Bo
hs
2339
(U
T)U
4741
6
Sola
nu
m a
myg
dal
ifo
lium
Ste
ud
.1Po
tato
eJa
smin
oso
lan
um
Arg
enti
na
Nee
& B
oh
s 50
840
(NY
)A
F500
800
Sola
nu
m a
ph
yod
end
ron
S. K
nap
p2
Sola
nu
mG
emin
ata
Co
lom
bia
RG
O S
-92
(WTU
)A
F500
801
Sola
nu
m a
pp
end
icu
latu
m D
un
al2
Pota
toe
Bas
arth
rum
Mex
ico
An
der
son
140
1(C
ON
N)
AF2
2406
2
Sola
nu
m a
rbo
reu
mD
un
al1
Sola
nu
mG
emin
ata
Co
sta
Ric
aB
oh
s 25
21 (
UT)
U47
417
Tax
ona
Subg
enus
bSe
ctio
nb
Col
lect
ion
Vou
cher
dG
enB
ank
loca
lity
cac
cess
ion
num
ber
30
A FESTSCHRIFT FOR WILLIAM G. D’ARCY
TA
BL
E2.
Sou
rces
of
taxa
seq
uen
ced
fo
r n
dh
F. a D
NA
ext
ract
s p
rovi
ded
by:
(1)
L.
Bo
hs,
Un
iver
sity
of
Uta
h,
Salt
Lak
e C
ity,
Uta
h;
(2)
R.
G.
Olm
stea
d,
Un
iver
sity
of
Was
hin
gto
n,
Seat
tle,
Was
hin
gto
n;
(3)
T. M
ion
e, C
entr
al C
on
nec
ticu
t St
ate
Un
iver
sity
, N
ew B
rita
in,
Co
nn
ecti
cut;
(4)
D.
Spo
on
er,
Un
iver
sity
of
Wis
con
sin
,M
adis
on
, Wis
con
sin
; (5)
A. B
run
eau
, McG
ill U
niv
ersi
ty, M
on
trea
l, C
anad
a. b
Acc
ord
ing
to
D’A
rcy
(197
2, 1
991)
un
less
no
ted
. c Acc
essi
on
nu
mb
ers
giv
en f
or
cult
ivat
ed c
olle
ctio
ns.
BIR
M =
cu
ltiv
ated
at
Un
iver
sity
of
Bir
min
gh
am, U
.K.;
NIJ
= c
ult
ivat
ed a
t U
niv
ersi
ty o
f N
ijmeg
en, T
he
Net
her
lan
ds;
PI =
U.S
.D.A
. Pla
nt
Intr
od
uct
ion
nu
mb
er; D
’Arc
y co
llect
ion
= c
ult
ivat
ed a
t M
O. d
Co
llect
or,
nu
mb
er, a
nd
her
bar
ium
acr
on
ym (
if k
no
wn
) o
f h
erb
ariu
m v
ou
cher
s. e C
hild
(19
98).
f Bo
hs
(199
0). g
Nee
(19
99).
hSy
mo
n (
1981
). i D
’Arc
y (1
992)
. j Kn
app
(20
00).

Sola
nu
m a
rgen
tin
um
Bit
ter
& L
illo
1M
ino
nH
olo
ph
ylla
Arg
enti
na
Bo
hs
2539
(U
T)U
7275
2
Sola
nu
m a
vicu
lare
G. F
ors
t.2
Arc
hae
sola
nu
mA
rch
aeso
lan
um
BIR
M S
.080
9n
on
eU
4741
8
Sola
nu
m b
etac
eum
Cav
.1G
enu
s C
yph
om
and
raPa
chyp
hyl
laB
oliv
iaB
oh
s 24
68 (
UT)
U47
428
Sola
nu
m b
revi
cau
le B
itte
r4Po
tato
ePe
tota
Bo
livia
PI 4
9811
5H
awke
s et
al.
6701
AF5
0080
3
Sola
nu
m b
ulb
oca
stan
um
Du
nal
4Po
tato
ePe
tota
Mex
ico
PI 3
4775
7Ta
rn 1
53A
F500
804
Sola
nu
m c
aesi
um
Gri
seb
.1So
lan
um
Sola
nu
mB
oliv
iaB
oh
s et
al.
2815
(UT)
AF5
0080
5
Sola
nu
m c
alile
gu
ae C
abre
ra1
Sola
nu
mg
Du
lcam
arag
Arg
enti
na
Nee
& B
oh
s 50
809
(NY
)A
F500
806
Sola
nu
m c
amp
anu
latu
m R
. Br.2
Lep
tost
emo
nu
mC
amp
anu
lata
BIR
M S
.038
7R
GO
S-7
8 (W
TU)
AF5
0080
7
Sola
nu
m c
amp
ech
ien
se L
.1Le
pto
stem
on
um
Un
clea
rC
ost
a R
ica
Bo
hs
2536
(U
T)A
F224
071
Sola
nu
m c
and
idu
m L
ind
l.2Le
pto
stem
on
um
Lasi
oca
rpa
BIR
M S
.097
5R
GO
S-1
00 (
WTU
)A
F224
072
Sola
nu
m c
apsi
coid
es A
ll.1
Lep
tost
emo
nu
mA
can
tho
ph
ora
Peru
Bo
hs
2451
(U
T)A
F500
808
Sola
nu
m c
aro
linen
se L
.2Le
pto
stem
on
um
Lath
yro
carp
um
BIR
M S
.181
6R
GO
S-7
7 (W
TU)
AF5
0081
1
Sola
nu
m c
hen
op
od
inu
m F
. Mu
ell.2
Lep
tost
emo
nu
mG
raci
liflo
raB
IRM
S.0
813
no
ne
AF5
0081
2
Sola
nu
m c
iner
eum
R. B
r.1Le
pto
stem
on
um
Mel
on
gen
ahN
IJ 9
0475
0120
Bo
hs
2852
(U
T)A
F500
813
Sola
nu
m c
itru
llifo
lium
A. B
rau
n2
Lep
tost
emo
nu
mA
nd
roce
ras
BIR
M S
.012
7R
GO
S-7
9 (W
TU)
AF5
0081
4
Sola
nu
m c
leis
tog
amu
m S
ymo
n2
Lep
tost
emo
nu
mO
ligan
thes
B
IRM
S.0
844
RG
O S
-80
(WTU
)A
F500
815
Sola
nu
m c
on
dit
um
C. V
. Mo
rto
n1
Lep
tost
emo
nu
mU
ncl
ear
Bo
livia
Bo
hs
& N
ee 2
733
(NY
)A
F500
816
Sola
nu
m c
ord
ove
nse
Ses
sé &
Mo
ç.1
Min
on
Exte
nsu
mC
ost
a R
ica
Bo
hs
2693
(U
T)U
7275
1
Sola
nu
m c
rin
itip
es D
un
al2
Lep
tost
emo
nu
mTo
rva
Co
lom
bia
RG
O S
-81
(WTU
)A
F500
817
Sola
nu
m c
rin
itu
m L
am.1
Lep
tost
emo
nu
mC
rin
itu
me
NIJ
924
7500
49B
oh
s 28
50 (
UT)
AF5
0081
8
Sola
nu
m c
risp
um
Ru
iz &
Pav
.2M
ino
nH
olo
ph
ylla
BIR
M S
.048
6n
on
eA
F500
819
Sola
nu
m d
efle
xum
Gre
enm
.1So
lan
um
Go
nat
otr
ich
um
Co
sta
Ric
aB
oh
s 27
15 (
UT)
AF5
0082
0
Sola
nu
m d
elit
esce
ns
C. V
. Mo
rto
n1
Min
on
Ho
lop
hyl
laA
rgen
tin
aN
ee &
Bo
hs
5081
0(N
Y)
AF5
0082
1
Sola
nu
m d
iplo
con
os
(Mar
t.)
Bo
hs1
Gen
us
Cyp
ho
man
dra
Pach
yph
ylla
Bra
zil
Bo
hs
2335
(U
T)A
Y04
9014
Sola
nu
m d
od
dsi
i Co
rrel
l4Po
tato
ePe
tota
Bo
livia
Spo
on
er e
t al
. 665
1A
F500
822
Sola
nu
m d
rym
op
hilu
m O
. E. S
chu
lz1
Lep
tost
emo
nu
mPe
rsic
aria
ePu
erto
Ric
oB
oh
s 24
61 (
UT)
AF5
0082
3
Sola
nu
m d
ulc
amar
a L.
2Po
tato
eD
ulc
amar
aU
.S.A
.n
on
eU
4741
9
Sola
nu
m e
chin
atu
m R
. Br.1
Lep
tost
emo
nu
mLe
pro
ph
ora
NIJ
954
7500
52B
oh
s 27
27 (
UT)
AF5
0082
4
Sola
nu
m e
laea
gn
ifo
lium
Cav
.2Le
pto
stem
on
um
Lep
rop
ho
raU
.S.A
.R
GO
S-8
2 (W
TU)
AF2
2406
7
Sola
nu
m e
tub
ero
sum
Lin
dl.4
Pota
toe
Peto
ta
Ch
ile P
I 498
311
UA
C 1
322
AF5
0082
5
MAJOR CLADES IN SOLANUM BASED ON ndhF SEQUENCE DATA
31

Sola
nu
m e
volv
ulif
oliu
m G
reen
m.1
Bas
sovi
aH
erp
ysti
chu
mg
Pan
ama
Kna
pp &
Mal
let 9
178
(BM
)A
F500
826
Sola
nu
m f
ero
ciss
imu
m L
ind
l.2Le
pto
stem
on
um
Gra
cilif
lora
BIR
M S
.081
9R
GO
S-8
3 (W
TU)
AF5
0082
7
Sola
nu
m f
ieb
rig
iiB
itte
r1So
lan
um
Sola
nu
mB
oliv
iaB
oh
s et
al.
2784
(UT)
AF5
0082
8
Sola
nu
m f
raxi
nif
oliu
m D
un
al1
Pota
toe
Bas
arth
rum
Co
sta
Ric
aB
oh
s 25
58 (
UT)
AF5
0081
0
Sola
nu
m f
urf
ura
ceu
m R
. Br.2
Lep
tost
emo
nu
mU
ncl
ear
BIR
M S
.144
2R
GO
S-8
4 (W
TU)
AF5
0082
9
Sola
nu
m g
lau
cop
hyl
lum
Des
f.2
Sola
nu
mG
lau
cop
hyl
lum
D’A
rcy
colle
ctio
nn
on
eU
7275
3
Sola
nu
m h
avan
ense
Jac
q.1
Sola
nu
mD
iam
on
on
eN
IJ 9
0475
0122
Bo
hs
3076
(U
T)A
F500
830
Sola
nu
m h
ercu
leu
m B
oh
s2g
enu
s Tr
igu
era
Mo
rocc
oJu
ry 1
3742
(R
NG
)A
F224
065
Sola
nu
m h
ind
sian
um
Ben
th.1
Lep
tost
emo
nu
mU
ncl
ear
Mex
ico
Bo
hs
2975
(U
T)A
F500
831
Sola
nu
m h
oeh
nei
C. V
. Mo
rto
n1
Lep
tost
emo
nu
mN
emo
ren
seB
razi
lFo
lli 1
668
(MO
)A
F500
832
Sola
nu
m in
eleg
ans
Ru
sby1
Min
on
Ho
lop
hyl
laB
oliv
iaN
ee e
t al
. 518
13(N
Y)
AF5
0083
3
Sola
nu
m ip
om
oeo
ides
Ch
od
at &
Has
sl.1
Sola
nu
mg
Du
lcam
arag
Bo
livia
Bo
hs
& N
ee 2
766
(UT)
AF5
0083
4
Sola
nu
m ja
mai
cen
seM
ill.2
Lep
tost
emo
nu
mEr
iop
hyl
laB
IRM
S.1
209
RG
O S
-85
(WTU
)A
F224
073
Sola
nu
m ja
smin
oid
es P
axto
n2
Pota
toe
Jasm
ino
sola
nu
mC
olo
mb
iaR
GO
S-8
6 (W
TU)
AF5
0083
6
Sola
nu
m ju
gla
nd
ifo
lium
Du
nal
4Po
tato
ePe
tota
Co
lom
bia
LA 2
788
AF5
0083
7
Sola
nu
m la
cin
iatu
mA
ito
n1
Arc
hae
sola
nu
mA
rch
aeso
lan
um
New
Zea
lan
dB
oh
s 25
28 (
UT)
U47
420
Sola
nu
m le
pid
otu
mD
un
al1
Min
on
Lep
ido
tum
Co
sta
Ric
aB
oh
s 26
21 (
UT)
AF5
0083
8
Sola
nu
m li
dii
Sun
din
g1
Lep
tost
emo
nu
mN
ycte
riu
mN
IJ 9
3475
0022
Bo
hs
2903
(U
T)A
F500
839
Sola
nu
m lu
teo
alb
um
Pers
.1G
enu
s C
yph
om
and
raC
yph
om
and
rop
sis
BIR
M S
.004
2B
oh
s 23
37 (
UT)
U72
749
Sola
nu
m ly
cop
ersi
cum
L.2
Gen
us
Lyco
per
sico
nLy
cop
ersi
con
U.S
.A. (
cult
.)n
on
eU
0892
1
Sola
nu
m m
acro
carp
on
L.2
Lep
tost
emo
nu
mM
elo
ng
ena
BIR
M S
.013
3R
GO
S-8
8 (W
TU)
AF2
2406
8
Sola
nu
m m
aho
rien
se D
’Arc
y &
Rak
ot.
1Le
pto
stem
on
um
Cry
pto
carp
um
iM
adag
asca
rB
oh
s 25
76 (
UT)
AF5
0084
1
Sola
nu
m m
amm
osu
m L
.2Le
pto
stem
on
um
Aca
nth
op
ho
raB
IRM
S.0
983
RG
O S
-89
(WTU
)A
F224
074
Sola
nu
m m
apir
ien
se B
itte
r1N
on
efA
llop
hyl
lum
fB
oliv
iaN
ee &
Sol
omon
303
05(U
T)A
F500
842
Sola
nu
m m
auri
tian
um
Sco
p.2
Min
on
Bre
van
ther
um
BIR
M S
.086
0R
GO
S-9
0 (W
TU)
AF5
0084
3
Tax
ona
Subg
enus
bSe
ctio
nb
Col
lect
ion
Vou
cher
dG
enB
ank
loca
lity
cac
cess
ion
num
ber
32
A FESTSCHRIFT FOR WILLIAM G. D’ARCY
TA
BL
E2
CO
NT
INU
ED.

MAJOR CLADES IN SOLANUM BASED ON ndhF SEQUENCE DATA
33
Sola
nu
m m
elo
ng
ena
L.2
Lep
tost
emo
nu
mM
elo
ng
ena
BIR
M S
.065
7R
GO
S-9
1 (W
TU)
AF2
2406
9
Sola
nu
m m
on
tan
um
L.1
Pota
toe
Reg
man
dra
NIJ
904
7502
05B
oh
s 28
70 (
UT)
AF5
0084
4
Sola
nu
m m
ult
ifid
um
Ru
iz &
Pav
.1Po
tato
eR
egm
and
raN
IJ 9
0475
0205
Bo
hs
2863
(U
T)A
F500
845
Sola
nu
m m
uri
catu
mA
ito
n2
Pota
toe
Bas
arth
rum
Co
lom
bia
RG
O S
-93
(WTU
)A
F500
846
Sola
nu
m n
emo
ren
se D
un
al1
Lep
tost
emo
nu
mN
emo
ren
seB
oliv
iaB
oh
s &
Nee
275
7(U
T)A
F500
847
Sola
nu
m n
itid
um
Ru
iz &
Pav
.1M
ino
nH
olo
ph
ylla
Bo
livia
Nee
319
44
(NY
)A
F224
075
Sola
nu
m o
chro
ph
yllu
m V
an H
eurc
k &
Mü
ll. A
rg.1
Sola
nu
mG
emin
ata
Bo
livia
Bo
hs
& N
ee 2
805
(UT)
AF5
0084
8
Sola
nu
m p
alit
ans
C. V
. Mo
rto
n1
Sola
nu
mPa
raso
lan
um
BIR
M S
.083
7/70
Bo
hs
2449
(U
T)A
F224
064
Sola
nu
m p
has
eolo
ides
Po
l.1B
asso
via
Her
pys
tich
um
Co
sta
Ric
aB
oh
s 24
85 (
UT)
AF5
0084
9
Sola
nu
m p
hys
alif
oliu
mR
usb
yva
r. n
itid
ibac
catu
m(B
itte
r) E
dm
on
ds1
Sola
nu
mSo
lan
um
U.S
.A.
Bo
hs
2467
(U
T)U
4742
1
Sola
nu
m p
inn
atis
ectu
m D
un
al4
Pota
toe
Peto
taM
exic
o P
I 347
766
Tarn
205
AA
F500
850
Sola
nu
m p
iura
e B
itte
r4Po
tato
ePe
tota
Peru
PI 3
1099
7H
awke
s 24
43A
F500
851
Sola
nu
m p
rin
op
hyl
lum
Du
nal
1Le
pto
stem
on
um
Olig
anth
esh
NIJ
904
7501
71B
oh
s 27
25 (
UT)
AF5
0085
2
Sola
nu
m p
seu
do
cap
sicu
m L
.2M
ino
nPs
eud
oca
psi
cum
BIR
M S
.087
0n
on
eU
4742
2
Sola
nu
m p
tych
anth
um
Du
nal
2So
lan
um
Sola
nu
mU
.S.A
.R
GO
S-9
4 (W
TU)
U47
423
Sola
nu
m p
ub
iger
um
Du
nal
1M
ino
nH
olo
ph
ylla
NIJ
904
7501
04n
on
eA
F500
853
Sola
nu
m p
yrac
anth
um
Lam
.2Le
pto
stem
on
um
Olig
anth
escu
lt.(
UCS
B Bo
t.G
ard.
)R
GO
S-9
5 (W
TU)
AF5
0085
4
Sola
nu
m q
uad
ran
gu
lare
L.f
.2So
lan
um
Qu
adra
ng
ula
reSo
uth
Afr
ica
RG
O 9
9-46
(W
TU)
AF5
0085
5
Sola
nu
m r
ioje
nse
Bit
ter1
Sola
nu
mEp
isar
cop
hyl
lum
Arg
enti
na
Nee
& B
oh
s 50
843
(NY
)A
F500
856
Sola
nu
m r
ost
ratu
mD
un
al1
Lep
tost
emo
nu
mA
nd
roce
ras
U.S
.A.
no
ne
U47
424
Sola
nu
m r
ovi
rosa
nu
m D
on
n. S
m.1
Sola
nu
mG
emin
ata
Co
sta
Ric
aB
oh
s 29
19 (
UT)
AF5
0085
7
Sola
nu
m r
ug
osu
m D
un
al1
Min
on
Bre
van
ther
um
Co
sta
Ric
aB
oh
s 30
11 (
UT)
AF5
0085
8
Sola
nu
m s
and
wic
ense
Ho
ok.
& A
rn.1
Lep
tost
emo
nu
mIr
eno
sola
nu
mH
awai
iB
oh
s 29
92 (
UT)
AF5
0085
9
Sola
nu
m s
chim
per
ian
um
Ho
chst
.2Le
pto
stem
on
um
Torv
aB
IRM
S.1
538
RG
O S
-97
(WTU
)A
F500
860
Sola
nu
m s
chle
chte
nd
alia
nu
m W
alp
.1M
ino
nEx
ten
sum
Co
sta
Ric
aB
oh
s 29
15 (
UT)
AF5
0086
1
Sola
nu
m s
eafo
rth
ian
um
An
dre
ws2
Pota
toe
Jasm
ino
sola
nu
mB
IRM
S.0
051
no
ne
U47
425
Sola
nu
m s
isym
bri
ifo
lium
Lam
.1Le
pto
stem
on
um
Cry
pto
carp
um
Arg
enti
na
Bo
hs
2533
(U
T)A
F500
862
Sola
nu
m s
ten
op
hyl
lidiu
m B
itte
r1Po
tato
ePe
tota
NIJ
904
7500
79B
oh
s 28
55 (
UT)
AF5
0080
2
Sola
nu
m s
tram
on
ifo
lium
Jac
q.5
Lep
tost
emo
nu
mLa
sio
carp
aPe
ruW
hal
en 8
60 (
HU
T)A
F500
863

34
A FESTSCHRIFT FOR WILLIAM G. D’ARCY
Tax
ona
Subg
enus
bSe
ctio
nb
Col
lect
ion
Vou
cher
dG
enB
ank
loca
lity
cac
cess
ion
num
ber
Sola
nu
m t
erm
inal
e Fo
rssk
.1So
lan
um
Afr
oso
lan
um
NIJ
814
7500
72B
oh
s 27
19 (
UT)
AF5
0086
4
Sola
nu
m t
hel
op
od
ium
Sen
dtn
.1U
ncl
earj
Un
clea
rjB
oliv
iaN
ee &
Bo
hs
5085
8(N
Y)
AF5
0086
5
Sola
nu
m t
olia
raea
D’A
rcy
& R
ako
t.1
Lep
tost
emo
nu
mU
ncl
eari
Mad
agas
car
Bo
hs
2574
(U
T)A
F500
866
Sola
nu
m t
orv
um
Sw.2
Lep
tost
emo
nu
mTo
rva
BIR
M S
.083
9R
GO
S-1
01 (
WTU
)L7
6286
Sola
nu
m t
rid
ynam
um
Du
nal
2Le
pto
stem
on
um
Nyc
teri
um
BIR
M S
.183
1R
GO
S-1
02 (
WTU
)A
F500
867
Sola
nu
m t
rifl
oru
m N
utt
.1So
lan
um
Para
sola
nu
mU
.S.A
.B
oh
s 30
62 (
UT)
AF5
0086
8
Sola
nu
m t
rip
arti
tum
Du
nal
1So
lan
um
Pa
raso
lan
um
BIR
M S
.070
8/71
Bo
hs
2465
(U
T)U
7275
0
Sola
nu
m t
rise
ctu
m D
un
al1
Pota
toe
No
rman
iaFr
ance
Bo
hs
2718
(U
T)A
F224
063
Sola
nu
m t
rizy
gu
m B
itte
r1B
asso
via
Pter
oid
eaC
ost
a R
ica
Bo
hs
2511
(U
T)U
7275
4
Sola
nu
m t
ub
ero
sum
L.2
Pota
toe
Peto
taU
.S.A
. (cu
lt.)
WR
F 16
10L7
6287
PI (
2457
93U
SDA
NR
SP-6
X 2
4579
6)
Sola
nu
m t
urn
ero
ides
Ch
od
at1
Sola
nu
mG
on
ato
tric
hu
mB
oliv
iaN
ee e
t al
. 517
16(N
Y)
AF5
0086
9
Sola
nu
m u
lean
um
Bit
ter1
Bas
sovi
aPt
ero
idea
D’A
rcy
colle
ctio
nB
oh
s 27
20 (
UT)
AF5
0087
0
Sola
nu
m v
esp
erti
lio A
ito
n2
Lep
tost
emo
nu
mN
ycte
riu
mB
IRM
S.2
091
RG
O S
-103
(W
TU)
AF2
2407
0
Sola
nu
m v
illo
sum
Mill
.1So
lan
um
Sola
nu
mIr
an P
I 304
600
Bo
hs
2553
(U
T)A
F224
066
Sola
nu
m w
alla
cei (
A. G
ray)
Par
ish
1Po
tato
eeC
alif
orn
iso
lan
um
eU
.S.A
.B
oh
s 24
38 (
UT)
U47
426
Sola
nu
m w
end
lan
dii
Ho
ok.
f.2
Lep
tost
emo
nu
mA
cule
iger
um
BIR
M S
.048
8n
on
eU
4742
7
Wit
her
ing
ia s
ola
nac
ea L
’Hér
.1C
ost
a R
ica
Bo
hs
2416
(U
T)U
7275
5
TA
BL
E2
CO
NT
INU
ED.

MAJOR CLADES IN SOLANUM BASED ON ndhF SEQUENCE DATA
extracted with the modified CTAB method werepurified using cesium chloride density gradientcentrifugation or a phenol-chloroform protocol.Amplification and sequencing of the ndhF geneused the primers and PCR program given in Bohsand Olmstead (1997). PCR products were cleanedusing QiaQuick spin columns and sequenced onan ABI automated sequencer. Sequences wereedited and contigs assembled using the programSequencher (Gene Codes Corp.). After a consen-sus sequence was obtained, it was aligned by eyeto a template sequence (Nicotiana tabacum L.).Base changes relative to the template sequencewere then double-checked against the chro-matograms. No alignment difficulties wereencountered in assembling the sequences into adata set in NEXUS file format. All new sequencesreported here have been submitted to GenBank(Table 2). The data set and resultant phylogenet-ic trees have been submitted to TreeBASE (acces-sion numbers S735 and M1167).
The data matrix was analyzed using unweightedparsimony with the program PAUP*4.0b10(Swofford, 2002). The analysis used the heuristicsearch algorithm with the TBR and MulTreesoptions, 714 random addition replicates withrearrangements limited to 100,000 per replicate,and gaps treated as missing data. Trees wererooted using Physalis alkekengi as the outgroup.Bootstrap analyses were performed with 500replicates using the heuristic search option, TBRand MulTrees, MaxTrees set to 1000, and1,000,000 rearrangements per replicate.
The data were also analyzed using the parsimonyratchet (Nixon, 1999) as implemented in the pro-gram PAUPRat (Sikes & Lewis, 2001). Five repli-cate searches of 200 iterations each were per-formed. The shortest trees from all searches wereretained and combined into a single consensustree.
The same data matrix was analyzed by maximumlikelihood using the program fastDNAml (Olsenet al., 1994) on a UNIX platform computer.Parameters used in the analysis were a transi-tion/transversion ratio of 1.0006 (estimated using
ML in PAUP from a neighbor-joining tree of the120-taxon data set), empirical base frequencies (A= 0.27665, C = 0.15518, G = 0.18366, T = 0.38450),and random addition order.
RESULTSThe ndhF sequences obtained for all taxa exceptLycianthes heteroclita, Solanum wendlandii, S. diploconos, and S. deflexum were 2086 basepairs long, corresponding to positions 24 through2109 in the tobacco ndhF sequence. Lycianthesheteroclita had a 15 bp insertion, S. wendlandiihad a 33 bp insertion, and S. diploconos had a 24bp insertion between positions 1476 and 1477.Solanum deflexum had a 9 bp deletion betweenpositions 1703 and 1711.
Of 2119 total characters in the data set, 541 werevariable and 288 of these were parsimony-informative. Pairwise sequence divergence calcu-lated using the Kimura 2-parameter modelranged from 3.4% between S. candidum versusLycianthes heteroclita to 0.048% in the closelyrelated species pairs S. ferocissimum versus S.chenopodinum, S. vespertilio versus S. liddii, S.doddsii versus S. stenophyllidium, and S. piuraeversus S. doddsii. Solanum schlechtendalianumand S. lepidotum had identical ndhF sequences.
The available memory capacity of PAUP on aPower Macintosh G4 was reached after saving18,200 most parsimonious trees from 714 randomaddition replicates. These trees were 1053 stepslong with a CI (excluding uninformative charac-ters) of 0.497 and RI of 0.819. PAUPRat saved 992trees of 1053 steps out of 1000 iterations. Thestrict consensus trees from the heuristic parsimo-ny and the PAUPRat searches were nearly identi-cal, differing only in greater resolution at two ofthe branch tips in the PAUPRat consensus tree(not shown). Likewise, the maximum likelihoodtopology (not shown) was virtually identical tothe parsimony trees and included the same taxain the major clades described below. This analysiswas completed overnight, examined 39,626 trees,and resulted in a tree with a log likelihood of–13487.40739.
35

36
A FESTSCHRIFT FOR WILLIAM G. D’ARCY
In these trees, Solanum forms a monophyleticclade, with members of the former generaLycopersion, Cyphomandra, Normania, andTriguera nested within it (Fig. 1). Species of allthese genera have been transferred to Solanum(Spooner et al., 1993; Bohs, 1995; Bohs &Olmstead, 2001). Capsicum plus Lycianthesemerges as the sister group to the Solanum cladewith bootstrap support of 70%. Solanum plus thegenera Jaltomata, Lycianthes, and Capsicum forma well-supported clade (bootstrap = 100%), andLycianthes plus Capsicum form a well-supportedgroup (bootstrap = 89%).
At least 12 major clades can be discerned withinSolanum (Fig. 1, see pp. 48–49). These clades aresupported with bootstrap values ranging from51% (Leptostemonum s.l.) to 100% (theRegmandra, Archaesolanum, and Normaniaclades). However, the relationships among thesemajor clades are unclear, because for the mostpart they form a polytomy at the base ofSolanum. Several of these clades conform toinfrageneric groups recognized by previoussystematists, but others do not.
These clades have been given informal cladenames and are briefly described below with a listof their constituent sections and non-molecularsynapomorphies that may define them. Asterisks(*) indicate sections or species groups that havebeen sampled in the present analysis. Othergroups listed under each clade are inferred tobelong there due to morphological similarity.Brief comparisons are made with reference toD’Arcy’s (1972) classification and with severalother schemes.
DISCUSSIONMajor clades defined by ndhF data:
1. Thelopodium clade
3 spp., South America
Included taxa:
Solanum thelopodium species group sensu Knapp (2000)*
This group is morphologically distinctive due toits enlarged roots, single-stemmed growth habit,reduced number of sympodia, and narrow,tapered, dimorphic anthers. It was revised recent-ly by Knapp (2000), who recognized three species.One of them, S. thelopodium, was included in thendhF analysis, where it forms a single branch atthe very base of Solanum. This placement is sur-prising and has not been suggested by recentSolanaceae systematists, although Bitter thoughtthat S. thelopodium was sufficiently distinct tomerit generic rank (Knapp, 2000). Dunal (1852)and Seithe (1962) placed S. thelopodium intoSolanum sect. Anthoresis (Dunal) Bitter, but thismeans little, as section Anthoresis is a catch-allgroup of disparate taxa. D’Arcy did not include itin either of his summary classifications (D’Arcy,1972, 1991). Nee (1999) put this species intoSolanum sect. Pteroidea (Potato clade), but thendhF data do not support this placement. Furthersampling is needed to determine if the basal posi-tion of this clade in Solanum is correct or is per-haps a long branch artifact.
2. Regmandra clade
ca. 7 spp., South America
Included taxa:
Solanum subg. Potatoe (G. Don) D’Arcy pro parte
Solanum sect. Regmandra (Dunal) D’Arcy*
D’Arcy (1972, 1991) placed this small group ofspecies from Pacific coastal deserts of SouthAmerica into Solanum subg. Potatoe. Nee (1999)also allied this section with the potatoes, where-as Child and Lester (2001) put it into Solanumsubg. Solanum, and Hunziker (2001) consideredits subgeneric position uncertain. Taxa ofSolanum sect. Regmandra included in the ndhFdata set are S. montanum and S. multifidum, andthey fall out together on a well-supported butisolated clade near the base of Solanum.
Non-molecular characters that may distinguishthis clade include herbaceous habit and usually

MAJOR CLADES IN SOLANUM BASED ON ndhF SEQUENCE DATA
pinnately dissected and rather thick leaves, some-times with winged petioles and stems. Plants ofSolanum montanum and S. multifidum grown inthe University of Utah greenhouse had nearlyrotate corollas and notably expanded stigmas.Solanum montanum is reported to bear tubers(Dunal, 1852; Macbride, 1962), but the ndhFresults do not suggest a direct relationshipbetween the Regmandra clade and the tuber-bearing members of the Potato clade.
3. Archaesolanum clade
ca. 8 spp., Australia, New Guinea, New Zealand
Included taxa:
Solanum subg. Archaesolanum Marzell
Solanum sect. Archaesolanum(Marzell) Danert*
This is a distinctive group with no obvious closerelatives within Solanum. It is distinguished by itsaneuploid chromosome number based on n = 23,a number unique in the genus. All species of thisgroup occur in Australia and the South Pacific(New Guinea, Australia, Tasmania, New Zealand).Aside from its chromosome number, possiblenon-molecular synapomorphies of this cladeinclude plurifoliate sympodial units, rotate corol-las with abundant interpetalar tissue, looselyerect anthers on relatively long filaments, andfruits with abundant stone cell aggregates. Thebasal position of this clade may indicate a rela-tively old radiation in the South Pacific.
The Archaesolanum clade has been recognized asdistinct by virtually all previous Solanum workers,including D’Arcy (1972, 1991), Bitter in Marzell(1927), Danert (1970), and Symon (1994).Olmstead and Palmer (1997) included S. avicularein their analysis of Solanum using chloroplastrestriction site data, and it formed a clade with76% bootstrap support along with S. ptychan-thum, S. crispum, S. dulcamara, and S. jasmi-noides. However, sampling within non-spinySolanum taxa was sparse in their study, with 17non-spiny representatives out of 36 totalSolanum species. Bohs and Olmstead (2001)
found that S. aviculare and S. laciniatum formeda well-supported basal clade in Solanum in analy-ses using nuclear ITS sequence data as well as ITScombined with ndhF data. It seems safe to saythat the Archaesolanum clade represents an iso-lated group whose closest relatives have not yetbeen identified.
4. Normania clade
3 spp., Macaronesia, Spain, NW Africa
Included taxa:
Solanum sect. Normania (Lowe) Bitter [genus Normania Lowe]* genus Triguera Cav.*
This clade includes two enigmatic groups endem-ic to Macaronesia and adjacent areas of Spainand northwestern Africa. Although these taxahave been recognized as the segregate generaNormania and Triguera, molecular data indicatethat both are nested within Solanum and thethree species of both genera have been trans-ferred to Solanum (Bohs & Olmstead, 2001).Francisco-Ortega et al. (1993) made a thoroughmorphological analysis of Normania and Trigueraand concluded that they were closely related.
Numerous non-molecular characters unite thespecies of the Normania clade, including herba-ceous or weakly woody habit, foliaceous andaccrescent calyces, zygomorphic corollas, sube-qual to very unequal stamens, anther dehiscenceby both apical pores and longitudinal slits,anthers with horned projections, fruits dry orwith sparse pulp, seeds large and few per fruitwith the seed coat cell walls radially expanded,and pollen grains with colpi joined at the poles.Affinities of the Normania clade within Solanumare presently obscure. In combined analyses ofndhF and ITS data this clade forms a group withmembers of the Potato and Morelloid/Dulcamaroid clade (Bohs & Olmstead, 2001), butthis placement is poorly supported, with a boot-strap value of 17%. As with the Archaesolanumclade, the Normania clade appears to form an iso-lated group within Solanum without obviousclose relatives.
37

38
A FESTSCHRIFT FOR WILLIAM G. D’ARCY
5. African non-spiny clade
ca. 7 spp., Africa
Included taxa:
Solanum subg. Lyciosolanum Bitter*
Solanum subg. Solanum pro parte
Solanum sect. Afrosolanum Bitter*
Solanum sect. QuadrangulareBitter*
Solanum sect. Benderianum Bitter
D’Arcy (1972, 1991) recognized Solanum subg.Lyciosolanum as monotypic, with S. aggregatumas its sole member, but the ndhF data indicatethat probably this group should be expanded toinclude members of Solanum sects. Afrosolanum,Quadrangulare, and perhaps Benderianum, allplaced by D’Arcy (1972, 1991) in Solanum subg.Solanum. This clade forms an isolated groupwithin Solanum. It is poorly known taxonomical-ly, but possible non-molecular synapomorphiesmay include shrubby or climbing habit,unbranched or dendritically branched hairs, andpurple or white stellate corollas. This group needsbetter molecular sampling and morphologicalcharacterization.
No DNA samples are available from representa-tives of Solanum sects. Lemurisolanum Bitter andMacronesiotes Bitter, two non-spiny sectionsendemic to Madagascar. Their affinities may liewith the African non-spiny clade or with theDulcamaroid clade.
6. Potato clade
ca. 200–300 spp., New World
Included taxa:
Solanum subg. Potatoe (G. Don) D’Arcy pro parte
Solanum sect. Petota Dumort.*
Solanum sect. AnarrhichomenumBitter*
Solanum sect. Basarthrum
(Bitter) Bitter*
Solanum sect. Lycopersicon (Mill.) Wettst.*
Solanum sect. NeolycopersiconCorrell
Solanum sect. Juglandifolium(Rydb.) A. Child*
Solanum sect. Etuberosum(Bukasov & Kamaraz) A. Child*
Solanum sect. Articulatum(Correll) A. Child
Solanum sect. TaeniotrichumA. Child
Solanum subg. Bassovia (Aubl.) Bitter pro parte
Solanum sect. HerpystichumBitter*
Solanum sect. PteroideaDunal*
This clade includes most of the groups of D’Arcy’ssubgenera Potatoe and Bassovia. Child’s treat-ment of subgenus Potatoe (Child, 1990; Child &Lester, 2001) included these groups, but his con-cept also encompassed a number of disparate ele-ments that are placed here in different clades,such as Solanum sect. Normania (here placed inthe Normania clade), the dulcamaroid taxa sensuChild and Lester (2001; sects. Dulcamara,Jasminosolanum, and Californisolanum, hereplaced in the Dulcamaroid clade), and the“anomalously prickly” taxa sensu Child (1990;sects. Aculeigerum, Nemorense, andHerposolanum, here placed in theWendlandii/Allophyllum and Leptostemonumclades). Nee’s recent Solanum scheme (Nee, 1999)considered the taxa that here belong to thePotato clade to represent two distinct evolution-ary lines. He included the potatoes and their rel-atives (sects. Petota, Anarrhichomenum,Basarthrum) in a large and morphologicallydiverse subgenus Solanum, along with othergroups such as sections Dulcamara, Solanum,

MAJOR CLADES IN SOLANUM BASED ON ndhF SEQUENCE DATA
Holophylla, Brevantherum, Regmandra, andArchaesolanum. He also included members ofsection Herpystichum in this clade. As Nee (1999)noted, the type of section Herpystichum is notknown with certainty and the group is not wellcircumscribed, but he listed S. phaseoloides and S.evolvulifolium as members of the section. Thesespecies are sampled in the ndhF analyses, andthey both fall out in the Potato clade.
On the other hand, Nee (1999) maintainedSolanum subg. Bassovia, amplifying it to includesections Cyphomandropsis and Pachyphylla of theCyphomandra clade and section Allophylla of theWendlandii/Allophyllum clade along with sectionPteroidea, which was placed in subgenus Bassoviaby previous workers such as Bitter (1921), Seithe(1962), Danert (1970), and D’Arcy (1972). Knappand Helgason (1997) revised the species of sectionPteroidea, but they were unsure of the higher-level relationships of the section.
The ndhF data indicate that section Pteroideabelongs to the Potato clade, and that the sam-pled representatives of the subgenera Potatoeand Bassovia sensu D’Arcy (1972) each formmonophyletic clades. Non-molecular synapomor-phies that may unite both of these groups includeherbaceous to weakly woody and often scandenthabit, exclusively unbranched hairs, presence ofrhizomes or tubers in many taxa, presence ofcompound leaves in most species, and lack ofstone cell aggregates in the fruits. The presenceof solanidine/tomatidine alkaloids may be themost consistent synapomorphy that defines thesubgenus Potatoe. Whether members of the sub-genus Bassovia possess these types of alkaloids isunknown.
Child (1990) placed Solanum evolvulifolium insection Anarrhichomenum, whereas Nee (1999)placed this species in section Herpystichum. ThendhF data show that S. evolvulifolium is moreclosely related to S. phaseoloides (sect.Herpystichum) than to S. appendiculatum (sect.Anarrhichomenum).
The placement of this monotypic Solanum sect.Rhynchantherum Bitter has been debated. Dunal(1852), D’Arcy (1972, 1991), and Hunziker (2001)assigned it to subgenus Potatoe, Bitter (1913a)proposed an affinity with S. reptans of sectionHerposolanum (cf. S. hoehnei in theLeptostemonum clade), and Miers (1855) andChild (1984b; Child & Lester, 2001) placed it in thegenus Cyphomandra (Cyphomandra clade).Although no DNA data are available, its pinnate-ly compound leaves and anther structure(described in Bohs, 1994) argue for placement inthe Potato clade.
7. Morelloid/Dulcamaroid clade
This group comprises two subclades, which willbe discussed separately. Bootstrap support for theassociation of the two groups is strong (94% ofbootstrap replicates) in the ndhF data set, butadditional molecular data from other genes areneeded to ascertain whether this group shouldbe better recognized as two separate clades. Forinstance, ITS data from a small subset of the taxaconsidered here provided weak support (19% ofbootstrap replicates) for the association of themorelloid and dulcamaroid subgroups (Bohs &Olmstead, 2001).
7a. Morelloid clade
ca. 75 spp., worldwide
Included taxa:
Solanum subg. Solanum pro parte
Solanum sect. Solanum*
Solanum sect. Campanulisolanum Bitter*
Solanum sect. ParasolanumA. Child*
Solanum sect. Episarcophyllum Bitter*
Solanum sect. Chamaesarachidium Bitter
39

40
A FESTSCHRIFT FOR WILLIAM G. D’ARCY
This clade includes the core of Solanum speciesoften known as the morelloid taxa. The four sec-tions exclusive of section Parasolanum are mor-phologically homogeneous, and sectional distinc-tions are not clear-cut. Three members ofSolanum sect. Parasolanum (S. tripartitum, S. pal-itans, S. triflorum) were sampled in the ndhFanalyses, and all are included in the morelloidclade. However, these three taxa do not fall outtogether, indicating that section Parasolanum ascircumscribed by Child (1984a) may not be amonophyletic group. In the ndhF analyses, S. tri-partitum and S. palitans form a strongly support-ed clade, which, in turn, is strongly associatedwith the rest of the Morelloid clade (95% boot-strap support). However, these two species form aseparate group distinct from the rest of theMorelloid clade in trees based on ITS sequences(Bohs & Olmstead, 2001). More extensive ITS sam-pling along with molecular data from additionalgenes may enhance the circumscription andplacement of section Parasolanum.
Some non-molecular characters that may serve tounite this clade include herbaceous or weaklywoody habit, 2- to 3-foliate sympodial units,pubescent filaments and styles in many taxa, andsmall stone cell aggregates in the fruits.
7b. Dulcamaroid clade
ca. 40 spp., worldwide
Included taxa:
Solanum subg. Potatoe(G. Don) D’Arcy pro parte
Solanum sect. Dulcamara Dumort.*
Solanum sect. JasminosolanumSeithe*
Solanum sect. CalifornisolanumA. Child*
Solanum subg. Solanum pro parte
Solanum sect. Lysiphellos (Bitter) Seithe
Solanum subg. Minon Raf. pro parte
Solanum sect. Holophylla Walp. pro parte*
This clade consists of elements from three ofD’Arcy’s subgenera. Sectional limits are not welldefined, and the majority of groups includedhere are in need of critical taxonomic revision andnomenclatural clarification. The ndhF results indi-cate that Solanum sect. Holophylla is not mono-phyletic as traditionally defined. Part of Solanumsect. Holophylla that includes the species S.aligerum, S. pubigerum, and members of the S.nitidum group [Knapp, 1989; equivalent to S. sub-sect. Nitidum A. Child (Child, 1998)] belongs tothe Dulcamaroid clade. At least part of theremainder of Solanum sect. Holophylla, repre-sented in the ndhF trees by S. argentinum,belongs to the Geminata clade. Morphologicalsynapomorphies of the Dulcamaroid clade mayinclude vining habit in many taxa, the presence ofunbranched, dendritic, or echinoid hairs, 3- tomany-foliate sympodial units, and fruits lackingstone cell aggregates.
The following clades form a large group inSolanum with 98% bootstrap support (Fig. 1).Although the majority of species in this groupbelong to the spiny Solanum subg.Leptostemonum (the Leptostemonum clade),four other predominantly non-spiny clades arerepresented here. This group is morphologicallyheterogeneous and has not been recognized for-mally at any rank.
8. Wendlandii/Allophyllum clade
ca. 10 spp., New World
Included taxa:
Solanum sect. Allophyllum(Child) Bohs*
Solanum subg. Leptostemonumpro parte
Solanum sect. Aculeigerum Seithe*

MAJOR CLADES IN SOLANUM BASED ON ndhF SEQUENCE DATA
This clade is perhaps the most unusual and sur-prising in all of Solanum. Thus far it consists oftwo groups whose relationships to otherSolanum taxa have been debated. Species ofSolanum sect. Allophyllum were previouslyplaced in the genus Cyphomandra (D’Arcy, 1973;Child, 1984b; Bohs, 1988), but Bohs (1989)showed that they did not have the characters ofthe Cyphomandra clade. The subgeneric place-ment of Solanum sect. Allophyllum, however, hasbeen obscure (Bohs, 1990). Solanum sect.Aculeigerum has usually been placed in subgenusLeptostemonum because the plants bear spines(D’Arcy, 1972, 1991; Whalen, 1984). However,they lack stellate hairs, a hallmark of the sub-genus, so some workers have placed this sectionin with the non-spiny species of Solanum in eithersubgenus Solanum (Seithe, 1962) or Potatoe(Child, 1990; Child & Lester, 2001). Molecular dataof Bohs and Olmstead (1997, 1999, 2001) showedthat Solanum sect. Aculeigerum probably doesnot belong in the spiny Solanum subg.Leptostemonum, but is instead allied to a spine-less group, section Allophyllum. The ndhF analy-ses presented here continue to support thatplacement. Species of Solanum sectionsAllophyllum and Aculeigerum are morphological-ly distinctive, but both groups have narrow,tapered anthers that dehisce by small terminalpores, exclusively unbranched hairs, and fre-quently have pinnately lobed leaves.
9. Cyphomandra clade
ca. 50 spp., New World
Included taxa:
Solanum sect. Pachyphylla (Dunal) Dunal [genus Cyphomandra Sendtn.]*
Solanum sect. Cyphomandropsis Bitter*
Solanum sect. Glaucophyllum A. Child*
The association of these three sections and theirrelationship to Solanum have been controversial.From 1845 to 1995, Cyphomandra was recog-nized as a separate genus (Sendtner, 1845; Bohs,1994, and references therein). However, molecu-lar data establish that it is nested within Solanum,
and all species of Cyphomandra were transferredto Solanum in 1995 (Bohs, 1995). Solanum sect.Cyphomandropsis was considered to be part ofCyphomandra by some workers (D’Arcy, 1972;Child, 1984b; Child & Lester, 2001), whereas oth-ers maintained this group in Solanum (Bitter,1913b; Seithe, 1962; Gilli, 1970; Danert, 1970;Morton, 1976). Within Solanum, its subgenericplacement has been debated, with Seithe (1962)placing it in subgenus Solanum and Smith andDowns (1966) and Morton (1976) placing it insubgenus Leptostemonum. Most authors haveconsidered S. glaucophyllum to belong toSolanum sect. Cyphomandropsis, but Child (1986)removed it to its own monotypic section andplaced it in subgenus Solanum. Hunziker (2001)disagreed with this view on morphologicalgrounds and placed it within Solanum subg.Potatoe. Morphological, cytological, and molecu-lar studies have confirmed the close association ofSolanum sections Pachyphylla, Cyphomandropsis,and Glaucophyllum (Morton, 1976; Moscone,1992; Bohs, 2001; Bohs & Olmstead, 2001), andmolecular data indicate that they form a distinctclade within Solanum whose close relatives areunclear (Fig. 1).
Species of the Cyphomandra clade are woodyshrubs or trees that often have enlarged or elab-orated anther connectives or dorsal anther sur-faces. The synapomorphy that unites this group isthe presence of very large chromosomes, whichhave been found in all species of the clade inves-tigated to date.
10. Geminata clade
ca. 140 spp., mainly New World
Included taxa:
Solanum subg. Solanum pro parte
Solanum sect. Geminata (G. Don) Walp.*
Solanum sect. Delitescens Hunz. & Barboza*
Solanum sect. Diamonon (Raf.) A. Child*
41

42
A FESTSCHRIFT FOR WILLIAM G. D’ARCY
Solanum subg. Minon Raf. pro parte
Solanum sect. Holophylla pro parte*
Solanum sect. Pseudocapsicum(Moench) Bitter*
Although placed by D’Arcy (1972, 1991) in sepa-rate subgenera of Solanum, both morphologicalstudies (Knapp, 2002) and the ndhF analyses con-firm that section Geminata and sectionPseudocapsicum are closely related. Both groupshave mainly leaf-opposed inflorescences andoften 1- to 2-foliate sympodial units. Yet otherelements belong to the Geminata clade, such as S.argentinum, S. delitescens, and S. havanense.Solanum argentinum has been placed in sectionHolophylla, but this group is apparently poly-phyletic, with at least part of the section belong-ing to the Dulcamaroid clade.
The systematic position of S. delitescens has beenunclear. Knapp (2002) includes it in her treatmentof Solanum sect. Geminata, but lists it under taxaof uncertain placement. Nee (1999) included itwithin the heterogeneous Solanum sect.Holophylla within subgenus Solanum. Hunzikerand Barboza (in Hunziker, 2000) created themonotypic Solanum sect. Delitescens to accom-modate this species and also placed it within sub-genus Solanum. The ndhF data indicate thatSolanum sections Geminata, Pseudocapsicum,and Delitescens are closely related to each otherand are not allied with the morelloid species thatmake up the core of subgenus Solanum.
Likewise, the affinities of Solanum havanensehave been uncertain. This species occurs in Cubaand Jamaica and, according to Knapp (2002), isallied to the Jamaican species S. troyanum Urb.Knapp (2002) excluded these two species fromSolanum sect. Geminata and regarded them as anisolated lineage in Solanum, which she called theS. havanense species group (Knapp, 2002). Child(1998) created the monotypic Solanum sect.Diamonon to accommodate S. havanense andhypothesized that it may belong near sectionPseudocapsicum. In the ndhF trees, S. havanensebelongs to the Geminata clade along with mem-bers of Solanum sections Geminata,
Pseudocapsicum, and Delitescens.
Characters that may unite the taxa of this cladeinclude woody habit, unbranched to dendriticallybranched hairs, oblong anthers with large terminal pores, and fruits lacking stone cellaggregates.
11. Brevantherum clade
ca. 60 spp., New World
Included taxa:
Solanum subg. Brevantherum (Seithe) D’Arcy pro parte [Solanum subg. Minonpro parte in D’Arcy (1991)]
Solanum sect. Brevantherum Seithe*
Solanum sect. Extensum D’Arcy*
Solanum sect. Lepidotum Seithe*
Solanum sect. StellatigeminatumA. Child*
Solanum sect. Cernuum Carvalho & G. J. Sheph.
Solanum subg. Solanum pro parte
Solanum sect. GonatotrichumBitter*
For the most part, this clade consists of a numberof morphologically similar groups that often havestellate hairs or lepidote scales, oblong antherswith large terminal pores, and green, yellow, orpurple fruits. D’Arcy (1991) used the subgenericname Minon to refer to an analogous group inSolanum, which, however, also included elementssuch as sections Holophylla and Pseudocapsicumthat are here referred to different clades. Sincethe type species of subgenus Minon is S. pseudo-capsicum, which belongs to the Geminata clade,the appropriate name for the Brevantherumclade at subgeneric rank would be Solanum subg.Brevantherum.
The sections of Solanum subg. Brevantherum arenot well demarcated. The three members ofSolanum sect. Brevantherum (S. abutiloides, S. mauritianum, S. rugosum) sampled in the ndhF

MAJOR CLADES IN SOLANUM BASED ON ndhF SEQUENCE DATA
trees do not form a monophyletic group, butadditional data and sampling are needed toresolve relationships in the Brevantherum clade.There are a number of species that fall outsidethe traditional limits of the established sectionslisted above. One example is Solanum inelegans,placed by Nee (1999) in the polymorphic and ill-defined Solanum sect. Holophylla and evidently amember of the Brevantherum clade according tothe ndhF data.
The odd group out from a morphological per-spective is Solanum sect. Gonatotrichum (S.adscendens, S. turneroides, S. deflexum). Itsplacement here is surprising, because Solanumsect. Gonatotrichum has few of the characterslisted above for the Brevantherum clade and hasbeen thought to be more closely related to theMorelloid clade (D’Arcy, 1972, 1991; Nee, 1999;Child & Lester, 2001) or to Solanum sect.Pseudocapsicum of the Geminata clade(Hunziker, 2001). Molecular data indicate thatSolanum sect. Gonatotrichum forms a distinctsubclade within the Brevantherum clade (Fig. 1),but it clearly does not belong to the Morelloidclade. The names S. adscendens and S. deflexummay be synonymous (Nee, 1989, 1999; D’Arcy,2001) but the two species exhibit a fair amount ofsequence divergence in ndhF (1.0%) and areapparently allopatric (Bitter, 1912).
12. Leptostemonum clade
ca. 450 spp., worldwide
Includes all spiny sections and species groups except Solanum sect. AculeigerumSeithe
Possibly includes Solanum sect. Herposolanum Bitter
Sampling to date includes at least 20 sections and 20 species groups sensuWhalen (1984)
This is the largest and most complex of the majorclades of Solanum and encompasses the vastmajority of species traditionally placed inSolanum subg. Leptostemonum. Data thus far
indicate that all the species of Solanum that bearspines form a clade with the exception of sectionAculeigerum mentioned above. Nearly all mem-bers of this group have stellate hairs as well asspines. The anthers are narrow and tapered withsmall terminal pores that do not enlarge into lon-gitudinal slits. Much work is still needed to revealthe phylogenetic structure within theLeptostemonum clade and to interpret patternsof character evolution and biogeography withinthe group. A more detailed analysis of theLeptostemonum clade using ndhF and nuclear ITSsequence data is under way (L. Bohs, unpublisheddata) and will be summarized in a later publica-tion.
The ndhF data indicate members of Solanum sec-tions Nemorense (S. nemorense) andHerposolanum (S. hoehnei) may represent thebasalmost branches in the Leptostemonum clade,but the bootstrap support for this grouping is low(51%). These taxa are similar to Solanum sect.Aculeigerum in that they have spines but lackstellate hairs. The placement of Solanum sect.Herposolanum has been particularly problematic;D’Arcy (1972, 1991) put it into Solanum subg.Bassovia, whereas Child (1983) suggested a rela-tionship with Solanum sect. Aculeigerum (theWendlandii/Allophyllum clade above) and provi-sionally placed it in Solanum subg. Potatoe (Child,1990; Child & Lester, 2001). Whalen (1984)merged Solanum sections Herposolanum andNemorense into his S. nemorense species group,which he considered to belong to Solanum subg.Leptostemonum. Nee (1999) included Solanumsect. Aculeigerum in section Herposolanum andregarded both as members of subgenusLeptostemonum. The ndhF data do not fullyresolve these questions, but Solanum sectionsHerposolanum and Nemorense apparently donot belong to the Potato clade and are not close-ly related to section Aculeigerum.
Solanum sect. Acanthophora (S. capsicoides, S.mammosum) also appears to be relatively basal inthe Leptostemonum clade. This group often hasunbranched or weakly stellate hairs in addition tospines. These have been interpreted as being
43

44
A FESTSCHRIFT FOR WILLIAM G. D’ARCY
reduced stellate hairs (Nee, 1979), but a thoroughexamination of the ontogeny of hairs in this cladeshould be undertaken with a phylogenetic per-spective to determine if these simple hairs repre-sent an ancestral rather than derived state in theLeptostemonum group.
GENERAL RECOMMENDATIONThis is not the last word on phylogenetic structure orevolutionary relationships in Solanum. The majorclades identified here, although well supported fromndhF data, need to be corroborated by data fromother genes. Additional sampling, especially frommorphologically unusual, underrepresented, and/orputatively isolated groups, is needed to test the distinctiveness of the major ndhF clades and to ascer-tain the phylogenetic position of enigmatic taxa. Forinstance, no molecular data are available for the twospecies placed in Solanum sect. Solanocharis (Bitter)A. Child. The two species may not be closely related(M. Nee, pers. comm.), and they may not belong toSolanum. The type of the section is S. albescens(Britton) Hunz., which apparently has longitudinalanther dehiscence and has been regarded by some asbelonging to the genera Solanocharis, Poecilo-chroma, or Saracha (Rusby, 1896; Bitter, 1918; M.Nee, pers. comm.). Molecular data will certainly aidin the interpretation of this puzzling group.
Morphological and biochemical characters alsoshould be examined, especially in the light ofmolecular findings, in order to identify non-molecular synapomorphies that support the ndhFclades. Taxonomic studies at lower levels todemarcate species limits are desperately neededfor many subgeneric groups. Many nomenclatur-al issues also need careful clarification.
In light of these uncertainties, new formal taxo-nomic designations for infrageneric categories inSolanum are strongly discouraged without moreextensive data and sampling. Progress will not befacilitated by the creation of yet more formalnames that must be sifted through by all subse-quent workers in the group. Informal names forspecies groups or clades (e.g., Whalen, 1984; Knapp, 1989, 2000, 2002; Bohs, 1994, 2001) are
encouraged until enough data have accumulatedto positively demarcate and define distinct evolu-tionary units within Solanum.
ACKNOWLEDGMENTSThis paper is dedicated to the late W. G. D’Arcy, apioneer in the field of Solanaceae systematics anda gracious and supportive colleague. His exten-sive knowledge and sage advice on many mattersof nomenclature, morphology, and evolution ofsolanaceous plants have been greatly appreciat-ed and are sorely missed. I especially acknowl-edge Bill’s constant support of my research andcareer, beginning from my graduate student dayswhen I knew next to nothing about Solanaceae.From revealing obscure field sites for rareSolanum species to providing advice for dealingwith difficult colleagues, Bill was unfailingly posi-tive, helpful, and human.
I also thank other friends and colleagues for theirhelp with this project: R. G. Olmstead, who hasshared freely of his expertise, lab facilities, sam-ples, sequences, and ideas; M. Nee, S. Knapp, andD. Spooner, who provide constant assistance of allkinds; M. Johnson, A. Freeman, S. King-Jones, A.Egan, A. Moore, and P. Reeves for technical assis-tance; D. Reed for help with the likelihood analy-sis; D. Spooner and an anonymous reviewer forcomments on the manuscript; the greenhousestaff of the University of Utah and DukeUniversity for maintaining living collections; J.Solomon of MO for granting permission toremove leaf material from herbarium specimens;T. Mione, A. Child, J. Franciso-Ortega, R. N. Lester,M. Welman, A. Egan, the Botanic Garden at theUniversity of Nijmegen, The Netherlands, andnumerous field companions for help in obtainingmaterial of Solanaceae. This research was sup-ported by National Geographic Society Grant6189-98 and National Science Foundation grantsDEB-9207359 and DEB-9996199.
LITERATURE CITED Bitter, G. 1912. Solana nova vel minus cognita. III.
X. Sectio: Gonatotrichum Bitter, nov.sect. Repert. Spec. Nov. Regni Veg. 11:230–234.

MAJOR CLADES IN SOLANUM BASED ON ndhF SEQUENCE DATA
45
—. 1913a. Solana nova vel minus cognita. X. XXIII.Sectio: Rhynchantherum Bitter, nov. sect.Repert. Spec. Nov. Regni Veg. 12: 61–65.
—. 1913b. Solana nova vel minus cognita XII.XXXVII. Sectio: Cyphomandropsis Bitter,nov. sect. Repert. Spec. Nov. Regni Veg.12: 461–467.
—. 1918. XXIII. Solanaceae quattuor austro-amer-icanae adhuc generibus falsis adscriptae.Repert. Spec. Nov. Regni Veg. 15:149–155.
—. 1919. Die papuasischen Arten von Solanum.Bot. Jahrb. 55: 59–113.
—. 1921. Aufteilung der Gattung Bassovia (imDunalschen Sinne) zwischen Solanum,Capsicum, und Lycianthes. Repert. Spec.Nov. Regni Veg. 17: 328–335.
Bohs, L. 1988. The Colombian species ofCyphomandra. Revista Acad. Colomb. Ci.Exact. 16: 67–75.
—. 1989. Solanum allophyllum (Miers) Standl. andthe generic delimitation of Cyphoman-dra and Solanum (Solanaceae). Ann.Missouri Bot. Gard. 76: 1129–1140.
—. 1990. The systematics of Solanum sectionAllophyllum (Solanaceae). Ann. MissouriBot. Gard. 77: 398–409.
—. 1994. Cyphomandra (Solanaceae). Fl. Neotrop.Monogr. 63.
—. 1995. Transfer of Cyphomandra (Solanaceae)and its species to Solanum. Taxon 44:583–587.
—. 2001. A revision of Solanum sectionCyphomandropsis (Solanaceae). Syst.Bot. Monogr. 61: 1–85.
— & R. G. Olmstead. 1997. Phylogenetic relation-ships in Solanum (Solanaceae) based onndhF sequences. Syst. Bot. 22: 5–17.
— & —. 1999. Solanum phylogeny inferred fromchloroplast DNA sequence data. Pp.97–110 in M. Nee, D. E. Symon, R. N.Lester & J. P. Jessop (editors), Solanaceae
IV: Advances in Biology and Utilization.Royal Botanic Gardens, Kew.
— & —. 2001. A reassessment of Normania andTriguera (Solanaceae). Pl. Syst. Evol. 228:33–48.
Child, A. 1983. Taxonomic studies in Solanum L. 1.Section Nemorense Child, sectio nova.Feddes Repert. 94: 35–42.
—. 1984a. Taxonomic studies in Solanum L. 2. Twonew infrageneric taxa for the subgenusSolanum. Feddes Repert. 95: 141–150.
—. 1984b. Studies in Solanum L. (and related gen-era) 3. A provisional conspectus of thegenus Cyphomandra Mart. ex Sendtner.Feddes Repert. 95: 283–298.
—. 1986. Taxonomic studies in Solanum L. (andrelated genera) 4. Cyphomandra casanaChild sp. nov. and Solanum sect.Glaucophyllum Child sect. nov. FeddesRepert. 97: 143–146.
—. 1990. A synopsis of Solanum subgenusPotatoe (G. Don) D’Arcy (Tuberarium(Dun.) Bitter (s.l.)). Feddes Repert.101(5–6): 209–235.
—. 1998. Studies in Solanum and related genera(6). New infrageneric taxa for the genusSolanum L. (Solanaceae). Feddes Repert.109(5–6): 407–427.
— & R. N. Lester. 2001. Synopsis of the genusSolanum L. and its infrageneric taxa. Pp.39–52 in R. G. van den Berg, G. W. M.Barendse, G. M. van der Weerden & C.Mariani (editors), Solanaceae V:Advances in Taxonomy and Utilization.Nijmegen Univ. Press, The Netherlands.
Danert, S. 1970. Infragenerische Taxa der GattungSolanum L. Kulturpflanze 18: 253–297.
D’Arcy, W. G. 1972. Solanaceae studies II:Typification of subdivisions of Solanum.Ann. Missouri Bot. Gard. 59: 262–278.
—. 1973. Solanaceae. Flora of Panama. Ann.Missouri Bot. Gard. 60: 573–780.

46
A FESTSCHRIFT FOR WILLIAM G. D’ARCY
—. 1986. The calyx in Lycianthes and some othergenera. Ann. Missouri Bot. Gard. 73:117–127.
—. 1991. The Solanaceae since 1976, with areview of its biogeography. Pp. 75–137in J. G. Hawkes, R. N. Lester, M. Nee & N.Estrada (editors), Solanaceae III:Taxonomy, Chemistry, Evolution. RoyalBotanic Gardens, Kew.
—. 1992. Solanaceae of Madagascar: Form andgeography. Ann. Missouri Bot. Gard. 79:29–45.
—. 2001. Solanaceae. Pp. 2376–2426 in W. D.Stevens, C. Ulloa Ulloa, A. Pool & O. M.Montiel (editors), Flora de Nicaragua.Monogr. Syst. Bot. Missouri Bot. Gard. 85(Tomo 3).
Doyle, J. J. & J. L. Doyle. 1987. A rapid DNA isola-tion procedure for small quantities offresh leaf tissue. Phytochem. Bull. 19:11–15.
Dunal, M.-F. 1813. Histoire des Solanum, et desgenres qui ont été confondus avec eux.Koenig, Paris.
—. 1816. Solanorum generumque affinium syn-opsis, Ed. 2. Renaud, Montpellier.
—. 1852. Solanaceae. Pp. 1–690 in A. P. deCandolle (editor), Prodromus SystematisNaturalis Regni Vegetabilis, Vol. 13(1).Victoris Masson, Paris.
Francisco-Ortega, J., J. G. Hawkes, R. N. Lester & J.R. Acebes-Ginovés. 1993. Normania, anendemic Macaronesian genus distinctfrom Solanum (Solanaceae). Pl. Syst.Evol. 185: 189–205.
Gilli, A. 1970. Bestimmungsschlüssel derSubgenera und Sektionen der GattungSolanum. Feddes Repert. 81: 429–435.
Hunziker, A. T. 2000. Miscellaneous novelties inthe taxonomy of Solanaceae. Kurtziana28: 55–64.
—. 2001. Genera Solanacearum. A. R. G. Ganter, Ruggell.
Knapp, S. 1989. A revision of the Solanumnitidum group (section Holophylla proparte): Solanaceae. Bull. Brit. Mus. (Nat.Hist.), Bot. 19: 63–112.
—. 2000. A revision of Solanum thelopodiumspecies group (section Anthoresis sensuSeithe, pro parte): Solanaceae. Bull. Nat.Hist. Mus. Lond. (Bot.) 30: 13–30.
—. 2002. Solanum section Geminata(Solanaceae). Fl. Neotrop. Monogr. 84.
— & T. Helgason. 1997. A revision of Solanum sec-tion Pteroidea: Solanaceae. Bull. Nat.Hist. Mus. Lond. (Bot.) 27: 31–73.
Linnaeus, C. 1753. Species Plantarum. [Facsimileof the first edition, 1957.] The RaySociety, London.
Macbride, J. F. 1962. Solanaceae. Flora of Peru.Field Mus. Nat. Hist., Bot. Ser. 13(5B):1–267.
Marzell, H. 1927. Solanaceae. Pp. 2548–2625 in G.Hegi (editor), Illustrierte Flora vonMittel-Europa. Vol. 5, Part 4. J. F.Lehmanns, Munich, Germany.
Miers, J. 1855. XVII. On the genera Pionandra,Cliocarpus, and Paecilochroma.Pionandra. Ann. Mag. Nat. Hist., ser. 2.15: 196–200.
Morton, C. V. 1976. A Revision of the ArgentineSpecies of Solanum. Academia Nacionalde Ciencias, Córdoba, Argentina.
Moscone, E. A. 1992. Estudios de cromosomasmeióticos en Solanaceae de Argentina.Darwiniana 31: 261–297.
Nee, M. 1979. A Revision of Solanum sectionAcanthophora. Ph.D. Thesis, Universityof Wisconsin, Madison.
—. 1989. Notes on Solanum sect. Gonatotrichum.Solanaceae Newslett. 3(1): 80–82.
—. 1999. Synopsis of Solanum in the New World.Pp. 285–333 in M. Nee, D. E. Symon, R. N.Lester & J. P. Jessop (editors), SolanaceaeIV: Advances in Biology and Utilization.Royal Botanic Gardens, Kew.

MAJOR CLADES IN SOLANUM BASED ON ndhF SEQUENCE DATA
Nixon, K. C. 1999. The parsimony ratchet, a newmethod for rapid parsimony analysis.Cladistics 15: 407–414.
Olmstead, R. G. & J. D. Palmer. 1992. A chloroplastDNA phylogeny of the Solanaceae:Subfamilial relationships and characterevolution. Ann. Missouri Bot. Gard. 79:346–360.
— & —. 1997. Implications for the phylogeny, clas-sification, and biogeography of Solanumfrom cpDNA restriction site variation.Syst. Bot. 22: 19–29.
—, J. A. Sweere, R. E. Spangler, L. Bohs & J. D.Palmer. 1999. Phylogeny and provisionalclassification of the Solanaceae based onchloroplast DNA. Pp. 111–137 in M. Nee,D. E. Symon, R. N. Lester & J. P. Jessop(editors), Solanaceae IV: Advances inBiology and Utilization. Royal BotanicGardens, Kew.
Olsen, G. J., H. Matsuda, R. Hagstrom & R.Overbeek. 1994. fastDNAml: A tool forconstruction of phylogenetic trees ofDNA sequences using maximum likeli-hood. Computer Applic. Biosci. 10:41–48.
Rusby, H. H. 1896. On the collections of Mr.Miguel Bang in Bolivia—Part III. Mem.Torrey Bot. Club 6: 1–130.
Seithe, A. 1962. Die Haararten der GattungSolanum L. und ihre taxonomischeVerwertung. Bot. Jahrb. 81(3): 261–336.
Sendtner, O. 1845. De Cyphomandra, novoSolanacearum genere tropicaeAmericae. Flora 28: 161–176.
Sikes, D. S. & P. O. Lewis. 2001. PAUPRat: PAUP*Implementation of the ParsimonyRatchet. Beta software, version 1.Distributed by the authors, Departmentof Ecology and Evolutionary Biology,University of Connecticut, Storrs.
Smith, L. B. & R. J. Downs. 1966. Solanáceas. Pp.1–321 in P. R. Reitz (editor), FloraIlustrada Catarinense. Itajaí, Brazil.
Spooner, D. S., G. J. Anderson & R. K. Jansen.1993. Chloroplast DNA evidence for theinterrelationships of tomatoes, pota-toes, and pepinos (Solanaceae). Amer. J.Bot. 80: 676–688.
Swofford, D. 2002. PAUP*. Version 4.0b10.Sinauer, Sunderland, Massachusetts.
Symon, D. E. 1981. A revision of the genusSolanum in Australia. J. Adelaide Bot.Gard. 4: 1–367.
—. 1994. Kangaroo apples: Solanum sect.Archaesolanum. State Herbarium ofSouth Australia, Adelaide, Australia.
Whalen, M. D. 1984. Conspectus of species groupsin Solanum subgenus Leptostemonum.Gentes Herb. 12: 179–282.
47

48
A FESTSCHRIFT FOR WILLIAM G. D’ARCY
Figure 1. Strict consensus of 18,200 trees of 1053 steps from parsimony analysis of ndhF data. Numbers above branches are bootstrap values (500 replicates). Major clades in Solanum discussed in the text are labeled.

MAJOR CLADES IN SOLANUM BASED ON ndhF SEQUENCE DATA
49
Figure 1 continued.