Lecture6,April 17,2019 From CC bonds –Transition metals CC …...e= 95.0 + 2*7.3 = 109.6 kcal/mol...
Transcript of Lecture6,April 17,2019 From CC bonds –Transition metals CC …...e= 95.0 + 2*7.3 = 109.6 kcal/mol...

© copyright 2011 William A. Goddard III, all rights reservedCh120a-Goddard-L07,08
From CC bonds – Transition metals CC RedNature of the Chemical Bond
with applications to catalysis, materialsscience, nanotechnology, surface science,
bioinorganic chemistry, and energy
1
Lecture 6, April 17, 2019
© copyright 2017 William A. Goddard III, all rights reserved
William A. Goddard, III, [email protected] 316Beckman Institute, x3093
Charles and Mary Ferkel Professor of Chemistry, Materials Science, and Applied Physics,
California Institute of Technology
Course number: Ch120aHours: 2-3pm Monday, Wednesday, Friday
Teaching Assistant: Yalu Chen <[email protected]>: (office hours: 3-4 monday)

© copyright 2011 William A. Goddard III, all rights reservedCh120a-Goddard-L07,08 2
Summary, bonding to form hydrides
General principle: start with ground state of AHn and add H to form the ground state of AHn+1
Thus use for SiH2 and CF2 use 1A1 AH2: get pyramidal AH3
Use 3B1 get planar AH3.
For less than half filled p shell, the presence of empty p orbitals allows the atom to reduce electron correlation of the (ns) pair by hybridizing into this empty orbital.
This has remarkable consequences on the states of the Be, B, and C columns.

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 3
Now combine Carbon fragments to form larger molecules (old chapter 7)
Starting with the ground state of CH3 (planar), we bring two together to form ethane, H3C-CH3.
As they come together to bond, the CH bonds bend back from the CC bond to reduce overlap (Pauli repulsion or steric interactions between the CH bonds on opposite C).
At the same time the 2pp radical orbital on each C mixes with 2s character, pooching it toward the corresponding hybrid orbital on the other C
107.7º
111.2º
1.526A
1.095A1.086A120.0º

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 4
Bonding (GVB) orbitals of ethane (staggered)
Note nodal planes from
orthogonalization to CH bonds on
right C

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 5
Staggered vs. Eclipsed
There are two extreme cases for the orientation about the CC axis of the two methyl groups
The salient difference between these is the overlap of the CH bonding orbitals on opposite carbons.
To whatever extent they overlap, SCH-CH Pauli requires that they be orthogonalized, which leads to a repulsion that increases exponentially with decreasing distance RCH-CH.
The result is that the staggered conformation is favored over eclipsed by 3.0 kcal/mol

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 6
Propane
Replacing an H of ethane with CH3, leads to propane
Keeping both CH3 groups staggered leads to the unique structure
Details are as shown. Thus the bond angles are
HCH = 108.1 and 107.3 on the CH3
HCH =106.1 on the secondary C
CCH=110.6 and 111.8
CCC=112.4,
Reflecting the steric effects

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 7
Bond energiesDe = EAB(R=∞) - EAB(Re)Get from QM calculations. Re is distance at minimum energyD0 = H0AB(R=∞) - H0AB(Re)H0=Ee + ZPE is enthalpy at T=0KZPE = S(½Ћw) This is spectroscopic bond energy from ground vibrational state (0K)Including ZPE changes bond distance slightly to R0Experimental bond enthalpies at 298K and atmospheric pressure D298(A-B) = H298(A) – H298(B) – H298(A-B)D298 – D0 = 0∫298 [Cp(A) +Cp(B) – Cp(A-B)] dT =2.4 kcal/mol if A and B are nonlinear molecules (Cp(A) = 4R). {If A and B are atoms D298– D0 = 0.9 kcal/mol (Cp(A) = 5R/2)}.(H = E + pV assuming an ideal gas)

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 8
Snap Bond Energy: Break bond without relaxing the fragments
Snap
Adiabatic
DErelax = 2*7.3 kcal/mol
DsnapDesnap (109.6 kcal/mol) De (95.0kcal/mol)

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 9
Bond energies for ethane
D0 = 87.5 kcal/mol
ZPE (CH3) = 18.2 kcal/mol,
ZPE (C2H6) = 43.9 kcal/mol,
De = D0 + 7.5 = 95.0 kcal/mol (this is calculated from QM)
D298 = 87.5 + 2.4 = 89.9 kcal/mol
This is the quantity we will quote in discussing bond breaking processes

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06
new
10

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 11
The snap Bond energy
In breaking the CC bond of ethane the geometry changes from CC=1.526A, HCH=107.7º, CH=1.095A
To CC=∞, HCH=120º, CH=1.079A
Thus the net bond energy involves both breaking the CC bond and relaxing the CH3 fragments.
We find it useful to separate the bond energy into
The snap bond energy (only the CC bond changes, all other bonds and angles of the fragments are kept fixed)
The fragment relaxation energy.
This is useful in considering systems with differing substituents.
For CH3 this relation energy is 7.3 kcal/mol so that
De,snap (CH3-CH3) = 95.0 + 2*7.3 = 109.6 kcal/mol

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 12
Substituent effects on Bond energiesThe strength of a CC bond changes from 89.9 to 70 kcal/mol as the various H are replace with methyls.Explanations given include:
•Ligand CC pair-pair repulsions
•Fragment relaxation
•Inductive effects

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 13
Ligand CC pair-pair repulsions:
Each H to Me substitution leads to 2 new CH bonds gauche to the original CC bond, which would weaken the CC bond.
Thus C2H6 has 6 CH-CH interactions lost upon breaking the bond,
But breaking a CC bond of propane loses also two addition CC-CH interactions.
This would lead to linear changes in the bond energies in the table, which is approximately true. However it would suggest that the snap bond energies would decrease, which is not correct.

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 14
Fragment relaxation
Because of the larger size of Me compared to H, there will be larger ligand-ligand interaction energies and hence a bigger relaxation energy in the fragment upon relaxing form tetrahedral to planar geometries.
In this model the snap bond enegies are all the same.
All the differences lie in the relaxation of the fragments.
This is observed to be approximately correct
Inductive effect
A change in the character of the CC bond orbital due to replacement of an H by the Me.
Goddard believes that fragment relaxation is the correct explanation

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 15
Bond energies: Compare to CF3-CF3
For CH3-CH3 we found a snap bond energy of
De = 95.0 + 2*7.3 = 109.6 kcal/mol
Because the relaxation of tetrahedral CH3 to planar gains 7.3 kcal/mol. Thus the adiabatic BE = 109.6-14.6=95
For CF3-CF3, there is no such relaxation since CF3 wants to be pyramidal, FCF~111º
Thus we might estimate that for CF3-CF3 the bond energy would be De = 109.6 kcal/mol, hence D298 ~ 110-5=105
Indeed the experimental value is D298=98.7±2.5 kcal/mol suggesting that the main effect in substituent effects is relaxation (the remaining effects might be induction and steric)

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 16
CH2 +CH2 è ethene
Starting with two methylene radicals (CH2) in the ground state (3B1) we can form ethene (H2C=CH2) with both a s bond and a p bond.
The HCH angle in CH2 was 132.3º, but Pauli Repulsion with the new s bond, decreases this angle to 117.6º (cf with 120º for CH3)

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 17
Comparison of The GVB bonding orbitals of ethene
and methylene

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 18
Twisted etheneConsider now the case where the plane of one CH2 is rotated by 90º with respect to the other (about the CC axis)This leads only to a s bond. The nonbonding pl and pr orbitals can be combined into singlet and triplet states
Here the singlet state is referred to as N (for Normal) and the triplet state as T.
Since these orbitals are orthogonal, Hund’s rule suggests that T is lower than N (for 90º). The Klr ~ 0.7 kcal/mol so that the splitting should be ~1.4 kcal/mol.
Voter, Goodgame, and Goddard [Chem. Phys. 98, 7 (1985)] showed that N is below T by 1.2 kcal/mol, due to Intraatomic Exchange (s,p on same center)

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 19
Twisting potential surface for ethene
The twisting potential surface for ethene is shown below. The N state prefers θ=0º to obtain the highest overlap while the T state prefers θ=90º to obtain the lowest overlap

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 20
geometries
For the N state (planar) the CC bond distance is 1.339A, but this increases to 1.47A for the twisted form with just a single s bond. This compares with 1.526 for the CC bond of ethane.
Probably the main effect is that twisted ethene has very little CH Pauli Repulsion between CH bonds on opposite C, whereas ethane has substantial interactions.
This suggests that the intrinsic CC single bond may be closer to 1.47A
For the T state the CC bond for twisted is also 1.47A, but increases to 1.57 for planar due to Orthogonalization of the triple coupled pp orbitals.

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 21
CC double bond energies
Breaking the double bond of ethene, the HCH bond angle changes from 117.6º to 132.xº, leading to an increase of 2.35 kcal/mol in the energy of each CH2 so that
Desnap = 180.0 + 4.7 = 184.7 kcal/mol
Since the Desnap = 109.6 kcal/mol, for H3C-CH3,
The p bond adds 75.1 kcal/mol to the bonding.
Indeed this is close to the 65kcal/mol rotational barrier.
For the twisted ethylene, the CC bond is De = 180-65=115 Desnap = 115 + 5 =120. This increase of 10 kcal/mol compared to ethane might indicate the effect of CH repulsions
The bond energies for ethene are
De=180.0, D0 = 169.9, D298K = 172.3 kcal/mol

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 22
bond energy of F2C=CF2
The snap bond energy for the double bond of ethene od
Desnap = 180.0 + 4.7 = 184.7 kcal/mol
As an example of how to use this consider the bond energy of F2C=CF2,
Here the 3B1 state is 57 kcal/higher than 1A1 so that the fragment relaxation is 2*57 = 114 kcal/mol, suggesting that the F2C=CF2 bond energy is Dsnap~184-114 = 70 kcal/mol.
The experimental value is D298 ~ 75 kcal/mol, close to the prediction

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 23
Bond energies double bondsAlthough the ground state of CH2 is 3B1 by 9.3 kcal/mol, substitution of one or both H with CH3 leads to singlet ground states. Thus the CC bonds of these systems are weakened because of this promotion energy.

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 24
C=C bond energies

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 25
CC triple bonds
Starting with two CH radicals in the 4S- state we can form ethyne (acetylene) with two p bonds and a s bond.
This leads to a CC bond length of 1.208A compared to 1.339 for ethene and 1.526 for ethane.
The bond energy is
De = 235.7, D0 = 227.7, D298K = 229.8 kcal/mol
Which can be compared to De of 180.0 for H2C=CH2 and 95.0 for H3C-CH3.

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 26
GVB orbitals of HCCH

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 27
GVB orbitals of CH 2P and 4S- state

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 28
CC triple bonds
Since the first CCs bond is De=95 kcal/mol and the first CCpbond adds 85 to get a total of 180, one might wonder why the CC triple bond is only 236, just 55 stronger.
The reason is that forming the triple bond requires promoting the CH from 2P to 4S-, which costs 17 kcal each, weakening the bond by 34 kcal/mol. Adding this to the 55 would lead to a total 2nd p bond of 89 kcal/mol comparable to the first
2P
4S-

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 29
Bond energies

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 30

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 31
Bonding at a transition metaal
Bonding to a transition metals can be quite covalent.
Examples: (Cl2)Ti(H2), (Cl2)Ti(C3H6), Cl2Ti=CH2
Here the two bonds to Cl remove ~ 1 to 2 electrons from the Ti, making is very unwilling to transfer more charge, certainly not to C or H (it would be the same for a Cp (cyclopentadienyl ligand)
Thus TiCl2 group has ~ same electronegativity as H or CH3
The covalent bond can be thought of as Ti(dz2-4s) hybrid spin paired with H1s
A{[(Tids)(H1s)+ (H1s)(Tids)](ab-ba)}

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 32
GVB orbitals for bonds to Ti
Covalent 2 electron TiH bond in Cl2TiH2
Covalent 2 electron CH bond in CH4
Ti ds character, 1 elect H 1s character, 1 elect
Csp3 character 1 elect H 1s character, 1 elect
Think of as bond from Tidz2 to H1s

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 33
But TM-H bond can also be s-like
Cl2TiH+
ClMnH
Ti (4s)2(3d)2
The 2 Cl pull off 2 e from Ti, leaving a d1
configuration
Mn (4s)2(3d)5
The Cl pulls off 1 e from Mn, leaving a d5s1
configurationH bonds to 4s because of exchange stabilization of d5
Ti-H bond character1.07 Tid+0.22Tisp+0.71H
Mn-H bond character0.07 Mnd+0.71Mnsp+1.20H

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 34
Bond angle at a transition metal
For two p orbitals expect 90°, HH nonbond repulsion increases it
H-Ti-H plane
76°
Metallacycle plane
What angle do two d orbitals want

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 35
Best bond angle for 2 pure Metal bonds using d orbitals
Assume that the first bond has pure dz2 or ds character to a ligand along the z axis
Can we make a 2nd bond, also of pure ds character (rotationally symmetric about the z axis) to a ligand along some other axis, call it z.
For pure p systems, this leads to q = 90°
For pure d systems, this leads to q = 54.7° (or 125.3°), this is ½ the tetrahedral angle of 109.7 (also the magic spinning angle for solid state NMR).

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 36
Best bond angle for 2 pure Metal bonds using d orbitals
Problem: two electrons in atomic d orbitals with same spin lead to 5*4/2 = 10 states, which partition into a 3F state (7) and a 3P state (3), with 3F lower. This is because the electron repulsion between say a dxy and dx2-y2 is higher than between sasy dz2 and dxy
.
Best is ds with dd because the electrons are farthest apart
This favors q = 90°, but the bond to the dd orbital is not as good
Thus expect something between 53.7 and 90°
Seems that ~76° is often best

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 37
How predict character of Transition metal bonds?Start with ground state atomic configuration
Ti (4s)2(3d)2 or Mn (4s)2(3d)5
Consider that bonds to electronegative ligands (eg Cl or Cp) take electrons from 4s
easiest to ionize, also better overlap with Cl or Cp, also leads to less reduction in dd exchange
(4s)(3d)5(3d)2
Now make bond to less electronegative ligands, H or CH3
Use 4s if available, otherwise use d orbitals

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 38
But TM-H bond can also be s-like
Cl2TiH+
ClMnH
Ti (4s)2(3d)2
The 2 Cl pull off 2 e from Ti, leaving a d1
configuration
Mn (4s)2(3d)5
The Cl pulls off 1 e from Mn, leaving a d5s1
configurationH bonds to 4s because of exchange stabilization of d5
Ti-H bond character1.07 Tid+0.22Tisp+0.71H
Mn-H bond character0.07 Mnd+0.71Mnsp+1.20H

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 39
Example (Cl)2VH3
+ resonance configuration

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 40
Example ClMo-metallacycle butadiene

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 41
Example [Mn≡CH]2+

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 42
Summary: start with Mn+ s1d5
dy2 s bond to H1sdx2-x2 non bondingdyz p bond to CHdxz p bond to CHdxy non bonding4sp hybrid s bond to CH

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 43
Summary: start with Mn+ s1d5
dy2 s bond to H1sdx2-x2 non bondingdyz p bond to CHdxz p bond to CHdxy non bonding4sp hybrid s bond to CH

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 44
Compare chemistry of column 10

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 45
Ground state of group 10 column
Pt: (5d)9(6s)1 3D ground statePt: (5d)10(6s)0 1S excited state at 11.0 kcal/molPt: (5d)8(6s)2 3F excited state at 14.7 kcal/mol
Pd: (5d)10(6s)0 1S ground statePd: (5d)9(6s)1 3D excited state at 21.9 kcal/molPd: (5d)8(6s)2 3F excited state at 77.9 kcal/mol
Ni: (5d)8(6s)2 3F ground stateNi: (5d)9(6s)1 3D excited state at 0.7 kcal/molNi: (5d)10(6s)0 1S excited state at 40.0 kcal/mol

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 46
Salient differences between Ni, Pd, Pt
2nd row (Pd): 4d much more stable than 5s è Pd d10 ground state
3rd row (Pt): 5d and 6s comparable stability è Pt d9s1 ground state

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 47
Salient differences between Ni, Pd, Pt
Ni Pd Pt
4s more stable than 3d 5s much less stable than 4d 6s, 5d similar stability3d much smaller than 4s(No 3d Pauli orthogonality)Huge e-e repulsion for d10
4d similar size to 5s (orthog to 3d,4s
Differential shielding favors n=4 over n=5,
stabilize 4d over 5s èd10
2nd row (Pd): 4d much more stable than 5s è Pd d10 ground state
3rd row (Pt): 5d and 6s comparable stability è Pt d9s1 ground state
Relativistic effects of 1s huge èdecreased KE ècontraction è stabilize and contract all ns èdestabilize and expand nd

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06
Next section
48
Theoretical Studies of Oxidative Addition and Reductive Elimination: J. J. Low and W. A. Goddard III J. Am. Chem. Soc. 106, 6928 (1984) wag 190
Reductive Coupling of H-H, H-C, and C-C Bonds from Pd Complexes J. J. Low and W. A. Goddard III J. Am. Chem. Soc. 106, 8321 (1984) wag 191
Theoretical Studies of Oxidative Addition and Reductive Elimination. II. Reductive Coupling of H-H, H-C, and C-C Bonds from Pd and Pt Complexes J. J. Low and W. A. Goddard III Organometallics 5, 609 (1986) wag 206

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 49
Mysteries from experiments on oxidative addition and reductive elimination of CH and CC bonds on Pd and Pt
Why are Pd and Pt so different

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 50
Mysteries from experiments on oxidative addition and reductive elimination of CH and CC bonds on Pd and Pt
Why is CC coupling so much harder than CH coupling?

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 51
Step 1: examine GVB orbitals for (PH3)2Pt(CH3)

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06
Stopped Apr 17
52

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 53
Analysis of GVB wavefunction

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 54
Alternative models for Pt centers

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 55

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 56

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 57
energetics
Not agree with experiment

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 58
Possible explanation: kinetics

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 59
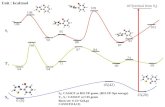
Consider reductive elimination of HH, CH and CC from Pd
Conclusion: HH no barrier
CH modest barrierCC large barrier

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 60
Consider oxidative addition of HH, CH, and CC to Pt
Conclusion: HH no barrier
CH modest barrierCC large barrier

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 61
Summary of barriers
But why?
This explains why CC coupling not occur for Pt while CH and HHcoupling is fast

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 62
How estimate the size of barriers (without calculations)

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 63
Examine HH coupling at transition state
Can simultaneously get good overlap of H with Pd sd hybrid and with the other H
Thus get resonance stabilization of TS è low barrier

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 64
Examine CC coupling at transition state
Can orient the CH3 to obtain good overlap with Pd sd hybrid OR can orient the CH3 to obtain get good overlap with the other CH3But CANNOT DO BOTH SIMULTANEOUSLY, thus do NOT get
resonance stabilization of TS è high barier

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 65
Examine CH coupling at transition state
H can overlap both CH3 and Pd
sd hybrid simultaneously but CH3 cannot
thus get ~ ½ resonance
stabilization of TS

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 66
Now we understand Pt chemistry
But what about Pd?
Why are Pt and Pd so dramatically different

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 67
Pt goes from s1d9 to d10 upon reductive eliminationthus product stability is DECREASED by 12 kcal/mol
Using numbers from QM

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 68
Ground state configurations for column 10
Ni Pd Pt

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 69
Pd goes from s1d9 to d10 upon reductive eliminationthus product stability is INCREASED by 20 kcal/mol
Using numbers from QM
Pd and Pt would be ~ same

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 70
Thus reductive elimination from Pd is stabilized by an extra 32 kcal/mol than for Pt due to the ATOMIC nature of the states
The dramatic stabilization of the product by 35 kcal/mol reduces the barrier from ~ 41 (Pt) to ~ 10 (Pd)
This converts a forbidden reaction to allowed

© copyright 2016 William A. Goddard III, all rights reservedCh120a-Goddard-L06 71
Summary energetics
Conclusion the atomic character of the metal can
control the chemistry













![Printed Growth Methanogenesis MethanosarcinaStrain 227 ... · free energy change for the hydrolysis of ATP (-7.6 kcal/mol[-31.8kJ/mol] [20]), the decar-boxylation of acetate to methane](https://static.fdocuments.net/doc/165x107/5e2cc83274b0e11acc28a896/printed-growth-methanogenesis-methanosarcinastrain-227-free-energy-change-for.jpg)



![東北大学 宮本研究室 [kcal/mol]0 5000 10000 15000 20000 25000 30000 35000 40000 E colors [kcal/mol] E DFT orE thermo [kcal/mol] DFT energy thermodynamic data Oxide Nitride](https://static.fdocuments.net/doc/165x107/5fb5bad201c96743e16ae224/oe-oecc-kcalmol-0-5000-10000-15000-20000-25000-30000-35000.jpg)
