Lecture 18: Introduction to Membranes Lipid Structure Properties of Lipid Bilayers.
-
date post
22-Dec-2015 -
Category
Documents
-
view
218 -
download
1
Transcript of Lecture 18: Introduction to Membranes Lipid Structure Properties of Lipid Bilayers.
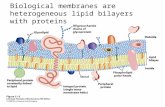
Mitochondria
Lysozome
Peroxisome
Golgi complex
Plasma membraneNucleus
EndoplasmicReticulum
Nutrients
Wastes
Biological Membranes Define the Boundaries of the Cell and its Compartments
Membranes are permeability barriers that keep the cellcontents in and unwantedsubstances out.
Membranes are selectivelypermeable: specific substancescan cross membranes in acontrolled way throughprotein-based transportsystems.
The various organelles andcompartments of the cells arebounded by membranes tocreate interior environmentssuitable for different functions.
Common Features of Biological Membranes
Membranes are sheet-like structures, 2 molecules thick, or 60 to 100Angstroms across.
Lipids and proteins are the major components of membranes, occurringin the ratio of 1:4 to 4:1. Both can be covalently modified by carbohydrates.
Lipids are small molecules with both hydrophobic and hydrophilic groups.They associate to form double layers called lipid bilayers, which areimpermeable to polar molecules.
Proteins associated with or embedded in the lipid bilayer carry out thedifferent functions of membranes. These proteins serve as pumps, channels,receptors, energy transducers, and enzymes.
Both lipid and protein components of membranes are held togetherby noncovalent bonds.
Membranes are asymmetric- the two layers are not equivalent. Lipids canflow and diffuse within a layer, but cannot in general cross to the other layer.
Most membranes are electrically polarized, enabling transport of molecules,energy conversion, and transmission of signals.
Nonpolar “tails”
Polar “head”
Schematic Structure of a Membrane Lipid
Schematic Structure of a Lipid Bilayer
Layer 1
Layer 2
Example Functions of Membranes
PERMEABILITY BARRIERS: Regulation of molecular and ionic compositions of cellsand intracellular organelles
a) channels & pumps (proteins that act as selective transport systems)b) electrical polarization of membrane (due to differences in ion
concentrations on opposite sides)
INFORMATION PROCESSINGbiological communication
a) signal reception by specific protein receptors (BINDING)b) transmission/transduction of signals (via protein conformational changes)
ENERGY CONVERSIONordered arrays of enzymes to organize of reaction sequences a) photosynthesis (conversion of light energy to provide chemical bond energy) b) oxidative phosphorylation (oxidation of fuel molecules to provide
chemical bond energy)
Lipids
Lipids are a diverse group of biological molecules which share the common solubility property of being insoluble in water but highly soluble in organic solvents.
Lipids can serve diverse biological roles (energy sources,signalling molecules) but we will focus on membrane lipids-lipids whose primary role is as components of biological membranes.
Three major types of membrane lipids:
Phospholipids
Glycolipids
Cholesterol
Fatty Acids
Fatty acids are components of phospholipids and glycolipids.They consist of long hydrocarbon chains that terminate witha carboxylic acid.
Palmitate:16 carbons
(fully saturated)
Oleate:18 carbons
(monounsaturated)
Kink at 9th carbon
The hydrocarbons of fatty acids vary in length (16 and 18 carbonsare the most common) and also in the number and position of double bonds.
Fatty Acid Nomenclature: Substitute oic for e in name of parent alkane: octadecane is an alkane with 18 carbons octadecanoic acid is the fatty acid derived from octadecane (18:0)
Substitute enoic for ene in name of parent alkene: octadecene is an alkene with 18 carbons, 1 double bond octadecenoic acid is the fatty acid derived from octadecene (18:1)
Fatty acids containing more than one double bond are given the suffixes -dienoic, -trienoic, etc. octadecadienoic acid has 18 carbons and 2 double bonds (18:2) octadecatrienoic acid has 18 carbons and 3 double bonds (18:3)
An alkane is a hydrocarbon with no double bonds.An alkene is a hydrocarbon containing one or more double bonds.
A shorthand notation to describe the degree of unsaturation:18:0 represents a fatty acid with 18 carbons and 0 double bonds18:1 represents a fatty acid with 18 carbons and 1 double bond18:2 represents a fatty acid with 18 carbons and 2 double bonds…
Description of Fatty Acids
The carbon atoms are numbered from the carboxyl group.The position of double bonds, and whether they are cis or trans, can be indicated by the notation, e.g. oleate can be referred to as cis-9-octadecenoate (cis double bond between carbons 9 and 10)
The last carbon is referred to as the carbon.
Consuming foods rich in -3 fatty acids, such as salmon, is believedto protect against heart disease.
trans cis Oleate
Phospholipids and Glycolipids
Phospholipids are a major class of membrane lipids, and are comprisedof a “platform” or central backbone compound (glycerol or sphingosine),fatty acids, a phosphate, and an alcohol. The presence of a phosphategroup is the primary identifier. These have charged head groups.
Glycerol:
CH CH CH2 2
OH OH OH
Sphingosine:
Glycolipids are another class of membrane lipids. The platform issphingosine, with a fatty acid linked to the amino group and one ormore sugars to the primary hydroxyl. These have polar head groups.
Phosphoglycerides
C O
O
R1
O
C O
R2
H
CH CH CH2
O
P O O
O
-
Fatty Acids
Phosphate
Alcohol
Glycerol
Phosphate-containing lipids built from glycerol are phosphoglycerides.They contain 2 fatty acids esterified to two of the hydroxyl groups onglycerol, and the third hydroxyl is esterified to phosphoric acid. Thephosphate group is typically further esterified to other alcohols.
Nonpolar fatty acid tails
Polar (charged) head group
The simplest phosphoglyceride isphosphatidate in which the phosphate is not esterified.
Other commonphosphoglyceridescan be formed by addition of hydroxyl-containing groups: serine, choline, ethanolamine, inositol.
(lecithin)
An example of phospholipids based on sphingosine are thesphingomyelins. The amino group of sphingosine forms anamide bond to a fatty acid and the primary hydroxyl is esterifiedto phosphoryl choline or phosphoryl ethanolamine.
Sphingophospholipids
Nonpolar tails Polar (charged)head group
GlycolipidsGlycolipids are based on sphingosine and also form an amide bondwith a fatty acid. The primary hydroxyl is esterified to one or more sugars.Cerebrosides have a single sugar and gangliosides have a branched chainof up to seven sugars.
Glycolipids are found on the exterior layer of the plasma membrane withthe sugars ouside the cell.
Nonpolar tails Polar head group
Phospholipids(charged)
Phosphoglycerides(based on glycerol)
Sphingophospholipids(based on sphingosine)
Phosphatidyl cholinePhospatidyl serine
Sphingomyelins
Glycolipids(based on sphingosine. uncharged)
CerebrosidesGangliosides
Nonpolar tailsPolar or charged
head group
Cholesterol
An important lipid of entirely different form is cholesterol. Its backbone isa steroid, a 4-ring structure, with a hydrocarbon tail, and a polar hydroxylgroup. It is particularly abundant in some kinds of nerve cell membranes.
Lipids Spontaneously Form Bilayers
In aqueous solution, the nonpolar tails of phospholipids and glycolipidstend to associate to minimize contact with water, but the polar headgroups tend to seek contact with water. Two ways to satisfy bothrequirements are to form micelles or bilayers.
Micelles are formed byfatty acids, detergents.Typically smaller than200 Angstroms.
Bilayers are formed by phospholipids.The 2 fatty acid chains are too large tofit in the interior of a micelle.Bilayers can be very large, upto 10 Angstroms (1 mm).7
Bilayer Self-Assembly
Bilayers are stabilized primarily by hydrophobic interactions between thenonpolar tails but also by Van der Waals interactions and also hydrogenbonding between the polar head groups and water.
Bilayers seek to avoid exposure of the hydrophobic tails of lipids to waterso can be very extensive.
To avoid such exposure they form closed compartments.
Any holes that form in the bilayer are energetically unfavorable sobilayers are self-sealing.
Formation of Lipid Vesicles The self-sealing property of bilayers allows creation of lipid vesiclesor liposomes- small membrane-bounded compartments containinga desired substance.These vesicles have clinical uses such as drug delivery.The ability to create membranes with concentration gradients across themis useful for the study of membrane proteins.
Permeability of Bilayers
Bilayers are nearly impermeable to ions and most polar molecules other than water.
The rate at which such substances traverse the membrane is correlated with their solubility in nonpolar solvents.
Polar or charged molecules must be desolvated (shed bound water) beforethey can spontaneously cross the membrane, which is unfavorable.
Summary:
Biological membranes are composed of lipids and proteins and form theboundary of the cell and its compartments.
Phospholipids and glycolipids are formed of fatty acids esterifiedto a platform molecule and contain other groups such as alcoholsor sugars.
Lipids spontaneously assemble into bilayers which are largelyimpermeable to charged and polar molecules and which formclosed compartments.
Key Concepts:Fatty acidsPhospholipidsGlycolipidsCholesterolMicellesBilayersVesiclesPermeability of bilayers