Lab 20 Aplastic Anemias
-
Upload
christina101 -
Category
Documents
-
view
2.101 -
download
0
Transcript of Lab 20 Aplastic Anemias

Lab 20 – Aplastic Anemias
GOAL
The student will be able to outline the causes, pathogenesis, pathology, clinical picture and outcome of aplastic anemia, anemia of chronic disease and anemia of renal failure
Objectives:
Upon completion of this case the student will be able to:
1. List the major causes of aplastic anemia. Describe the two mechanisms postulated to explain the failure of stem cells in aplastic states
2. List the causes of anemia due to bone marrow infiltrations, and describe the expected blood findings3. Define myelophthisic anemia4. Describe the epidemiology of aplastic anemia5. Describe the clinical presentation of aplastic anemia6. Understand importance of bone marrow aspiration and biopsy in the diagnosis of aplastic anemia7. Describe the bone marrow cellularity and peripheral blood counts in aplastic anemia8. Discuss the prognosis of aplastic anemia9. Review the pathogenesis and hematologic findings of anemia of chronic disease10. Understand the mechanism of anemia of renal failure and how this differs from all other forms of anemia
RESOURCES
Robbins & Cotran Pathologic Basis of Disease (7th edition) Chapter 13, pp. 646-649
Ronald Hoffman (editor) Hematology: Basic Principles and Practice (3rd edition) Churchill Livingstone, 2000, pp. 383-388
CASE HISTORYPatricia D., age 62, comes to you complainng that she's experienced gradually increasing tiredness and lack of energy over the past month. She's seeking medical help after unsuccessful self-medication with stress-formula vitamins, at her husband's insistence.
Her only other medical problem is osteoarthritis, for which she has been taking phenylbutazone (100 mg TID) for the past 6 months, as well as aspirin on a PRN basis (generally 4-6 regular strength tablets daily).
The physical examination is unremarkable except for pallor, several bruises on various parts of the body and findings typical for osteoarthritis. However, the laboratory tests reveal a red blood cell count of 2.5 million/mm3 (normal 4.2-6.2), a hemoglobin of 6.2 gr/dL (normal 13-18), a reticulocyte count of 0.5% (normal 0.5-1.5% of red blood cells), a white blood count of 1800/mm3 (normal 4,500-11,000) with 50% neutrophils (normal 57-67% of leukocyte differential) and a platelet count of 35,000/mm3(normal 150,000-350,000).
On the basis of the above findings, a bone marrow aspiration and biopsy were performed at the posterior iliac crest and spine. The aspirate revealed moderately hypocellular marrow with reduced granulocyte and red blood cell precursors

and megakaryocytes. The biopsy also indicated decreased marrow cellularity (~35-40%) with fatty cell replacement. Diagnostic considerations include drug-induced aplastic anemia.
Clinical diagnosis: Aplastic anemia, most probably induced by phenylbutazone
1. Aplastic Anemia: DefinitionAplastic anemia is a disorder of pluripotential stem cells that leads to bone marrow failure. The disorder features hypocellular bone marrow and pancytopenia (decreased circulating levels of all formed elements in the blood). The term “pancytopenia” is used when there is a reduction in all three marrow lineages (granulocytes, platelets and red blood cells). “Bicytopenia” is a reduction in two of three bone marrow cell lines. “Monocytopenia” or “single cytopenia” is reduction in only one of three cell lines.
Aplastic anemia is usually characterized by a severe diminuition in bone marrow function that affects all the hematopoietic lineages (pancytopenia). Granulocyte, platelet and red blood cell levels may not be depressed uniformly and sometimes pancytopenia is not observed but odd combinations of bicytopenia and even monocytopenias are observed instead.
2. Aplastic Anemia: EtiologyThere are three etiologic groups of aplastic anemia:
1) Idiopathic aplastic anemia (2/3 of all cases)
2) Acquired aplastic anemia, i.e. secondary to:
Drugs used in cancer chemotherapy Idiosyncratic effect of drugs such as
phenylbutazone, gold (used to treat joint diseases) and chloramphenicol (antibiotic)
Chemicals (benzene, insecticides) Viral infections (hepatitis viruses, Ebstein- Barr
virus, HIV, parvovirus)
3) Inherited (rare)
Fanconi anemia

3. Aplastic Anemia: Pathogenesis
Theoretically, the bone marrow failure may result from two mechanisms: either a) damage to or defects in the stem and progenitor cells in bone marrow; or b) damage to or defects in the bone marrow stromal cells. Damaged stem cells can produce progeny expressing neo-antigens that evoke an autoimmune reaction, or give rise to a clonal population with reduced proliferative capacity. Either pathway could lead to marrow aplasia.
A consistent laboratory finding in aplastic anemia is the very low numbers of assayable bone marrow progenitor and stem cells. Their total numbers are dramatically decreased. Colony formation by bone marrow cells of patients with aplastic anemia remains unresponsive even to high levels of hepatopoietic growth factors.
The survival and proliferation of hematopioetic cells are dependent on stromal marrow cells. Stromal cell function is not defective in aplastic
anemia and they produce normal quantities of hematopoietic growth factors. Adequate stromal function is implicit in the success of bone marrow transplantation in aplastic anemia, because important stromal elements remain of host origin.
The pathogenesis of aplastic anemia is not fully understood. Indeed it is unlikely that a single mechanism underlies all causes of marrow aplasia. Two major mechanisms have been invoked to explain acquired aplastic anemia:
1) Direct hematopoietic injury:
Certain chemical or physical agents injury directly both proliferating and quiescent hematopoietic cells. Such agents cause significant damage to DNA to result in apoptosis.
Approximately 25% of all the cases of aplastic anemia could be blamed on drug use. There are two classes of drugs that cause bone marrow failure:
A) Drugs used in cancer chemotherapy (cisplatin, mercaptopurine, vincristine, etc.) regularly induce, in a dose-dependent manner, marrow aplasia; this is an expected effect and it is counterbalanced by the benefit these drugs provide in cancer treatment.
B) Most of aplastic anemias associated with medical drugs is described as idiosyncratic, i.e. it is unexpected, and caused by drugs that are not known to induce regularly marrow failure. The term “idiosyncracy” in this context means an unusual individual reaction to a drug or a food. Most marrow failure complications from these drugs occur after a few weeks of initiation of drug use.
Viruses have also been hypothesized to be involved in the pathophysiology of aplastic anemias. In addition to their direct toxic effects, chemicals and viruses may induce complex immune reactions leading to bone marrow failure in aplastic anemia.
2) Immune-mediated bone marrow failure.

Aplastic anemia shares numerous clinical and pathophysiologic features with other autoimmune disorders. Each of these disorders is characterized by T-cell mediated, specific organ destruction. Common to all these disorders there is a cytotoxic T-lymphocyte activation, cytokine production and specific target cell elimination.
In aplastic anemias, chemicals and viral antigens are thought to initiate the autoimmune process.
It is postulated that stem cells are first antigenically altered by exposure to drugs, infectious agents, or other unidentified environmental insults. This evokes a cellular immune response, during which activated T cells produce cytokines such as interferon-γ and TNF that prevent normal stem cell growth and development.
This scenario is supported by several observations. Immunosuppressive therapy with antithymocyte globulin combined with drugs such as cyclosporine produces responses in 60% to 70% of patients. Successful bone marrow transplantation requires "conditioning" with high doses of myelotoxic drugs or radiation. In both instances, it is hypothesized these therapies work by suppressing or killing autoreactive T-cell clones. The target antigens for T-cell attack are not well defined.
The blood marrow and blood from aplastic anemia patients contain elevated numbers of activated cytotoxic T-lymphocytes. Activated cytotoxic T-cells overproduce both interferon gamma (IFN-γ) and tumor necrosis factor (TNF). These two cytokines inhibit hematopoietic proliferation of progenitor and stem cells.
The accumulation of activated cytotoxic T-lymphocytes and the secretion of lymphokines are specifically localized to the affected target tissue, i.e., the bone marrow. The inhibitory effects on hematopoietic proliferation are far more potent when those lymphokines are secreted into the bone marrow microenvironment than when they are simply added to the cultures.
INFγ and TNF not only can suppress hematopoiesis by inhibitory effects on cell proliferation, but an important component of their inhibitory activity is cell death by induction of apoptosis. Apparently, immune-mediated cell cycle blockade and apoptosis can lead to the dramatic elimination of hematopoietic progenitor and stem cells in aplastic anemias.
The early immune system events that precede the global destruction of hematopoietic cells are much less clear. The initial antigen and the events that lead to breached tolerance and the subsequent process of spreading autoimmunity remain undetermined.
4. Aplastic Anemia: EpidemiologyThe annual incidence of aplastic anemia in North America is 2 cases per million population.
Aplastic anemia is much more frequently seen in the Orient (6 cases per million population in Thailand; 14 cases per million population in Japan).
The male-to-female ratio in acquired aplastic anemia is approximately 1:1.
Aplastic anemia occurs in all age groups, with two peaks of incidence. One is observed in people aged 20-25 years; the subsequent peak is observed in people older than 60 years.

5. Aplastic Anemia: PathologyThe bone marrow in aplastic anemia shows variably reduced cellularity, depending on the clinical stage of the disease. There is a decrease in the number of cells of myeloid, erythroid, and megakaryocytic lineage, with a relative increase in lymphocytes and plasma cells. As the cellularity decreases, there is a corresponding increase in bone marrow fat.
Anemia, leukopenia (primarily granulocytopenia) and thrombocytopenia characterize aplastic anemia. Circulating red cells have a normal shape but are often mildly macrocytic. Despite elevated erythropoietin levels, reticulocytosis is not present. Increased fetal hemoglobin can be demonstrated in the red cells in some cases. As in the bone marrow, a relative lymphocytosis is observed in the blood.
6. Aplastic Anemia: Laboratory Studies
Peripheral blood smears will a paucity of platelets, red blood cells, granulocytes and monocytes, but with normal red blood cell morphology (in contrast to hemolytic anemias and sickle cell disease). The reticulocyte count is low, less than 0.5% of red blood cells (normal is 0.5%-1.5%).
Bone marrow aspirations and biopsy are assessed qualitatively (bone marrow aspiration) and quantitatively (bone marrow biopsy) for cellularity and morphology of residual cells.
To assess bone marrow cellularity, the percentage of marrow space occupied by hematopoietic elements plus stroma is visually estimated in bone marrow biopsy.
The reference age for marrow cellularity decreases with age (from 90% in infants to 50% in the elderly) and so the value must be interpreted in light of the patient’s age.
In aplastic anemia both the bone marrow aspiration and biopsy specimen are hypocellular or acellular. The disease is considered severe if the bone marrow cellularity is reduced less than 30%.
Pathological examination of bone marrow biopsy specimen shows fat cell replacement of the bone marrow.
7. Normal Bone Marrow Biopsy Specimen: Low Magnification
A bone marrow biopsy of a normal specimen (above) will normally show approximately 50% to 60% of the marrow space as hematopoietic elements; the fat cells make up the remaining 40%. These percentages are age-dependent, the proportion of marrow fat increasing with age. This biopsy section is stained with hematoxylin and eosin, which allows recognition of the different cell lineages present e.g., erythroid, granulocytic and megakaryocytic.

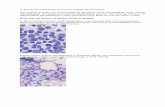
8. Aplastic Anemia Bone Marrow Biopsy: Low & High Magnification
This interactive image depicts a bone marrow biopsy specimen in aplastic anemia. At low magnification (10x) the marrow space is seen to be virtually devoid of hematopoietic elements. The higher magnification image (40x) corroborates the hypocellularity (<5%) of the marrow. The residual cellular composition includes some myeloid elements, plasma cells, and lymphocytes.
9. Aplastic Anemia: Peripheral Blood Smear
The hypocellularity of the marrow is reflected in the peripheral blood smear, which shows granulocytopenia (the nucleated cell shown is a lymphocyte) and leukopenia. Normally, at this magnification (about 40x) you should be able to identify 5 to 7 leukocytes. Also notice that there is ample space between red cells, a reflection of this patient's anemia; and there are few, if any, platelets. All these features reflect the suppression of formation of all the cell lines in the marrow.
10. Aplastic Anemia: Clinical Features
The clinical presentation of aplastic anemia includes symptoms related to the decrease in bone marrow production of hematopoietic cells. The onset is insidious, with the initial symptom relating to anemia or bleeding or neutropenia.

Anemia may manifest as pallor, dyspnea (breathing difficulty) or fatigue. Thrombocytopenia may present as mucosal or gingival bleeding or skin bruises. Neutropenia may manifest as overt or recurrent infection. Levels of serum erythropoietin are elevated proportionally to the degree of anemia.
11. Aplastic Anemia: Treatment
Transfusion: Patients with aplastic anemia require transfusion support until the diagnosis is established and specific therapy can be instituted.
Treatment of infections with broad-based empirical antibiotic therapy.
Bone marrow transplantation from a HLA-matched sibling donor is the treatment of choice for patients less than 60 years old. Immunosuppressive therapy is used if the patient is older than 60 years.
12. Aplastic Anemia: Prognosis
Aplastic anemia has greater than a 75% mortality rate with supportive care alone. It represents a hematological emergency and care should be instituted promptly.
The 5-year survival rate for the patients receiving matched sibling donor bone marrow transplantation is greater than 90%. The 5-year survival rate for patients receiving only immunosuppressive therapy is 75%.
13. Which of this patient's drugs is most likely to have caused her aplastic anemia?
Phenylbutazone, a nonsteroidal antiinflammatory drug (NSAID) used for the treatment of pain of osteoarthritis, is probably the culprit. Phenylbutazone is a well known cause of aplastic anemia, and the risk is greater when phenylbutazone is taken regularly and for a sustained period.
14. Anemia of Renal Disease
Anemia of chronic renal insufficiency reflects decreased production of erythropoietin by the damaged kidneys. The severity of anemia is proportional to the underlying degree of renal insufficiency.
In addition to renal disease (from varying causes) causing decreased production of erythropoietin and subsequent development of anemia, it has been suggested that there is a “uremic toxin,” capable of suppressing erythroid precursors, as well as a minor hemolytic component produced. These have been implicated as contributing to the anemia of chronic renal disease, but the proof of this hypothesis is lacking.
The anemia of chronic renal disease is normocytic and normochromic. In some cases, echinocytes (crenated cells, burr cells) with scalloped cell membranes can be seen. If the renal insufficiency is secondary to malignant hypertension, red cell fragmentation with formation of schistocytes may also be seen on smears.
Administration of recombinant erythropoietin is the treatment of choice.

15. Anemia of Renal Disease: Peripheral Blood Smear, Medium Magnification
The anemia of renal failure results directly from a deficiency in erythropoietin production, making it unique among the many types of anemia. Because of the decreased erythropoietin, there is decreased proliferation of erythroid precursors in the marrow. Thus, anemia of renal failure falls into the broad category of hypoproliferative anemias. The anemia is generally normochromic/normocytic with little anisopoikilocytosis, as illustrated in the above image.
16. Anemia of Chronic Disease: Pathology & Abnormalities of Iron Distribution
Anemia of chronic disease arises in association with chronic inflammatory and malignant conditions. The pathogenesis of nnemia of chronic disease results from the release of inflammatory cytokines (IL-1, TNF, IFN-gamma) in inflammatory or neoplastic processes. It is thought that these cytokines suppress erythropoiesis through multiple mechanisms.
Thus, anemia of chronic disease represents another hypoproliferative anemia. The cytokines also cause abnormalities of iron metabolism, including increased storage iron (second feature) and decreased serum iron. These abnormalities of iron distribution are generally not felt to be a significant cause of the anemia in this disorder, but are useful as laboratory markers. The increased storage iron is reflected as increased serum ferritin; thus, increased serum ferritin in
conjunction with decreased serum iron is characteristic of anemia of chronic disease.
The red cells in anemia of chronic disease are reduced in number and may be normocytic as shown above left, or mildly microcytic and minimally hypochromic as shown at right. The anemia of chronic disease is mild to moderate, and the red cells are often microcytic. Prussian blue staining (left) demonstrates normal or often increased amounts of storage iron. Serum iron levels tend to be reduced.
However, in contrast to iron deficiency anemia, total iron binding capacity also tends to be decreased (as is the serum albumin level). Successful treatment of the

underlying chronic disease is associated with restoration of normal hemoglobin levels. The cytokines also cause abnormalities of iron metabolism, including increased storage iron such in this image of bone marrow aspiration and decreased serum iron. These abnormalities of iron distribution are generally not felt to be a significant cause of the anemia of chronic disease, but are useful as laboratory markers. The increased storage iron is reflected as increased serum ferritin; thus, increased serum ferritin in conjunction with decreased serum iron is characteristic of anemia of chronic disease.
17. Myelophthisic Anemia - Anemia Associated with Bone Marrow Infiltration
Myelophthisic anemia refers to hypoproliferative anemia associated with infiltration of the bone marrow by a variety of processes.
Pathogenesis: Any infiltrative process (such as myelofibrosis, hematological malignancies, metastatic carcinoma, or granulomatous disease) can replace normal hematopoietic elements and cause anemia (and often leukopenia and thrombocytopenia). In an attempt to maintain blood cell production, foci of extramedullary hematopoiesis may appear, most prominently in the spleen and liver.
Pathology: Bone marrow infiltration results in moderate to severe normocytic anemia, with anisopoikilocytosis and teardrop cells. Circulating immature granulocytes and nucleated red blood cells (leukoerythroblastosis) are frequently identified.

Quiz
1) Which of the following features in the CBC count does not agree with a diagnosis of aplastic anemia?
A) NeutropeniaB) Normocytic anemiaC) Microcytic anemiaD) ThrombocytopeniaE) Lack of reticulocytosis
2) The diagnosis of aplastic anemia can be made without a bone marrow biopsy if the CBC count shows severe pancytopenia.
A) TrueB) False
3) A 35-year-old man presents with a 2-month history of headache and was noted to be pale by a friend. On examination, petechia, hepatosplenomegaly and supraclavicular adenopathy are present. The CBC count confirms severe pancytopenia. Aplastic anemia is the likely diagnosis.
A) TrueB) False
4) Which of the following is neither a complication of aplastic anemia neither a consequence of pancytopenia?
A) ThrombosisB) PallorC) SepsisD) Tiredness and malaiseE) Ecchymoses
5) A 35-year-old man presents with petechia, ecchymoses, fatigue, and a low-grade fever. His physical examination is otherwise unremarkable. A complete blood cell count reveals a moderately severe normocytic anemia, thrombocytopenia, and absolute neutropenia. The reticulocyte count is less than 0.5 %. The serum blood urea nitrogen and creatinine are normal. The next step in the management of this patient is to order which one of the following tests?
A) Direct Coombs testB) Sugar water hemolysis testC) Serum vitamin B12 level measurementD) Bone marrow aspirate and biopsyE) Serum iron and ferritin test