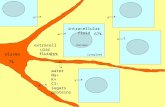
Intracellular vs. extracellular concentrations
description
Transcript of Intracellular vs. extracellular concentrations

1
Intracellular vs. extracellular concentrations
Note:Na+, K+, Cl-, phosphate,- & protein-

2
[IC] vs. [EC] important points
*Intracellular cations = Intracellular anions (mEq/L)
*Extracelluar cations = Extracellular anions (mEq/L)
*miniscule, unmeasurable differences
Intracellular particles = Extracellular particles
i.e. IC osmolality = EC osmolality

3
Membrane transport overview
No carrier:
simple diffusion (lipid soluble substances)
diffusion through ion channels
diffusion through water channels
Carrier mediated transport
facilitated diffusion (passive)
primary active transport (active, uses ATP)
secondary active transport (active, uses ion gradient)
Endocytosis & exocytosis

4
Simple diffusion
Through phospholipid bilayer
Lipid soluble substances
e.g. O2, CO2, NH3, N2, fatty acids, steroids, ethanol,
Passive (down concentration gradient)
No carrier ( no saturation, competition)

5
Simple diffusion
fig 4-2

6
Simple diffusion (flux)
At equilibrium:compartment 1 concentration = compartment 2 concentrationone-way flux (left right) = one-way flux (right left)net flux = 0
fig 4-3

7
Simple diffusion (graph of Ci vs. time)
Graph shows that transport is passivei.e. over time Ci will reach, but never exceed Co
fig 4-4

8
Simple diffusion (graph of rate vs. concentration)
Graph shows that transport is not carrier mediated;because no saturation of transport rate

9
Transport through ion channels
fig 4-7

10
Properties of ion channels
Usually (not always) highly specific for the ion
Ion transport is passiveions are chargedtherefore, gradient depends on concentration & chargecombination is “electrochemical gradient”
Channels open and close spontaneously
Percentage of “open time” can be regulated (gating)
Open time regulated by:binding of ligands to the channels (ligand gating)voltage difference across membrane (voltage gating)stretch of membrane (mechanical gating)covalent alteration of channel protein

11
Facilitated diffusion
fig 4-8

12
Facilitated diffusion (properties)
Passive, carrier mediated
Examples: glucose into most cells (not luminal membrane of kidney or intestine), urea, some amino acids
Kinetics:
shows: passive shows: carrier mediated

13
Non-mediated vs. mediated transport
fig 4-9

14
Primary active transport (Na+/K+ ATPase pump)
3 Na+’s out, 2 K+’s in, 1 ATP hydrolyzed
fig 4-11

15
Primary active transport properties
Active (energy from direct hydrolysis of ATP)
Carrier mediated
Used when:many ions moved (e.g. 5 for Na+/K+ ATPase pump)ions moved against steep gradient (Ca++ ATPase in muscle, H+/K+ ATPase in stomach, H+ ATPase in kidney)

16
Primary active transport kinetics
shows active transport shows carrier mediated

17
Effect of Na+/K+ ATPase pump
fig 4-12

18
Secondary active transport
fig 4-13

19
Secondary active transport properties
Active (energy from ion gradient, usually Na+)
Carrier mediated
Can be cotransport (symport) or countertransport (antiport)
Examples (many):Na+/amino acids, Na+/glucose (luminal membrane kidney, GI tract), *Na+/H+ kidney, *Ca++/3Na+ muscle, *Cl-/HCO3
- red cell. (* = countertransport)
Kineticssee primary active transport graphs

20
Transport, the big picture
fig 4-15

21
Table 4-2

22
Water transport (aka osmosis)
Water moves through aquaporin channels
Water moves passively down its own concentration gradient
Dissolving solute in water reduces the water concentration
Water therefore moves from a dilute solution to a more concentrated solution
The “solute concentration” depends on the number of particles
The number of particles is called “osmolarity” (?osmolality?)
The units of osmolarity are milliosmoles/L (mOsm/L)

23
The osmolarity of a 100 mM glucose solution is 100 mOsm/L
A 100 mM NaCl solution dissociates into 100 mM Na+ and 100 mM Cl-; its osmolarity is therefore 200 mOsm/L
Assuming complete dissociation, calculate the osmolarity of the following solutions:
1. 100 mM NaCl, 50 mM urea
2. 200 mM glucose, 30 mM CaCl2
Calculation of osmolarity
Answer: 250 mOsm/L
Answer: 290 mOsm/L

24
Red cells in solution
Notes: nonpenetrating solutes, cell osmolarity ~300 mOsm/L
fig 4-19

25
Crenated red cells

26
Osmolarity and tonicity
Osmolarity is a measure of the total number of particles
Tonicity is a measure of the solute particles which do not cross the cell membrane “non-penetrating solutes”
Tonicity therefore depends on the properties of the solute and the cell membrane
For example, urea crosses most cell membranes, and will enter the cell down its concentration gradient
A solution of 300 mM urea is isosmotic to red cells but is hypotonic

27
Osmolarity and tonicity problems
1. Consider a solution of 100 mM NaCl and 200 mM urea. How does its osmolarity and tonicity compare with red cells having an osmolarity of 300 mOsm/L?
Answer: hyperosmolar and hypotonic
2. Consider a solution of 125 mM NaCl and 50 mM urea. How does its osmolarity and tonicity compare with red cells having an osmolarity of 300 mOsm/L?
Answer: isosmolar and hypotonic

28
Osmolarity (important concept)
Because cells contain abundant aquaporin channels, water rapidly equilibrates across the cell membrane
Therefore, the osmolarity of virtually all body cells is equal, and equal to the osmolality of extracellular fluid

29
Drinking water

30
Endocytosis and exocytosis
fig 4-20

31
Endocytosis and exocytosis properties
Endocytosis:
pinocytosis, phagocytosis
specificity conferred by receptor mediated endocytosis
route: see next slide
Exocytosis:
release of neurotransmitters, hormones, digestive enzymes
route: rough er Golgi secretory vesicles
release usually triggered by cytosolic [Ca++]
insertion of glucose transporters (insulin),
insertion of water channels (ADH)

32
Endocytosis route
fig 4-21

33
Epithelial transport (Na+)
fig 4-22

34
Epithelial transport (water)
fig 4-24

35
Epithelial transport (glucose in kidney, GI tract)
fig 4-23