InsulinResistance, Hyperinsulinemia, and Obesity-Associated
Transcript of InsulinResistance, Hyperinsulinemia, and Obesity-Associated

1064 Volume 3 ‘ Number 5’ 1992
Insulin Resistance, Hyperinsulinemia, andObesity-Associated Hypertension1
Michael W. Brands2 and John E. Hall
MW. Brands, J.E. Hall, Department of Physiology and
Biophysics. University of Mississippi Medical Center,
Jackson, MS
(J. Am. Soc. Nephrol. 1992; 3:1064-1077)
ABSTRACTRecent work to elucidate the cause of obesity-asso-ciated hypertension has focused on insulin resist-ance and hyperinsulinemia. A significant amount ofepidemiologic and correlational evidence suggestsa link between these factors and obesity-associatedhypertension, and acute insulin infusion studies haverevealed renal, neural, and cardiovascular effectsof this hormone that, if maintained chronically, couldcause hypertension. However, correlations andacute effects may not reliably predict a chroniccause-and-effect relationship, and the fundamentalquestion of whether chronic increases in plasma in-sulin concentration per so can produce a sustainedincrease in arterial pressure has not been corn-pletely resolved. Recent studies designed to addressthis question directly have found no evidence of a
hypertensive effect of insulin in normal dogs, or in
dogs with a 70% reduction in kidney mass and givena high sodium intake. Chronic hyperinsulinemia alsodid not potentiat� the pressor effects of angioten-sin II or norepinephrine. In fact, hyperinsulinemia
caused significant reductions in total peripheral vas-cular resistance in dogs and a decrease in arterialpressure. Furthermore, induction of insulin resistancein dogs made obese by being fed a high-fat dieteliminated the decrease in peripheral vascular re-sistance during chronic insulin infusion but did notuncover a pressor effect of hyperinsulinemia. In con-trast, insulin infusion for up to 7 days produced asustained increase in arterial pressure in rats. Al-though the mechanism for this pressor response is
‘Received April 24, 1992. Accepted July 22, 1992.2 Correspondence to Dr. M.W. Brands, Department of Physiology and Biophys�
cs, University of Mississippi Medical Center, 2500 North State Street. Jackson,
MS 39216.
1046-6673/0305- 1064$03.00/0Journal of the American society of NephrologyCopyright © 1992 by the American Society of Nephrotogy
unknown, these data indicate either that there aremajor species differences in the chronic blood pres-sure response to insulin or that specific, presentlyunknown, conditions must exist in order for insulin toraise blood pressure. Also, it is not clear whetherhumans respond more like rats or dogs with respectto blood pressure changes during chronic hyperin-sulinemia. However, it is apparent that obesity hy-pertension is probably much too complex to be
ascribed to insulin resistance and hyperinsulinemiaalone.
Key Words: Kidney, insulin, blood pressure. sodium excretion,
sympathetic nervous system
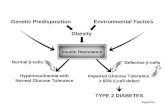
H Igh blood pressure has become recognized as a
potential clinical complication associated withobesity. Several studies have reported a correlationbetween body weight and blood pressure (1 -4). andweight loss lowers blood pressure in obese hyperten-
sives (5- 1 0), even when sodium intake Is maintainedat a high level (6). Furthermore, excessive weight gainin experimental animals produces a significant riseIn blood pressure that Is reversed by weight loss( 1 1 , 1 2). However, the mechanisms responsible forweight-related changes in blood pressure are unclear.
One factor that has been advanced as a potentiallink between obesity and hypertension is insulin(13-20). Insulin resistance and compensatory hy-perinsullnemla are common features of obesity
(1 4, 1 7,2 1 ,22), and in addition to its blood pressure-lowering effect, weight loss decreases plasma insulinlevels (5, 1 2). Hyperinsulinemia and insulin resist-
ance also have been reported to be associated withhypertension independent of obesity (14,15,21-24),
further suggesting that these abnormalities can con-tribute to high blood pressure. Moreover, acute stud-
ies have reported multiple effects of insulin on thekidney, the sympathetic nervous system, and the
cardiovascular system that, if maintained chron!-cally, could lead to hypertension. Thus, epidemiologic
and correlational evidence linking insulin to obesityand hypertension, and evidence from acute studiesfor potential pressor effects of insulin, have beeninterpreted as suggesting that hyperinsulinemia isimportant in the development of hypertension asso-ciated with obesity. However, most of these studieshave not addressed directly the question of whether
a chronic rise in plasma insulin concentration per se

Brands and Hall
Journal of the American Society of Nephrology 1065
is capable of producing a sustained increase in arte-na! pressure, and there have been several recentreports that have questioned whether hyperinsu-linemia and Insulin resistance are key to hyperten-
sion associated with obesity.
EPIDEMIOLOGIC AND CORRELATIONALEVIDENCE LINKING INSULIN RESISTANCE,HYPERINSULINEMIA, AND HYPERTENSION
A positive correlation between insulin and bloodpressure has been reported in obese patients(1 4, 1 7,2 1 ,22) and also may occur independent ofobesity (14,21-24). Christlieb et at. (14), in a study
of 1 95 patients with Impaired glucose tolerance, re-ported that serum Insulin was significantly elevatedin hypertensive patients, and this relationship per-
sisted after correction for body weight. In a randompopulation sample of approximately 2,500, Modan etat. (2 1 ) reported a strong association between hyper-
tension, glucose intolerance, and obesity and alsonoted that fasting and postglucose-load insulin levelsin 1 ,24 1 hypertensives were significantly elevatedindependent of obesity. Ferrannini et at. (24) re-ported that hyperinsulinemia was consistently asso-dated with hypertension independent of obesity in asurvey of nearly 3,000 subjects in the San AntonioHeart Study. However, the slope of the relationshipbetween blood pressure and postglucose-load plasmainsulin concentration in 2,241 normotenslve, non-
diabetic subjects predicted that a 200 .tU/mL increasein plasma insulin concentration could account foronly a 1 mm Hg rise in blood pressure (24), thussuggesting minimal sensitivity of blood pressure toinsulin levels per Se.
A positive relationship between insulin resistance,insulin, and blood pressure also has been reported inanimal models of hypertension . Hyperinsulinemiaand insulin resistance have been measured in severalgenetic models of hypertension, including the spon-taneously hypertensive rat (25,26) and the Milanhypertensive rat (27). Furthermore, maintainingSprague-Dawley rats on a high intake of simple sug-ars has been reported to cause insulin resistance,raise plasma insulin levels, and increase tail-cuffsystolic pressure (15,28,29) and blunting the rise in
insulin by administering somatostatin appears to de-
crease systolic pressure (28). Hyperinsulinemia andinsulin resistance also have been measured in obesehypertensive dogs (11,12,30,31). Thus, a large bodyof correlational data exists to suggest that insulinresistance, hyperinsulinemia, and hypertension maybe related.
Insulin resistance has been postulated to be fun-
damental in this relationship by inducing a compen-satory rise in plasma insulin concentration, whichthen acts through various mechanisms to increaseblood pressure (13-20). However, insulin resistance
also has been suggested to act by mechanisms thatincrease peripheral vascular resistance independent
of hyper!nsul!nemia (1 9,20,32-34). Regardless of the
proposed mechanisms linking insulin resistance, hy-
perinsulinemia, and hypertension, It is important tonote that not all hypertensives are insulin resistantand that many patients with hyperinsulinemia andinsulin resistance are not hypertensive (35-37). Sim-ilarly, obese Zucker rats have been reported to benormotensive despite significant hyperinsulinemia
(38) and some forms of hypertension, such as reno-vascular hypertension, are not associated with hy-perinsulinemia or insulin resistance (39,40). In ad-
dition, it is Important to remember that a cause-and-effect relationship cannot be inferred or refuted fromcorrelational evidence. In experimental and humanhypertension, many variables are highly correlatedwith blood pressure, even though their Importance in
causing hypertension may be questionable. For cx-ample, increased total peripheral resistance is highlycorrelated with hypertension, with almost all formsof hypertension being characterized by elevated totalperipheral resistance. However, this association
often leads to erroneous conclusions regarding theImportance of Increased vascular resistance in caus-
ing the rise In blood pressure, because the rise Inperipheral vascular resistance often occurs second-ary to various autoregulatory adjustments after theonset of hypertension (4 1 ). Therefore, one must cx-ercise caution before embracing the concept thatinsulin resistance and hyperinsulinemia can causehypertension solely on the basis of correlational cvi-dence.
ACUTE EFFECTS OF INSULIN ON BLOODPRESSURE CONTROL MECHANISMS
Perhaps the most widely accepted hypothesis link-Ing insulin, insulin resistance, and hypertension isthat the onset of insulin resistance necessitates a
compensatory rise in plasma insulin concentrationin order to maintain glucose homeostasis. The rise inplasma insulin is then postulated to cause effects on
the kidney and sympathetic nervous system thateventually lead to Increased blood pressure. However,support for these effects of Insulin is derived mainly
from acute studies.Early studies in humans reported an antinatri-
uretic response to insulin administration (42,43), andwithdrawal of insulin therapy from diabetic patientsincreased sodium excretion (42). This effect of insu-lin appears to be direct because acute, Intrarenalinsulin infusions have been reported to decrease so-dium excretion (16), even in isolated kidneys (44).The mechanism for the antinatriuretic action is un-clear, but micropuncture data suggest that Insulinhas a direct effect to stimulate sodium reabsorptionat a site beyond the proximal tubule (16,45). This

Hyperinsulinemia and Hypertension �
1066 Volume 3’ Number 5’ 1992
effect of insulin to decrease renal sodium excretorycapability, if maintained chronically, could increaseblood pressure through sodium retention and expan-
sion of the extracellular fluid volume. The rise inblood pressure eventually would offset the antinatri-uretic effect of insulin. via pressure natriuresis, sim-
ilar to the effect of aldosterone (4 1 ). Thus, in the
steady-state, sodium balance would be reattained,but at the expense of an elevated arterial pressure.This putative response to hyperinsulinemia, how-
ever, has been proposed based on the results fromacute insulin infusions (16) and has not been dem-onstrated chronically.
Another action of insulin that has been postulatedto raise blood pressure is stimulation of the sympa-thetic nervous system. There is evidence that in-creased caloric intake produces parallel increases in
plasma insulin levels and tissue norepinephrineturnover, although high fat or carbohydrate diets alsocan stimulate sympathetic activity even if caloricintake is not increased (18,46). Troisi et at. (47) re-ported that, in 572 men from the Normative AgingStudy, total caloric intake was independently corre-lated with 24-h urinary norepinephrine excretionand that norep!nephrlne excretion was higher in hy-perinsulinemic subjects. In addition, high sucrose,fructose, or glucose intake in rats stimulates sym-pathetic nervous system activity and increases bloodpressure ( 1 5,48,49), and also produces insulin resist-ance and hyperinsulinemia (15,29). Thus, there ap-pears to be a positive correlation between hyperin-sulinemia and increased sympathetic activity.
The sympathetic nervous system could chronicallyraise blood pressure by increasing tubular sodiumreabsorption and preglomerular resistance (4 1 ,50,5 1 ). A peripheral vasoconstrictor effect of sympa-thetic stimulation could shorten the time for devel-opment of hypertension and may underlie the pressorresponse to insulin infusion observed during acuteexperiments. Many studies have demonstrated thatinsulin-induced hypoglycemia can increase plasma
epinephrine concentration (18,52-54), but insulinalso may have a direct effect to stimulate sympathetic
nervous system activity independent of hypoglyce-mia (18,52,55,56). Pereda et at. (57) reported an ef-fect of acute, lv infusion of large doses of insulin toincrease arterial pressure and cardiac output in dogs.This response occurred in the absence of hypoglyce-mia, could be duplicated by infusing insulin in thecarotid artery, and was inhibited by ganglionic oradrenergic receptor blockade. Liang et at. (52) re-ported that epinephrine and norepinephrlne levels
rose if hypoglycemia occurred during acute insulininfusion in dogs but that only norepinephrine in-
creased when euglycemia was maintained. Bloodpressure also increased slightly (approximately 5 mmHg) with the euglycemic insulin infusions at high
rates (52). Similar results have been obtained in hu-mans (55). These observations are consistent withthe hypothesis that hyperinsulinemia may increaseblood pressure by stimulating the sympathetic nerv-ous system.
A potential problem with most of these studies is
that large, nonphysiologic doses of insulin were usedand only administered acutely. Recently, Anderson
et at. (57) reported that acute insulin infusion, atrates that produced plasma insulin levels in thepathophysiologic range, increased sympathetic nerve
activity in humans. It is important to note, however,that acute insulIn infusion did not increase arterialpressure in that study and also that the demonstra-
tion of an acute relationship between two variables(e.g. , insulin and sympathetic nervous system actlv-ity) does not provide evidence that this effect can be
sustained chronically. Furthermore, the correla-tional evidence isjust that: evidence that insulin andthe sympathetic nervous system are positively cor-
related, not evidence for a cause-and-effect relation-ship. Therefore, a role of the sympathetic nervous
system in mediating any putative, chronic hyperten-sive effect of hyperinsulinemia remains to be estab-lished.
Thus, there is a significant amount of evidence
relating hyperinsulinemia in insulin-resistant states,such as obesity. to the development of hypertension.
Correlational studies are consistent with the involve-ment of the sympathetic nervous system that couldmediate this effect by combined antinatriuretic andvasoconstrictor actions. Acute studies support astimulatory effect of Insulin on the sympathetic nerv-ous system and also provide evidence for a directantinatriuretic action. However, regardless of the
mechanism , a cause-and-effect relationship betweeninsulin and chronic hypertension has not been defin-itively established.
CHRONIC EFFECTS OF HYPERINSULINEMIA ONBLOOD PRESSURE
Although the demonstration that insulin has acuteeffects on the sympathetic nervous system and on
the cardiovascular system is consistent with a rolefor hyperinsulinemia in hypertension , short-termstudies do not address directly the question ofwhether chronic hyperinsulinemia can increaseblood pressure. An example of the potential dangerinvolved when attempting to extrapolate informationderived from acute studies to a chronic setting is theacute versus chronic effects of some vasoconstrictors
such as vasopressin. Vasopressin is one of the mostpowerful vasoconstrictors: yet, although the acute
pressor effect of vasopressin can be demonstratedreadily, chronic infusion of vasopressin causes onlysmall increases in arterial pressure as long as kidney

function is not impaired (58,59). Thus, the acute
effect of this hormone does not accurately predict thelong-term response. To address the question of
whether chronic hyperinsul!nemia per se can pro-duce hypertension, we conducted several series ofexperiments in dogs and rats (60-64).
Chronic Hyperinsulinemia in DogsThese studies were designed to test whether
chronic increases in plasma insulin concentration,comparable to those found In obese hypertensives,would cause sustained elevations in blood pressure.
In these experiments, insulin was continuously in-fused lv at a dose of 1 mU/kg/mm; plasma insulinconcentration increased approximately fivefold tosixfold, but plasma glucose concentration was main-tamed within the normal range by a simultaneous ivglucose infusion. The effects of insulin infusion for7 days in chronically instrumented, conscious dogsmaintained on a normal sodium intake are illustratedin Figure 1 (61). The most important finding was that
7 days of hyperinsulinemia did not increase blood
pressure but, instead, produced an approximate 10mm Hg fall in blood pressure. This occurred despite
a transient decrease in urinary sodium and waterexcretion and the cumulative retention of approx!-
mately 120 mEq of sodium after 7 days of insulininfusion. The antinatriuretic effect of insulin wasdue to increased tubular sodium reabsorption rather
110
100
90
80
70
100
80
60
40
20
0.4 .2 0 2 4 6 8 10 12 14
TIME (days)
Figure 1. Effects of insulin infusion (1.0 mU/kg/mm iv) onmean arterial pressure and urinary sodium excretion innormal, conscious dogs (N = 6). Adapted with permissionfrom reference 61.
than decreased filtered sodium load, because GFR
and effective RPF increased by 1 5 to 20%.One mechanIsm through which insulin has been
postulated to raise blood pressure is by causing renalsodium and volume retention (16). Because the hy-pertensive actions of other antinatriuretic hormones,such as aldosterone, are exacerbated by either a high-salt intake or reduced kidney mass (4 1 ), we alsotested the possibility that hyperinsulinemia mightexert a hypertensive effect when renal function isimpaired or sodium Intake is high (60). This possibil-
!ty also has clinical relevance, because many obesehypertensives are elderly and there is a gradual re-duction in the number of functional nephrons withaging (65).
The effects of chronic hyperinsulinemia in dogswith kidney mass surgically reduced to approxi-mately 30% of normal are shown in Figure 2 (68).The dogs were maintained on a high sodium intake
(over 300 mEq/day) to further increase their suscep-tibility to any potential hypertensive effect of in-
sulin, and insulin was infused for 28 days to becertain that sufficient time was allowed for insulinto exert a hypertensive action. Insulin infusion
caused marked sodium retention and, similar to theresponse in normal dogs, GFR increased, Indicating
that the insulin infusion increased tubular sodiumreabsorption. However, despite the sodium retention,these dogs responded to insulin with an approximate
1 0 mm Hg decrease in blood pressure. Although bloodpressure returned towards control levels after 2 to 3wk of hyperinsulinemia, there was no evidence of
hypertension. Chronic hyperinsulinemia also did notincrease blood pressure in reduced kidney mass,high-salt dogs that were continuously infused with
ang!otcnsln II and mildly hypertensive (60), eventhough some studies suggest that Insulin potent!atesthe acute pressor and aldosterone-stimulating effects
of angiotensin II (66).Because insulin infusion caused significant so-
dium retention in normal dogs and dogs with reducedkidney mass and high salt intake, the absence ofhypertension and, more specifically, the decrease Inblood pressure that was measured, appear at first to
be paradoxical. Chronic infusions of some antinatri-uretic hormones, such as anglotensin II (ANG II) andaldosterone, cause marked hypertension mainly be-cause of their direct renal actions (4 1 ,67). In contrast,insulin infusion caused transient antlnatriuresis and
a reduction in blood pressure. The fact that the anti-natriuresis was transient is not surprising, becauseeven the most powerful antinatriuretic hormonescause only a temporary decrease in sodium excretion.This is because sodium retention initiates variouscompensatory mechanisms, especially increases inblood pressure and pressure natriuresis, which thenhelp to return sodium excretion to normal and restore
Brands and Hall
Journal of the American Society of Nephrology 1067
MEANARTERIALPRESSURE(mmHg)
URINARYSODIUMEXCRETiON(mEq/day)

Hyperinsulinemia and Hypertension � � �
HIGH SODIUM + 4 KIDNEY MASS
5
4
-4 0 4 8 12 16 20 24 28 32
TiME (days)
2
1
1068 Volume 3 ‘ Number 5’ 1992
MEANARTERIALPRESSURE(mmHg)
URINARYSODIUMEXCRETION(mEq/day)
Figure 2. Effects of insulin infusion (1.0 mU/kg/mm iv) on mean arterial pressure and urinary sodium excretion in dogs withreduced kidney mass maintained on a high-sodium intake of approximately 319 mEq/day (N= 7). Adapted with permissionfrom Reference 60.
sodium balance. The observation that the antinatri-uresis during hyperinsulinemia was accompanied bya decrease in blood pressure is reminiscent of thesodium retention that often occurs as a compensa-tion for low blood pressure in circumstances such as
hemorrhage or administration of a peripheral vaso-dilator such as nitroprusside. In fact, we reportedrecently that hyperinsuilnemia in dogs significantlydecreased total peripheral resistance and increasedcardiac output by 30 to 40%, indicative of peripheralvasodilation (Figure 3) (62).
This information suggests that in these studies
much of the sodium retention likely was secondaryto the fall in renal perfusion pressure (41,67). Thisis supported by the observation that chronic intra-renal insulin infusion in dogs, at rates that produced
renal arterial plasma insulin concentrations compa-rable to those attained with the iv insulin infusionsbut that caused no decrease in blood pressure, causedmuch less sodium and water retention than that
measured during iv insulin infusion (Figure 4) (68).Thus, insulin does not appear to have a major directantinatrluretic effect that can be sustained suffi-
ciently to cause hypertension in dogs (60-62,68).Because insulin has been observed to acutely acti-
vate the sympathetic nervous system and increaseplasma norepinephrine concentration, even whenplasma glucose concentration is maintained constant(52), we examined the potential for interaction be-
tween insulin and catecholamines to increase bloodpressure. During chronic insulin infusion in normaldogs. however, we found no effect of hyperinsuline-

MEANARTERIALPRESSURE(mmHg)
CARDIACOUTPUT(% control)
100
80
60
40
20
140
120
100
80
110
100
MEANARTERIALPRESSURE 90(mmHg)
80
70
100
80
URINARYSODIUM 60EXCRETION(mEq/day) �
20
0-4 -2 0 2 4 6 8 10 12 14
TIME (days)
Figure 4. Effects of intrarenal insulin infusion (0.6 mU/kg/mm)on mean arterial pressure and urinary sodium excretion inuninephrectomized dogs (N = 8). Adapted with permissionfrom Reference 68.
C� �.8 �2.5mmHga/m(n
Brands and HaIl
Journal of the American Society of Nephrology 1069
120
TOTAL 100PERIPHERALRESISTANCE(% control)
C�2.3jO.1
60
.4 .2 1 3 5 7 2 4 6 8 11
TIME (days)
Figure 3. Effects of insulin infusion (1.0 mU/kg/mm iv) onmean arterial pressure, cardiac output, and total periph-eral resistance in normal, conscious dogs (N= 6). C, aver-age values for cardiac output and total peripheral resist-once during the control period. Adapted with permissionfrom Reference 62.
mia on plasma catecholamines (61). In addition, hy-perinsulinemia did not potentiate the pressor effectsof norepinephrlne when norepinephrlne was chron-ically infused In dogs to produce a sustained, 1 7-fold
increase in plasma noreplnephrine concentration(61). However, these findings do not rule out thepossibility that increased sympathetic activity may
accompany Insulin Infusion in dogs, because we ob-served consistent increases in heart rate duringchronic hyperlnsulinemla (61,62). The mechanismsresponsible for the rise in heart rate are still unclearand also could be related to a direct chronotropiceffect of Insulin on the heart or to withdrawal ofparasympathetic tone; further study will be neededto resolve this Issue.
Thus, several studies in dogs have directly ad-dressed the question of whether chronic increases inplasma Insulin concentration per se could raise bloodpressure and revealed no evidence of a hypertensiveeffect. In fact, these studies demonstrated that the
direct antinatriuretic action of insulin was mild andthat much of the sodium retention occurred duringsystemic Insulin administration may have been due
to a peripheral vasodilatory and depressor effect of
hyperinsulinemia.
Chronic Hyperinsulinemia in Rats
Several genetic models of hypertension in rats areassociated with insulin resistance and hyperinsu-
linemia (25-27). In addition, correlational evidencefrom Reaven et at. (15,28,29) suggests that hyperin-sulinemia may be responsible for the increase In tail-
cuff systolic pressure measured in rats maintainedon a high intake of simple sugars. Furthermore, low-
ering plasma Insulin concentration during a period
of high-fructose diet In rats, by continuously infusinga somatostatin analog, lowered systolic pressure (28).Similar results have been obtained In human studies,
where somatostatln has been reported to lower bloodpressure and plasma insulin in insulin-resistant, hy-perinsulinemic subjects with hypertension (69,70).However, somatostatin has many other effects be-
sides Inhibition of Insulin secretion that were not
controlled for in those studies, thus leaving somequestion as to whether the depressor response wasdue solely to lower insulin levels. Furthermore, the
use of the tail-cuff technique may have had a con-founding effect on the assessment of blood pressure

in the rat studies (15,28,29). Under conditions ofheightened sympathetic nervous system activity. as
has been reported during the high Intake of simplesugars (1 5, 1 8,48,49). blood pressure could be morelabile, such that any acute measurement of blood
pressure-especially in restrained animals with a
tail cuff-would be more likely to register a highpressure.
To directly test the chronic effect of insulin onblood pressure in rats. insulin was infused continu-ously in conscious, chronically instrumented ratswhile decreases in plasma glucose concentrationwere prevented (63,64). An important feature ofthese studies was that potential errors due to the
acute measurement of blood pressure commonly
used, whether from a catheter or by the tail-cufftechnique, were avoided by continuously measuringarterial pressure from an aortic catheter, 1 9 h/day,
from rats undisturbed in their metabolic cages. Theresults from these studies indicated that sustainedhyperinsuhlnemla produced an increase in arterial
pressure that could be maintained for at least 7 days(Figure 5: [701). The elevated blood pressure could notbe attributed to significant sodium retention (63,64)
or to increased activity of the renin-angiotensin sys-tem (64), but further study is needed to determinethe role of other mechanisms, such as the sympa-thetic nervous system, in mediating the hypertensiveeffect of chronic insulin infusion in rats.
Another interesting finding in these studies was
that the control mean arterial pressures were normal
(93 ± 1 mm Hg [63J and 86 ± 2 mm Hg [641), althoughthe rat chow we used (like most commercially avail-able. pehleted rat chows) was high in sucrose. Con-sistent with other reports ( 1 5, 28, 29) on the effectsof high-sugar intakes, the rats also appeared hyper-
insulinemic during the control period (63). As to whyarterial pressure was normal despite insulin resist-ance and modest hyperinsulinemia, It is possible that
1 3 5
Figure 5. Effect of insulininfusion (1.5 mU/kg/mm) on meanarterial pressure in rats on a normal sodium intake of 3.0mEq/day (N = 6). Adapted with permission from Reference
110
100
MEANARTERIALPRESSURE(mmHg)
90
70
-4 -2
TIME (days)
64.
Hyperinsulinemia and Hypertension
1070 Volume 3 ‘ Number 5’ 1992
the Insulin levels simply were not high enough toactivate the mechanisms that produced the pressure
rise during the Insulin infusion.
POSSIBLE EFFECTS OF VERY LONG-TERMHYPERINSULINEMIA
One other potential effect of hyperinsulinemia. ofconsiderably longer duration, may relate to mitogeniceffects of insulin on vascular smooth muscle (7 1 ,72)and/or to atherosclerotic lesions resulting from thedyslipidemic actions of insulin (20,73). These effects
of hyperinsulinemia, particularly those on plasmatriglyceride and cholesterol profiles, have been 1mph-cated in the development of atherosclerosis and inincreased risk for coronary artery disease, but theirrole in altering structure and function in the vascu-lature and in the development of hypertension isunclear.
It is important to recall that primary changes in
the vasculature, if their sole effect is to raise totalperipheral resistance, could not lead to chronic hy-pertension (41). This is because any increase in ar-terial pressure not accompanied by a decrease inrenal sodium excretory capability will result in apressure natriuresis that serves to return pressure tonormal (41 ,67). If structural changes were to occur
in the renal vasculature, particularly in preglomeru-lar vessels, increases in blood pressure could result.However, it is noteworthy that RBF was increased
rather than reduced In obese, hyperinsuhinemic dogs,
in which hypertension was induced by 5 wk of a
high-fat diet (74). Moreover, renal vascular resistanceis lower and RBF is higher in obese hypertensivepatients (75). Thus, although protracted hyperinsu-hinemia may be able to promote changes in vascularstructure, currently there is little evidence to suggest
that this effect can lead to hypertension.Thus, epidemiologic and correlational studies im-
phicate an effect of hyperinsulinemia to produce a
chronic increase in arterial pressure. but only a fewstudies have directly addressed this possibility exper-imentally (60-64,68). Most of these studies have
been in dogs and have found no evidence of a directhypertensive effect of insulin (60-62,68). However,studies in rats (63,64) suggest that hyperinsulinemia
can increase blood pressure, but the associated con-ditions that allow the expression of this pressor effectare unknown. Also, it is not clear whether humansrespond more like rats or dogs with respect to the
chronic blood pressure effects of insulin.Acute insulin infusions cause vasodilation in hu-
7 9 ii mans (57,76), and chronic, physiologic increases inplasma insulin decrease total peripheral resistancein normal dogs (62). In addition, patients with insu-
linoma and plasma insulin levels approximately sev-enfold higher than normal showed no evidence ofhypertension (77) and no increase in blood pressure

Brands and Hall
Journal of the American Society of Nephrology 1071
was measured with chronic, similar percent in-creases in insulin concentration in dogs (60-62). Fer-rannini et at. (24) also reported in human subjectsthat blood pressure was relatively insensitive to in-sulin levels, such that a 200 �U/mL increase inplasma insulin concentration would account for onlya 1 mm Hg rise in blood pressure. However, normalhumans and dogs are not insulin resistant, whereasinsulin resistance is a feature of obese hypertensives( 1 4, 1 7,2 1 ,22) and many essential hypertensives
(23,24), and evidence suggests that the high-sucrose-fed rats in our insulin infusion studies (63,64) wereinsulin resistant. Thus, features of both experimen-tal models of chronic hyperinsulinemia draw someparallels with humans. However, although insulin
resistance might appear to be a key element in deter-mining a pressor response to hyperinsulinemia, there
is no convincing evidence that this condition per secan directly cause chronic hypertension, as dis-cussed below.
THE EFFECT OF INSULIN RESISTANCE ONBLOOD PRESSURE
Initial hypotheses relating insulin resistance to hy-pertension proposed that renal and cardiovasculareffects of the compensatory hyperinsuhinemia pro-vided the causative link. However, It was clearly dem-onstrated that chronic hyperinsulinemia per se did
not cause hypertension in dogs (60-62) or humanswith insuhinoma (77). A concept now emerging is thathyperinsuhinemia can raise blood pressure only un-
der conditions of insulin resistance, therefore sug-
gesting that some effect of insulin resistance, mdc-pendent of hyperinsulmnemia, provides an environ-ment that permits the pressor effect of insulin to be
expressed.A fundamental action of insulin is to Increase pe-
ripheral tissue glucose uptake and utilization. Oneeffect of an increase in peripheral tissue glucoseuptake is an Increase In metabolic rate (78), whichwould be expected to cause peripheral vasodilationin order to allow tissue blood flow to rise sufficientlyto meet the increased metabolic demands (4 1 ). Thus,
one effect of Insulin infusion under conditions wherethe tissues have normal sensitivity to the metabolicactions of Insulin should be vasodilation and a de-crease in total peripheral resistance.
Evidence from chronic insulIn infusions in normaldogs suggests that they have normal insulin sensitiv-ity even after prolonged hyperinsuhinemia, becausewhen insulin was infused for up to 28 days, the
glucose infusion rate required to maintain normalplasma glucose concentrations remained constant(60-62). If insulin resistance had developed duringthe insulin infusion, a decrease In the glucose Infu-sion rate would have been required to prevent plasma
glucose levels from rising. This finding is in agree-ment with the observation that chronic hyperinsu-linemia does not cause insulin resistance in humans(77) and is consistent with the concept that hyper-insuhinemia is a compensatory response to insulinresistance, rather than a simple down-regulation ofinsulin receptors. The fact that normal dogs retaintheir sensitivity to the metabolic effects of insulinprobably explains our finding that systemic insulin
infusion produced a marked and progressive fall intotal peripheral resistance and a 30 to 40% increasein cardiac output (Figure 3) (62).
In contrast to normal dogs, there is evidence (pre-
sented above) that the rats used in our studies (63,64)were insulin resistant. One predicted effect of periph-
eral tissue insulin resistance is a decrease in the
ability of Insulin to stimulate glucose uptake, whichcorrespondingly should diminish the requirement foran Increase in tissue blood flow during hyperinsu-
hinemia. Recent studies in humans Indicate that theeffect of insulin to increase local blood flow in normalhuman subjects (57,76) was blunted in insulin-re-sistant subjects (76). Therefore, one potential expla-nation for the pressor effect of insulin infusion inrats could be that the hypertensive actions of insulin
were unopposed by the metabolic, depressor actionsbecause of peripheral tissue insulin resistance.
If this concept Is correct, one could speculate that
if dogs were made insulin resistant, such that the
peripheral vasodilatory effects of insulin were absentor attenuated, hyperinsulinemia would cause hyper-
tension. Conversely, If insulin sensitivity were im-proved in rats, insulin infusion should decrease ar-terial pressure similar to the response in normal,insulin-sensitive dogs. Because the underlying pos-tulate is that systemic vasodilation prevents the hy-
pertensive actions of insulin from raising blood pres-sure, we conducted a study in which the effects ofinsulin’s direct renal actions were measured In theabsence of peripheral vasodilation (68).
In that study (68), insulin was infused chronicallyinto the artery of the remaining kidney in unlne-
phrectomized dogs. Because the intrarenal infusionof other antinatriuretic hormones has been demon-
strated to raise blood pressure independent of sys-temic mechanisms (79), this protocol allowed us totest whether the direct antinatrIuretic effect of In-sulin was capable of elevating blood pressure In theabsence of insulin’s systemic metabolic actions.However, although significant increases in systemicplasma insulin concentration were avoided, no In-crease in blood pressure was measured. The insulininfusion did cause some sodium retention, but thiseffect was mild and transient, thus suggesting thatthe direct, antinatriuretic effect of insulin was inca-
pable of increasing blood pressure even when thevasodilatory response was prevented. However, this

Hyperinsulinemia and Hypertension
1072 Volume 3’ Number 5’ 1992
finding does not rule out the possibility that Indirectrenal and circulatory effects of systemically infusedInsulin, possibly mediated by the sympathetic nerv-
ous system, for example. could cause hypertension Ifthe decrease In peripheral resistance was blocked
because of insulin resistance.To test this possibility. we examined the effects of
chronic hyperinsulmnemla In obese dogs that wereresistant to the metabolic effects of Insulin (31). In
these experiments. dogs were placed on a high-fatdiet for 6 wk, causing obesity, increased blood pres-sure, and Insulin resistance. The subsequent infu-
sion of Insulin for 7 days in these obese dogs causedno significant changes in total peripheral resistanceor cardiac output. This contrasts sharply with themarked decrease in total peripheral resistance andincrease In cardiac output measured in insulin-sen-
sitive, normal dogs (62) and suggests that inducinginsulin resistance in dogs indeed prevented the sys-temic vasodilation, because of insulin’s metaboliceffects, from occurring during iv insulin infusion.However, despite insulin resistance, there were no
significant increases In blood pressure in obese dogswhen Insulin was infused for 7 days (31). In fact,there was actually a small decrease In blood pressure,similar to that found in normal dogs. Thus, the pres-ence of Insulin resistance does not markedly alterthe blood pressure response to chronic hyperinsu-linemia in dogs and is therefore unlikely to explainentirely the differences in blood pressure responsesto insulin in dogs versus rats.
Potential Effects of Insulin Resistance on BloodPressure Independent of Hyperinsulinemia
In the above discussion, the hypertensive effect ofinsulin resistance has been considered mainly toresult from various pressor effects of Insulin, withInsulin resistance playing a permissive role in thedevelopment of hypertension. Insulin resistance, bypreventing vasodilation during hyperinsuhinemla,would allow other effects of Insulin to raise bloodpressure. However, it also has been suggested thatinsulin resistance could maintain, or even increase,vascular tone through a more direct interaction withthe regulatory mechanisms for vascular smooth mus-dc contraction (1 9,20,32-34). This hypothesis notonly might explain how vasodilation Is preventedfrom hampering insulin’s putative pressor actions.but also provides the potential for a direct effect ofinsulin resistance to increase total peripheral resist-
ance.Insulin has been reported to attenuate the contract-
ile response of vascular smooth muscle to constrictoragonists (80) and may have a direct vasodilatory ac-tIon (57.76,8 1). Insulin may stimulate sodium-potas-sium ATPase (81), which might decrease vascular
tone by hyperpolarizing the vascular smooth muscleand increasing sodium-calcium exchange. In addi-tion, Insulin may stimulate calcium ATPase activity
(1 9). Presumably, In Insulin-resistant states, these
actions of insulin to decrease intracellular calciumconcentration would be blunted, allowing calciumlevels to rise and causing Increased vascular smoothmuscle tone. Recent studies have reported that intra-cellular calcium Is Increased In vascular smooth mus-
dc In insulin-resistant animals such as the Zuckerobese rat (19), although these changes In cell mem-brane ion transport also may be unrelated to insulinor Insulin resistance. Theoretically. however, thisprovides another mechanism, in addition to reducedmetabolic effects, whereby vasodilation during hy-perinsuhinemia may be prevented in insulin-resistantstates. Additionally. the premise that Insulin has aphysiologic effect to lower calcium concentration Invascular smooth muscle cells has provided the basisfor suggesting that Insulin resistance, Independentof hyperinsuhinemla, could cause hypertension bycausIng vasoconstrlction.
Several investigators have suggested that vasocon-strlction caused by Insulin resistance could contrib-ute to the development of hypertension (19,20,32-34). However, although Increased total peripheral re-sistance Is a hallmark of most forms of establishedhypertension , experimental and theoretical studieshave shown that increased peripheral vascular re-
sistance per se Is unlikely to cause hypertensionunless the constriction also occurs in renal vessels
and shifts pressure natrluresis to higher blood pres-sures (41 .67,82). This is because, if there Is no de-crease In renal sodium excretory capabIlity. the risein arterial pressure resulting from systemic vasocon-striction will increase renal sodium excretion anddecrease extracellular fluid volume until blood pres-sure returns to normal (Figure 6) (41 ,67,82). Theincrease in peripheral vascular resistance measuredin many forms of hypertension may be the result ofautoregulatory adjustments to maintain normal tis-
sue blood flow In the face of increased perfusionpressure (67).
Also arguing against the hypothesis that a primaryincrease in total peripheral resistance, initiated byinsulin resistance, Is a cause of obesity-induced hy-pertension Is the finding that cardiac output is dc-
vated, as is blood flow to many tissues (e.g. . skeletalmuscle), rather than reduced in obese hypertensives( 1 2,83). Experimental forms of hypertension associ-
ated with potent vasoconstriction are usually asso-dated with normal or reduced cardiac output andtissue blood flows, rather than Increases, as occursin obesity (41). Thus, It seems unlikely that a mech-anism that causes peripheral vasoconstriction couldbe the explanation for hypertension In obese individ-uals, unless the vasoconstriction also occurred in

A�
4
3
2
1
0
150
100
3
2
URINARYSODIUMOUTPUT(x NORMAL)
MEANARTERIALPRESSURE(mmHg)
URINARYSODIUMOUTPUT(x NORMAL)
________________ Considerable evidence that an abnormality in renalsodium handling exists in all forms of hypertension
has been previously reviewed (41 ,67,82,84). In hy-
pertenslon, the relationship between arterial pres-sure and sodium excretion, the pressure natriuresismechanism, is shifted to a higher pressure such that
. sodIum balance is maintained at an elevated pressure
. (41 .67.82). If It were not, the high arterial pressure
would cause unabated natriuresis, culminating Incirculatory collapse. Thus, pressure natriuresis must
be shifted In chronic hypertension associated with� obesity. The question that remains to be answered� : �: ; � :� 4 is: what is the cause of the decrease In renal sodium
excretory capability that leads to the rise in arterial
TIME (days) pressure?
The pressure natriuresis relationships for normal
300
200
URINARYSODIUMEXCRETION OBESE(mmol/24 hrs)
100
70 80 90 100
MEAN ARTERIAL PRESSURE(mmHg)
Brands and Hall
Journal of the American Society of Nephrology 1073
.7.J........P�.
50 100 150 200
MEAN ARTERIAL PRESSURE (mmHg)
STIMULUS I
Figure 6. Probable chronic relation between arterial pres-sure and urinary sodium excretion under the influence of apowerful peripheral vasoconstrictor that acutely raisesblood pressure from A to B but has no effect on renal sodiumexcretory capability (i.e., does not shift the renal functioncurve).
renal vessels and shifted the renal-pressure natrI-uresis mechanism (41 ,67,82). Recent studies sug-gest. however, that obesity and Insulin resistance donot cause renal vasoconstriction (30,75). In fact,
marked Increases in RBF and GFR have been notedIn obese, insulin-resistant dogs (30,74).
In summary. insulin resistance has been postu-lated to play a role in the etiology of hypertensionprimarily by causing a compensatory hyperinsulmne-
mia. The effects of chronic hyperinsulinemia onblood pressure regulation, however, remain unclear.Initial studies In rats have suggested that insulin canproduce a sustained increase in blood pressure, butmuch study Is needed to delineate the mechanism for
that effect. In contrast, an extensive series of studiesin dogs has provided strong evidence that hyperin-sulinemla per se does not cause hypertension. These
studIes, however, do not rule out the possibility that
some other, as yet unknown, factor or factors coex-istent with hyperInsuhmnemia may allow the expres-sion of a hypertensive effect. One candidate for this“insulin cofactor” has been InsulIn resistance. How-ever, although much correlational evidence suggests
an Important role for insulin resistance in defining
the relationship between Insulin and blood pressureregulation, recent experiments designed to address
this Issue directly found no supportive evIdence. Al-though further Investigation Is needed to fully under-stand the effects of insulin and insulin resistance onblood pressure regulation, clearly other mechanismsalso should be pursued to understand the cause ofobesity-associated hypertension.
RENAL MECHANISMS OF OBESITY-ASSOCIATEDHYPERTENSION
Figure 7. Steady-state relation between arterial pressure
and urinary sodium excretion for nonobese adolescents (N= 18), obese adolescents (N= 60). and obese adolescentsafter weight loss (N = 36). Adapted with permission fromreference 7.

Hyperinsulinemia and Hypertension
1074 Volume 3 ‘ Number 5’ 1992
individuals, obese adolescents, and obese adoles-
cents after weight loss were recently compared byRoechini et at. (7) and are presented in Figure 7. Innormal subjects. the relationship Is very steep. mdi-cating that blood pressure Is insensitive to changesin sodium intake over a wide range of intakes. Inobese subjects, the pressure natriuresis curve wasshifted to higher pressures, Indicating that renal so-
dium excretory capability was reduced and the slopeof the curve was decreased (7). In many forms ofhypertension characterized by a decrease In the slope
of the pressure natrluresis curve, the reduction Inrenal sodium excretory capability and the elevated
blood pressure appear to be Initiated by increasedtubular reabsorption, such as In mineralocorticoidhypertension. Thus, although the mechanisms re-sponsible for the obesity-induced shift of pressure
natriuresis have not been elucidated, it is possiblethat some factor that tends to increase renal tubularsodium reabsorptIon Is Involved.
In support of this possibility, we have observed Inpreliminary studies that obesity-induced hyperten-sion in dogs is associated with increased renal tubu-
lar sodium reabsorption, because marked sodium re-tention occurred despite large Increases in GFR andeffective RPF (74). The focus of this discussion hascentered on the possibility that Insulin, pathologi-cally Increased to compensate for Insulin resistance,might increase tubular reabsorption. However, nu-merous studies In dogs suggest that hypermnsuhmne-
mia per se cannot produce the changes In renalfunction required to cause chronic hypertension (3 1,60-62,68).
Recent studies in our laboratory indicate that obe-sity-induced hypertension is associated with in-creased PRA. Because ANG II has powerful effects toIncrease renal tubular sodium reabsorption (85), It ispossible that part of the shift of pressure natriureslsobserved in obesity hypertension may be mediatedby the renin-angiotensin system. However, we alsohave found that weight-Induced changes In bloodpressure in dogs can occur independently of changesin ANG II formation (30). Therefore, additional fac-tors besides hyperinsuhmnemia and Increased ANG II
must be considered as possible mediators of the re-duced sodium excretory capability and shift of pres-sure natriuresis in obesity-Induced hypertension.
Recent, preliminary observations (74) in our labo-ratory also have revealed potential changes in renal
pathology in obese dogs that are suggestIve of another
mechanism for shifting pressure natriuresis. Meas-urements of renal interstitial fluid hydrostatic pres-sure (RHIP) in anesthetized, obese dogs are consid-
erably higher than In anesthetized, normal dogs. Pre-vious studies have demonstrated a natriuretic effect
of small increases in RHIP (86-88). However, Burnettand Knox (88) demonstrated that greater Increases
In RHIP actually decreased sodium excretion, and themeasurements of RHIP In obese dogs thus far arecomparable with those antinatriuretlc levels of RHIP.A mechanism through which high RHIP could de-crease urinary sodium excretion could be throughcompression of renal tubular structures (88), whichwould Increase tubular transit time, thereby increas-ing tubular sodium reabsorption. Histologic studies
suggest that tubular compression In kidneys fromobese dogs may be due to proliferation of extracellu-lar matrix material as well as increased numbers ofinterstitial cells in the medulla. Although further
study is needed to confirm these observations andelucidate the mechanism for the Increase in RHIP Inobese dogs, such changes in renal function are con-sistent with a mechanism for producing a shift inpressure natriuresis caused by increased tubular so-
dium reabsorption.Thus, the shape of the pressure natrluresis curve
In obese humans and measurements of renal func-tion in obese humans and dogs suggest similar
causes, involving increased tubular sodium reab-sorption, in the development of hypertension withobesity In these specIes. Considerable evidence sug-gests that insulin resistance and hyperinsulinemiaper se cannot cause the changes in renal functionnecessary to produce chronic hypertension. How-
ever, early studies in rats have suggested that undersome conditions, hyperInsuhmnemia can produce asustained Increase In arterial pressure. Therefore,
how might sodium excretory capability be reduced inhyperinsulmnemic rats and how might this relate toobesity hypertension in humans?
The rise in blood pressure in rats was not sensitiveto changes In sodium Intake. thus resulting In aparallel shift of the pressure natriuresis curve (64).
This response is characteristic of hypertension pro-duced primarily by Increased preglomerular resist-ance, such as one-kidney, one-clip Goldbhatt hyper-
tension (4 1 ,67,82). Interestingly, norepinephrine,with relatively weak effects on tubular sodium reab-sorption (5 1 ) and a preferential constrictor effect on
the afferent arterioles (89), also produces a shift inpressure natriuresis that is relatively Insensitive to
changes in sodium Intake (5 1). Taken together withthe large body of correlational evidence that the sym-pathetic nervous system is more active in hyperin-suhinemic states (1 5, 1 8,46,47), Increased sympa-thetic activity could play a role In causing hyperten-
sion in rats chronically Infused with insulin (64).
However, the parallel shift In the pressure natriuresiscurve found in Insulin-infused rats is not consistentwith the decreased slope of the pressure natriuresis
curve found in obese hypertensive humans (7), fur-ther suggesting that another factor besides, or Inaddition to. hypermnsuhinemla Is Involved In causingobesIty hypertension.

3�*�W � Brands and Hall
Journal of the American Society of Nephrology 1075
SUMMARY
Analyses of obesity-induced hypertension and itspotential causes have proven to be much more com-
plex than the Initial hypotheses that were based on
correlational studies and acute experiments linkinghyperinsuhmnemia to hypertension. However, the re-
sults from chronic animal studies, which have ad-dressed directly the question of whether hyperinsu-linemla can produce sustained increases in arterialpressure, have shed new light on the subject andhave pointed toward a new direction of research inthis area. Fundamental to our understanding of themechanism for developing hypertension in obesity isthe concept that a decrease in renal sodium excretorycapability-a shift in pressure natriuresis-mustoccur and be maintained in order for chronic hyper-tension to occur. When viewed in this light, it isdifficult to envision a mechanism whereby insulinresistance per Se, purportedly via effects on periph-
era! vascular resistance, could lead to chronic in-creases in blood pressure. However, the original sup-position that hypermnsulinemia, occurring as com-
pensatlon for Insulin resistance, Is responsible forhigh blood pressure in obese subjects has not beencompletely resolved.
What has evolved from chronic insulin infusionstudies in normal dogs is the concept that hyperin-suhinemia per se does not cause an increase in bloodpressure. clearly indicating that other factors alsoshould be considered. The inability of obesity-induced insulin resistance to uncover a hypertensiveeffect of hyperinsulinemia in dogs strongly suggestsinsulin resistance is not the answer. The data fromchronic studies in rats, on the other hand. lend sup-port to the concept that under certain conditionshyperinsulmnemia can increase blood pressure. Thequestion now becomes: what are the conditions thatallow hyperinsuhinemia to chronically raise bloodpressure and which model best represents humans?The parallel shift of the pressure natriuresis curve
In insulin-hypertensive rats suggests that mecha-nisms for constricting the preghomerular vasculatureshould be investigated. but how this will relate tohumans is unclear. It is Interesting to note, however,that the pressure natriuresis curve in obese hyper-tensive humans is shifted with a decrease in slope,suggesting that increased tubular sodium reabsorp-tion may underlie the shift. The specific intrarenaland neurohumora! mechanisms that shift pressurenatriuresis in obese hypertensives remain an Impor-tant area for further Investigation.
ACKNOWLEDGMENTS
The authors research was supported by grants HL39399. HL23502.
and HL1 1678 from the NIH. MW. Brands is a recipient of National
Heart. Lung. and Blood Institute National Research Service Award
HLO8 17 1.
REFERENCES1 . Stamler RA, Stamler J, Reidlinger WF, Algera
G, Roberts RH: Weight and blood pressure find-ings in hypertension screening of 1 millionAmericans. JAMA 1978;240: 1607-16 10.
2. Havilk RJ, Hubert HB, Fabsitz RR, Feinleib M:Weight and hypertension. Ann Intern Med1 983;98:855-859.
3. Chiang BW, Penman LV, Epstein FH: Over-weight hypertension: A review. Circulation1969;39:403-42 1.
4. Dustan HP: Mechanisms of hypertension asso-ciated with obesity. Intern Med 1983;98:860-864.
5. Rocchini AP, Katch V. Schork A, Keich RP:Insulin and blood pressure during weight loss inobese adolescents. Hypertension 1 987; 10:267-273.
6. Reisen E, Abel R, Modan M, Silverberg DS,Eliahou HE, Modan B: Effect of weight loss with-out salt restriction on the reduction of bloodpressure in overweight hypertensive patients. NEngl J Med 1978;298:1-6.
7. Roechini AP, Key J, Bordie D, et at: The effectof weight loss on the sensitivity of blood pressureto sodium in obese adolescents. N Engl J Mcd1 989;32 1:580-585.
8. Alexander J, Dustan HP, Sims EAH, Tarazi R.Report of the Hypertension Task Force. Vol. 9.DHEW Publication No. 70- 1 63 1 . Bethesda, MD:National Institutes of Health; 1 979:pp 61-77.
9. Fletcher AP: The effect of weight reduction uponthe blood pressure of obese hypertensive women.Q J Med 1954;23:331-345.
10. Sowers JR, Nyby M, Stern N, et at: Blood pres-sure and hormone changes associated withweight reduction in the obese. Hypertension1982;4:686-69 1.
1 1 . Rocchini AP, Moorehead CP, Deremer 5, Bon-die D: Pathogenesis of weight related changes inblood pressure in dogs. Hypertension 1989;13:922-928.
12. Rocchini AP, Moorehead CP, Wentz E, Dc-Remer 5: Obesity-induced hypertension in thedog. Hypertension 1 987;9(suppl. III):III-64-III-68.
13. Berglund GS, Ljungman 5, Hartford M, Wil-helmsen L, Bjorntorp P: Type of obesity andblood pressure. Hypertension 1 982;4:692-696.
14. Christlieb AB, Krolewski AS, Warram JH,Soeldner JS: Is insulin the link between hyper-tension and obesity? Hypertension 1985;7(suppl. II):II-54-II-57.
1 5. Hwang 1-5, Ho H, Hoffman BB, Reaven GM:Fructose-Induced Insulin resistance and hyper-tension in rats. Hypertension 1 987; 10:512-516.
16. DeFronzo RA: The effects of insulin on renalsodium metabolism. Diabetologia 1 98 1 ;2 1:165-171.
1 7. Lucas CP, Estigarribia JA, Darga LL, ReavenGM: Insulin and blood pressure in obesity. Hy-pertension 1 985;7:702-706.
1 8. Landsberg L, Krieger DR: Obesity. metabolism.and the sympathetic nervous system. Am J Hy-pertens 1989;2: 1255- 1�25.
19. Sowers JR, Standley PR, Ram JL, Zemel MB,Resnick LM: Insulin resistance, carbohydratemetabolism, and hypertension. Am J Hypertens1991 ;4:466S-472S.
20. DeFronzo RA, Ferrannini E: Insulin resist-

Hyperinsulinemia and Hypertension
1076 Volume 3’ Number 5’ 1992
ance-a multifaceted syndrome responsible forNIDDM, obesity, hypertension, dyslipidemia. andatherosclerotic cardiovascular disease . DiabetesCare 1991;14:174-194.
2 1 . Modan M, Halkin H, Almog S. et at. : Hyperin-sulinemia: A link between hypertension. obesityand glucose intolerance. J Clin Invest 1985:809-817.
22. Manicardi V. Cannellini I, Bellodi G, CoscelliC, Ferrannini E: Evidence for an association ofhigh blood pressure and hyperinsulinemia inobese man. J Clin Endocrinol Metab 1986:62:1302-1304.
23. Ferrannini E, Buzzigoli G, Bonadonna R, et at.:Insulin resistance and hypertension. N Eng! JMed 1987:317:350-357.
24. Ferrannini E, Haffner SM, Stern MP: Essentialhypertension: an insulin resistant state. J Car-diovasc Pharmacol 1 990; 1 5(suppl. 5):5 18-525.
25. Reaven GM, Chang H: Relationship betweenblood pressure. plasma insulin and triglycerideconcentration, and insulin action in sponta-neous hypertensive and Wistar-Kyoto rats. AmJ Hypertens 1991:4:34-38.
26. Gaboury CL, Karanja N, Holcomb SR, Torok J,McCarron DA: Patterns of insulin secretion andresponsiveness in Wistar-Kyoto and sponta-neously hypertensive rats. Am J Hypertens1991:4:661-666.
27. Da1l’A�lio E, Tosini P. Ferrari P. Zavaroni I,Passeri M, Reaven GM: Abnormalities of insulinand lipid metabolism in Milan hypertensive rats.Am J Hypertens 1991:4:773-775.
28. Reaven GM, Ho H, Hoffman BB: Somatostatininhibition of fructose-induced hypertension. Hy-pertension 1989:14:117-120.
29. Reaven GM, Ho H: Sugar-induced hypertensionin Sprague-Dawley rats. Am J Hypertens 1991:4:610-614.
30. Hall JE, Brands MW, Hildebrandt DA, MizelleHL: Obesity-associated hypertension: hyperin-sulInemia and renal mechanisms. Hypertension1992; 19[suppl 11:1-45-1-55.
3 1 . Hall JE, Brands MW, Dixon WN, Mizelle HL,Hildebrandt DA: Hyperinsulinemia does not el-evate blood pressure in obese hypertensive dogs[Abstract). FASEB J 1991;5:A737.
32. Resnick LM: Hypertension and abnormal glu-cose metabolism. Am J Med 1989:87(suppl.6A):l75-215.
33. Reaven GM: Insulin and hypertension. Clin ExpHypertens Theory Pract 1 990:A 12:803-816.
34. Landsberg L: Insulin resistance. energy bal-ance. and sympathetic nervous system activity.Clin Exp Hypertens Theory Pract 1990:A12:817-830.
35. Cambien F, Warner J-M, Eachwege E, Jacque-son A, Richard JL, Rosselin G: Body mass,blood pressure. glucose, and lipids: Does plasmainsulin explain their relationships? Artcrioscle-rosis 1987:7:197-202.
36. Mbanya JC, Thomas TH, Wilkinson R, AlbertiKGMM, Taylor R: Hypertension and hyperinsu-linemia: A relation in diabetes but not essentialhypertension. Lancet 1988:1:733-744.
37. Grugni G, Ardizzi A, Dubini A, Guzzaloni G,Sartorio A, Morabito F: No correlation betweeninsulin levels and high blood pressure in obesesubjects. Hormone Metab Res 1990:22:124-1 25�.
38. Pawloski CM, Kanagy NL, Mortensen LH, FinkGD: Obese Zucker rats are normotensive on nor-mal and increased sodium intake. Hypertension1 992; 1 9(suppl I):I-90-I-95.
39. Kotchen TA, Zhang HY, Covelli M, Bleh-schmidt N: Insulin resistance and blood pres-sure in Dahl rats and In one-kidney. one-cliphypertensive rats. Am J Physiol1991 ;261 :E692-E697.
40. Buchanan TA, Sipos GF. Yip K-P, Marsh DJ,Hsueh W, Bergman RN: Glucose tolerance andinsulin action In rats with renovascular hyper-tension. Hypertension 1 99 1 ; 18:341-347.
41 . Guyton AC. Circulatory Physiology III. ArterialPressure and Hypertension. Philadelphia: WBSaunders Co; 1980:1-553.
42. Atchley DW, Loeb RF, Richards DW, BenedictEM, Driscoll ME: On diabetic acidosis. J ClinInvest 1933;12:297-326.
43. Miller JH, Bodgonoff MD: Antidiuresis associ-ated with administration of insulin. J App! Phys-lol 1954;6:509-512.
44. Nizet A, Lefebore P. Crabbe J: Control by in-sulin of sodium, potassium, and water by theisolated dog kidney. Pfluegcrs Arch 1 97 1 ;323:11-20.
45. Kirchner KA: Insulin Increases loop segmentchloride reabsorption in the euglycemic rat. AmJ Physiol 1988;255:Fl206-F1213.
46. Tuck ML: Obesity, the sympathetic nervous sys-tern , and essential hypertension . Hypertension1992; 19[suppl I):I-67-I-77.
47. Troisi RJ, Weiss ST. Parker DR. Sparrow D,Young JB, Landsberg L: Relation of obesity anddiet to sympathetic nervous system activity. Hy-pertension 199 1 ; 17:669-677.
48. Fournier RD. Chiueh CC, Kopin IJ, Knapka JJ,Dipette D, Preuss HG: Refined carbohydrate in-creases blood pressure and catecholamine secre-tion in SHR and WKY. Am J Physiol 1986;250:E38 1 -E385.
49. Kaufman LN, Peterson MM, Smith SM: Hyper-tension and sympathetic hyperactivity inducedin rats by high-fat or glucose diets. Am J Physiol1991;260:E95-E100.
50. Kopp UC, DiBona GF. The neural control ofrenal function. In: Seldin DW, Giebisch G, eds.The Kidney: Physiology and Pathophysiology.New York: Raven Press; 1992:1157-1204.
5 1 . Cowley AW Jr. Lohmeier TE: Changes in renalvascular sensitivity and arterial pressure asso-ciated with sodium intake during long-term in-trarenal norepinephrine infusion in dogs. Hyper-tension 1979; 1:549-558.
52. Liang CS, Doherty JV, Faillace R, et al. : Insulininfusion in conscious dogs: Effects on systemicand coronary hemodynamics. regional bloodflows and plasma catecholamines. J Clin Invest1982:69:1321-1336.
53. Christensen NJ, Alberti KGMM, Brandsborg 0:Plasma catecholamines and blood substrate con-centrations: Studies In insulin induced hypo-glycaemia and after adrenaline infusions. Eur JClin Invest 1975;5:415-423.
54. DiSalvo RJ, Bloom WL, Brust AA, FergusonRW, Ferris EB: A comparison of the metabolicand circulatory effects of epmnephrine, nor-epi-nephrine and insulin hypoglycemia with obser-vations on the influence of autonomic blockingagents. J Clin Invest 1956:35:568-577.

Brands and Hall
Journal of the American Society of Nephrology 1077
55. Rowe JW, Young JB, Mimaker KL, Stevens AL,Pallotta J, Landsberg L: Effect of insulin andglucose infusions on sympathetic nervous sys-tern activity in normal man. Diabetes 1981 ;30:219-225.
56. Pereda SA, Eckstein JW, Abboud FM: Cardio-vascular responses to insulin in the absence ofhypoglycemia. Am J Physiol 1962:202:249-252.
57. Anderson EA, Hoffman RP, Babon TW, SinkeyCA, Mark AL: Hyperinsulinemia produces bothsympathetic neural activation and vasodilationin normal humans. J Clin Invest 1991;87:2246-2252.
58. Smith MJ Jr. Cowley AW Jr. Guyton AC, Man-ning R Jr: Acute and chronic effects of vaso-pressin on blood pressure, electrolytes, and fluidvolumes. Am J Physiol 1979;237:F232-F240.
59. Hall JE, Montani J-P, Woods LL, Mizelle HL:Renal escape from vasopressmn: role of pressurenatriuresis. Am J Physiol 1986;250:F907-F916.
60. Hall JE, Coleman TG, Mizelle HL, Smith MJ Jr:Chronic hyperinsulmnemia and blood pressureregulation. Am J Physiol 1990;258:F722-F731.
6 1 . Hall JE, Brands MW, Kivlighn SD, Mizelle HL,Hildebrandt DA, Gaillard CA: Chronic hyper-insulinemia and blood pressure: Interactionswith catecho!amines? Hypertension 1990;15:519-527.
62. Brands MW, Mizelle HL, Gaillard CA, Hilde-brandt DA, Hall JE: the hemodynamic responseto chronic hypermnsulmnemia in conscious dogs.Am J Hypertens 1991:4:164-168.
63. Brands MW, Hildebrandt DA, Mizelle HL, HallJE: Sustained hyperinsulinemia increases arte-na! pressure in conscious rats. Am J Physio!1991 ;260:R764-R768.
64. Brands MW, Hildebrandt DA, Mizelle HL, HallJE: Hypertension during chronic hyperinsu-linemia in rats is not sart-sensitive. Hyperten-sion 1992;19(Supp! I):I-83-I-89.
65. Dunnill MS. Halley W: Some observations on thequantitative anatomy of the kidney. J Pathol1973;1 10:113-121.
66. Rocchini AP, Moorehead C, DeRemer 5, Good-friend TL, Ball DL: Hypermnsulmnemia and thealdosterone and pressor responses to angioten-sin II. Hypertension 1 990; 15:861-866.
67. Hall JE, Mizelle HL, Hildebrandt DA, BrandsMW: Abnormal pressure natriuresis: A cause orconsequence of hypertension? Hypertension1990; 15:547-559.
68. Hall JE, Brands MW, Mizelle HL, Gaillard CA,Hildebrandt DA: Chronic intrarenal hyperinsu-linernia does not cause hypertension. Am J Phys-iol 199 1 ;260:F663-F669.
69. Izumi Y, Honda M, Tsuchiya M, Ueda Y, HatanoM: Effect of sornatostatin on plasma renin activ-ity and blood pressure in patients with essentialhypertension. Endocrinology 1 980;27:505-5 1 1.
70. Caretta R, Fabris B, Fischetti F, et at.: Re-duction of blood pressure in obese hyperin-sulinernic hypertensive patients during so-matostatin infusion. J Hypertens 1989;7(suppl.6):S1 96-S 197.
71. Stout RW, Bierman E, Ross R: Effect of insulinon the proliferation of cultured primate arterialsmooth muscle cells. 1975;36:3 19-327.
72. Nakao J, Ito H, Kanayasu T, Murota SI: Stim-ulatory effect of insulin on aortic smooth muscle
cell migration induced by 1 2-L-hydroxy-5,8, 10,1 4-eicosatetraenoic acid and its modulation byelevated extracellular glucose levels . Diabetes1985;34:185-191.
73. Reaven GM: Role of abnormalities of carbohy-drate and lipoprotemn metabolism in the patho-genesis and clinical course of hypertension. JCardiovasc Pharm 1 990; 1 5(suppl. 5): 54-57.
74. Hall JE, Dixon WN, Brands MW, Mizelle HL,Hildebrandt DA: Obesity hyperinsulmnemia, andhypertension: control of renal function and sys-temic hemodynamics [Abstract]. J Am Soc Ne-phrol 1990:1:490.
75. Reisin E, Messerli FH, Ventura HO, FrohlichED. Renal hemodynamics in obese and lean es-sential hypertensive patients. In: Messerli FH.ed. Kidney in Essential Hypertension. Boston:Martinus Nijhoff Publishing; 1984:125-129.
76. Laakso M, Edelman SV, Brechtel G, Baron AD:Decreased effect of insulin to stimulate skeletalmuscle blood flow in obese man: A novel mech-anism of insulin resistance. J Clin Invest1990:85:1844-1852.
77. Tsutsu N, Nunoi K, Kodama T, Nomiyama R,Iwase M, Fujishima M: Lack of association be-tween blood pressure and insulin in patientswith insulinoma. J Hypertens 1990;8:479-482.
78. Ravussin E, Bogardus C, Schwartz RS, et at.:Thermic effect of infused glucose and insulin inman. J Clin Invest 1983:72:893-902.
79. Lohmeier TW, Cowley AW Jr: Hypertensive andrenal effects of chronic low level intrarenal an-giotensin infusion in the dog. Circ Res 1979:44:154- 160.
80. Yagi S. Takata S. Koyokawa H, Yamamoto M,Noto Y, Ikeda T, Hattori N: Effects of insulin onvasoconstrictive responses to norepinephrineand angiotensmn II in rabbit femoral artery andvein. Diabetes 1988:37:1064-1067.
8 1 . Ferrannini E, Taddei S. Santoro D, et at. : In-dependent stimulation of glucose metabolismand Na+-K+ exchange by insulin in the humanforearm. Am J Physiol 1988:255:E953-E958.
82. Guyton AC: Kidneys and fluids in pressure reg-ulation. Hypertension 1 992: 1 9(suppl. I):I-2-I-8.
83. Messerli FH, Christie B, DeCarvalbo JGR, etat.: Obesity and essential hypertension: Herno-dynamics, intravascular volume, sodium excre-tion, and plasma renin activity. Arch Intern Med198 1; 141:81-85.
84. Hall JE, Granger JP, Hester RL, Montani J-P:Mechanisms of sodium balance in hypertension:role of pressure natriuresis. J Hypertens 1986:4(suppl. 4):S57-S65.
85. Hall JE: Control of sodium excretion by angio-tensin II: Intrarenal mechanisms and blood pres-sure regulation. Am J Physiol 1986;250:R960-R972.
86. Granger JP: Pressure natriuresis: Role of renalinterstitial hydrostatic pressure. Hypertension1 992; 1 9(suppl. I):I-9-I- 17.
87. Granger JP: Effect of direct increases in renalinterstitial hydrostatic pressure on sodium cx-cretion. Am J Physiol 1988;254:F527-F532.
88. Burnett JC, Knox FG: Renal interstitial pres-sure and sodium excretion during renal vein con-striction. Am J Physiol 1980:238:F279-F282.
89. Loutzenhiser R, Epstein M: Calcium antago-nists and the kidney. Hosp Pract 1987:22:63-76.