Infectious disease p4
-
Upload
medico-legal-institute -
Category
Documents
-
view
747 -
download
0
description
Transcript of Infectious disease p4

Lyme Disease : caused by spirochete Borrelia burgdorferi. transmitted from rodents to people by ticks. involves multiple organ systems and is divided into
three stages. stage 1:• spirochetes multiply and spread in dermis at site of
tick bite.• causing an area of redness, often with pale center,
called erythema chronicum migrans.• may be accompanied by fever and lymphadenopathy, but usually disappears in 4 to 12 weeks.

stage 2: ( Early disseminated stage )• spirochetes spread hematogenously throughout
the body.• causes secondary skin lesions, lymphadenopathy,
migratory joint and muscle pain, cardiac arrhythmias, and meningitis often with cranial nerve involvement.
stage 3: ( late disseminated stage )• 2 or 3 years after initial bite.• causes chronic arthritis sometimes with severe damage
to large joints and an encephalitis that varies from mild to debilitating.

Pathogenesis: initial immune response is stimulated by binding of
bacterial lipoproteins to toll-like receptor 2 expressed by macrophages.
In response, macrophages release proinflammatory cytokines (IL-6 and TNF) and generate bactericidal nitric oxide, reducing but usually not eliminating the infection.
The adaptive immune response to Lyme disease is mediated by CD4+ T-helper cells and B-cells.
however B. burgdorferi escapes the antibody response through antigenic variation.

Histopathology showing Borrelia burgdorferi spirochetes in Lyme disease. Dieterle silver stain
Histopathology of erythema migrans. Note the thickened stratum corneum with a trailing scale, tiny spongiotic foci within the epidermis, and a “cuff-like” perivasuclar infiltrate composed of predominantly of lymphocytes in the superficial dermis. (Hematoxylin–eosin stain).

ANAEROBIC BACTERIA :Clostridial Infections : Clostridium species are Gram-positive bacilli that grow
under anaerobic conditions and produce spores that are present in soil.
Four types : Clostridium perfringens:• Cellulitis.• myonecrosis of traumatic and surgical wounds (gas
gangrene).• uterine myonecrosis .• mild food poisoning.• severe sepsis of ischemic small bowel.

Clostridium tetani:• proliferates in puncture wounds and umbilical stump of
newborn infants.• releases a potent neurotoxin called tetanospasmin, that
causes tetanus Clostridium botulinum:• grows in inadequately sterilized canned foods.• releases a potent neurotoxin that blocks synaptic release
of acetylcholine and causes a severe paralysis of respiratory and skeletal muscles (botulism).
Clostridium difficile:• overgrows other intestinal flora in antibiotic-treated
patients( e.g, lincomycin ).• releases toxins, and causes pseudomembranous colitis .

Clostridia gram-positive bacilli from a culture of C. perfringens.

Pathogenesis: C. perfringens:• will not grow in presence of oxygen, so tissue death
allows the bacteria to proliferate within the host.• These bacteria release collagenase and hyaluronidase
that degrade extracellular matrix proteins and contribute to bacterial invasiveness.
• but their most powerful virulence factors are many toxins they produce. C. perfringens secretes 14 toxins, the most important of which is α-toxin.
C. botulinum and C. tetani :• The neurotoxins produced by both inhibit release of
neurotransmitters, resulting in paralysis.

C. difficile : produces :• toxin A, an enterotoxin , that stimulates chemokine
production and thus attracts leukocytes.• toxin B, a cytotoxin, which causes cytopathic effects in
cultured cellsMorphology: Clostridial cellulitis :• differentiated from infection caused by pyogenic cocci by:
its foul odor, its thin discolored exudate, and wide tissue destruction.
Clostridial gas gangrene:• marked edema and enzymatic necrosis of involved
muscle cells .

• Gas bubbles caused by bacterial fermentation appear within gangrenous tissues.
• As the infection progresses, the inflamed muscles become soft, blue-black, friable.
• On microscopic examination, there is severe myonecrosis, extensive hemolysis, and marked vascular injury, with thrombosis.
Despite severe neurologic damage caused by botulinum and tetanus toxins, the neuropathologic changes are subtle and nonspecific.

Pathologic findings of muscle tissue. Postoperative pathologic findings of muscle tissue obtained during surgical removal. Hämatoxylin-Eosin-stain, zoom 100×. Infected tissue with gas-inclusion between the muscle-fibres can be seen, caused by Clostridium welchii (also known as Clostridium perfringens) Bacteria..

OBLIGATE INTRACELLULAR BACTERIA : Chlamydial Infections : Chlamydia trachomatis is a small Gram-negative
bacterium which is obligate intracellular . Mode of Transmission: Mainly sexually transmitted
disease , much less common via hand to eye contact The various diseases caused by C. trachomatis infection
are associated with different serotypes of bacteria: ocular infection of children , the trachoma , by
(serotypes A, B, and C). urogenital infections, and inclusion conjunctivitis
(serotypes D through K). Lymphogranuloma venereum (serotypes L1, L2, and L3).

Pathogenesis: C. trachomatis exists in two forms during its life cycle. The infectious form, Elementary body (EB),
is a metabolically inactive, spore like structure. The EB is taken up by host cells, primarily by
receptor-mediated endocytosis. The bacteria prevent fusion of endosome and lysosome,
but the mechanism of this is not known. Inside the endosome, the EB differentiates into
a metabolically active form, called Reticulate body (RB). Using energy sources and amino acids from host cell,
the RB replicates and ultimately forms new EBs that are capable of infecting additional cells.

Lymphogranuloma venereum: • Initially small, often unnoticed, papule on genital mucosa
or nearby skin.• Two to 6 weeks later, draining lymph nodes show swollen
tender lymph nodes, which may coalesce and rupture.• If not treated, cause fibrosis and strictures in anogenital
tract.• In active lesions, diagnosis made by demonstration of
organism in biopsy sections or smears of exudate.• In more chronic cases, by demonstration of antibodies
to chlamydial serotypes in patient's serum.

Morphology: C. trachomatis urethritis :
mucopurulent discharge containing a predominance of neutrophils.
lymphogranuloma venereum : mixed granulomatous inflammatory reaction and foci of necrosis and neutrophilic infiltration (stellate abscesses) later on: nonspecific chronic inflammatory infiltrates and extensive fibrosis.

a necrotic focus with neutrophils in the center , surrounded by epithelioid histiocytes (stellate abscesses)

Rickettsial Infections : are vector-borne obligate intracellular bacteria. Cause: epidemic typhus (by Rickettsia prowazekii);
scrub typhus (by Orienta tsutsugamushi); and spotted fevers (by Rickettsia rickettsii)
Pathogenesis: Rickettsiae do not produce significant toxins. The rickettsiae that cause typhus and spotted fevers
infect vascular endothelial cells, especially in lungs and brain.
enter endothelial cells by endocytosis. proliferate in endothelial cell cytoplasm.

manifestations of rickettsial infection are due to vascular leakage secondary to endothelial cell damage.
This causes hypovolemic shock with peripheral edema, as well as pulmonary edema, renal failure, and CNS manifestations including coma.
IFN-γ and TNF from activated natural killer cells; and CD4+, and CD8+ T lymphocytes, stimulate production of bactericidal nitric oxide, and reducing bacterial proliferation.
Rickettsial infections are diagnosed by immunostaining of organisms , or by detection of antirickettsial antibodies in serum.

Gimenez stain of hemolymph cells infected with R. rickettsii.

Fungal Infections : Fungal infections are called mycoses. Fungi are eukaryotes , grow by budding (yeasts), or
by filamentous extensions called hyphae (molds). Dimorphic fungi have both yeast form (at human body
temperature) , and mold form (at room temperature).YEASTS: Candidiasis : Residing normally in skin, mouth, gastrointestinal tract,
and vagina. Infections range from superficial lesions in healthy
persons, to disseminated infections in immunocompromised patients .

Pathogenesis: Candida produce a large number of adhesins , that
adherence to host cells. These adhesins include: (1) an integrin-like protein. (2) a protein that resembles transglutaminase. (3) several agglutinins . The immune response to Candida:• Innate immunity and T-cell responses against mucosal
and cutaneous Candida infection.• Neutrophils and mononuclear phagocytes for resistance
to systemic Candida infections.

Morphology: In tissue sections, C. albicans can appear as yeastlike
forms (blastoconidia), pseudohyphae, and , less commonly true hyphae.
The organisms may be visible with routine hematoxylin and eosin stains.
but a variety of special "fungal" stains (Gomori methenamine-silver, periodic acid-Schiff) are used.
Clinical picture: Superficial infection on mucosal surfaces of oral cavity
(thrush). Candida esophagitis is commonly seen in AIDS patients
and in those with hematolymphoid malignancies.

Candida vaginitis is common in women who are diabetic or pregnant or on oral contraceptive pills.
Cutaneous candidiasis can present in different forms:
• nail proper ("onychomycosis")• nail folds ("paronychia")• hair follicles ("folliculitis")• webs of fingers and toes ("intertrigo")• penile skin ("balanitis). ").• "Diaper rash" in perineum of infants.

Invasive candidiasis:• caused by blood-borne dissemination of
organisms to various tissues or organs.• Common patterns include:(1) renal abscesses.(2) myocardial abscesses and endocarditis.(3) brain involvement ( meningitis, parenchymal microabscesses ).(4) endophthalmitis ( any eye structure).(5) hepatic abscesses.(6) Candida pneumonia.

Gram-stain of vaginal smear showing Candida albicans ,epithelial cells ,and many gram-negative rods.

Cryptococcosis : Cryptococcus neoformans is an encapsulated yeast that
causes meningoencephalitis in normal individuals. but more frequently presents as an opportunistic
infection in immunosuppressed patients.Pathogenesis: C. neoformans is present in soil and in bird (particularly
pigeon) droppings and infects patients when it is inhaled. Several virulence factors enable it to evade host
defenses. The virulence factors include : (1) a polysaccharide capsule. (2) melanin production. (3) enzymes.

Morphology: In contrast to Candida, cryptococcus has yeast , but
not pseudohyphal or hyphal forms. cryptococcal yeast has a thick gelatinous capsule that is
valuable for diagnosis. Capsular polysaccharide stains intense red with periodic
acid-Schiff ( PAS ) ,and mucicarmine stains in tissues.Clinical picture: Although the lung is primary site of localization,
pulmonary infection with C. neoformans is usually mild and asymptomatic.
C. neoformans, however, may form a solitary pulmonary granuloma.

The major lesions caused by C. neoformans are in central nervous system, involving meninges, cortical gray matter, and basal nuclei (meningoencephalitis ).
In immunosuppressed patients, organisms may evoke no inflammatory reaction.
In non-immunosuppressed patients the fungi induce a chronic granulomatous reaction composed of macrophages, lymphocytes, and foreign body type giant cells. Neutrophils and suppuration also may occur.
In severely immunosuppressed persons, C. neoformans may disseminate widely to skin, liver, spleen, adrenals, and bones.

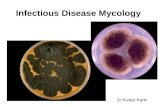
Cryptococcus neoformans in brain, H & E stain

MOLDS :Aspergillosis : causes allergies (brewer's lung) in healthy people. sinusitis, pneumonia, and fungemia in
immunocompromised individuals. The major factors that predispose to Aspergillus infection
are neutropenia and corticosteroids. This saprophytic fungus sporulates and produces
abundant conidia (asexual spores) that are readily aerosolized.

Pathogenesis: transmitted by airborne conidia. lung is the major portal of entry. The small size of Aspergillus fumigatus spores
( approximately 2 to 3 µm ) enables them to reach alveoli.
In lung, Aspergillus conidia are encountered initially by alveolar macrophages which can engulf and kill the conidia and secrete cytokines and chemokines to elicit adaptive immune responses.
T- lymphocytes confer protective immunity.

Aspergillus produces several virulence factors including adhesins, antioxidants, enzymes, and toxins.
The carcinogen aflatoxin is made by Aspergillus species growing on surface of peanuts and may be a major cause of liver cancer.
Sensitization to Aspergillus spores produces allergic alveolitis by TH2 reactions.
Allergic bronchopulmonary aspergillosis, often occurs in asthmatic patients, may eventually result in chronic obstructive lung disease.

Morphology: Colonizing aspergillosis ( aspergilloma ) implies
growth of fungus in pulmonary cavities with minimal or no invasion of tissues .
The cavities usually result from preexisting tuberculosis, bronchiectasis, old infarcts, or abscesses.
Proliferating masses of fungal hyphae called fungus balls form brownish masses lying free within the cavities.
The surrounding inflammatory reaction may be sparse, or there may be chronic inflammation and fibrosis.

Invasive aspergillosis is an opportunistic infection that is confined to immunosuppressed patients.
The primary lesions are usually in lung, but widespread hematogenous dissemination with involvement of heart valves, brain, and kidneys is common.
The pulmonary lesions take the form of necrotizing pneumonia.
Aspergillus forms fruiting bodies (particularly in cavities), and septate filaments branching at acute angles.


Zygomycosis (Mucormycosis): is an opportunistic infection caused by "bread mold
fungi" including Rhizopus, Absidia, Cunninghamella, and Mucor, which belong to class Zygomycetes.
they infect immunosuppressed patients. The major predisposing factors are neutropenia,
corticosteroid use, diabetes mellitus and breakdown of cutaneous barrier (e.g., as a result of burns, surgical wounds, trauma).

Pathogenesis: Similar to Aspergillus , zygomycetes are transmitted by
airborne asexual spores. Most commonly, inhaled spores produce infection in
sinuses and lungs. but spores can also lead to infection following
percutaneous exposure or ingestion. Macrophages provide initial defenses by phagocytosis
and oxidative killing of spores. Neutrophils have a key role in killing fungi during
established infection. Proteolytic and lipolytic enzymes and mycotoxins have
been identified for some zygomycetes.

Morphology: Zygomycetes form nonseptate wide fungal hyphae
with right-angle branching. in diabetics, fungus may spread from nasal sinuses to
orbit and brain, giving rise to rhinocerebral mucormycosis.
The zygomycetes cause local tissue necrosis, invade arterial walls, and penetrate periorbital tissues and cranial vault.
Meningoencephalitis follows, sometimes complicated by cerebral infarctions when fungi invade arteries and induce thrombosis.
The lung lesions combine areas of hemorrhagic pneumonia with vascular thrombi and distal infarctions.

Nasopharyngeal mucormycosis (zygomycosis) in conjunction with diabetic ketoacidosis is shown here microscopically. Note the broad, branching non-septated hyphae.

THANK YOU