Improvement in early human embryo development using new formulation sequential stage-specific...
-
Upload
simon-cooke -
Category
Documents
-
view
220 -
download
0
Transcript of Improvement in early human embryo development using new formulation sequential stage-specific...

Improvement in early human embryodevelopment using new formulationsequential stage-specific culture media
Simon Cooke, B.Sc.Agr.,a Patrick Quinn, Ph.D.,b Lee Kime, B.Sc.,a
Cheryl Ayres, M.Med.Sci.,a John P. P. Tyler, Ph.D.,a and Geoff L. Driscoll, M.D.a
CityWest IVF, Westmead, New South Wales, Australia
Objective: To determine whether altering selected components of sequential culture media can improve earlydevelopment variables of human embryos.
Design: Prospective, randomized, sibling oocyte split trial.
Setting: Private ART center.
Patient(s): Two hundred eight undergoing treatment with in vitro fertilization or microinjection.
Intervention(s): Oocytes from each patient were randomly allocated to fertilization and cleavage media of acontrol and a trial culture medium formulation.
Main Outcome Measure(s): Rates of fertilization, cleavage, and uncontrolled division; average embryomorphology score; blastomeres per embryo; embryo score parameter (number of blastomeres � embryomorphology grade); and embryo utilization.
Result(s): The trial media resulted in a higher fertilization rate, higher cleavage rate, lower rate of uncon-trolled division, higher number of blastomeres per embryo, higher average embryo morphology score, a higherembryo score parameter, and higher embryo utilization rate compared to the control media. All differenceswere statistically significant.
Conclusion(s): Improved sequential stage-specific culture media can reduce the occurrence of severe humanembryo fragmentation and improve developmental variables in early IVF- and ICSI-generated embryos.(Fertil Steril� 2002;78:1254–60. ©2002 by American Society for Reproductive Medicine.)
Key Words: Sequential culture media, early embryo development, embryo morphology, in vitro develop-ment, uncontrolled division
The embryology laboratory plays a criticalrole in determining the success rate of a humanIVF program. One of the most important com-ponents that influences the success of the lab-oratory is the quality and composition of theculture medium used.
Human tubal fluid (HTF) (1), has been usedin many IVF programs worldwide, but thisculture medium is now being replaced by me-dia based on more current physiologic evi-dence of the composition of the female repro-ductive tract environment (2). Glucoseconcentrations of 2.8 mM are sufficient tomaintain sperm velocity in fertilization me-dium (3), and glucose is needed in cleavagestage embryos to satisfy such requirements asthe pentose phosphate pathway. Phosphateshould be removed entirely to minimize thenegative effects of the combination of glucose
and phosphate (1, 4). Magnesium concentra-tion should be increased to compensate for anincrease in intracellular calcium induced by invitro culture that has been shown to be detri-mental to embryo development in vitro (5, 6).
Other ingredients, such as taurine, havebeen shown to be beneficial for in vitro cultureof embryos (7, 8), and both taurine and ethyl-enediamine tetraacetic acid (EDTA) provideantioxidant activity. However, elevated con-centrations of EDTA may be responsible forgranularity in human embryos (Quinn P. Un-published data). Inclusion of citric acid may beimportant, as it has been demonstrated to sup-port early mouse preimplantation development(9) and is a component in the tricarboxylic acidcycle (10).
Glutamine, and in particular, the type ofglutamine, has been shown to be beneficial for
Received January 11,2002; revised andaccepted April 16, 2002.Reprint requests: SimonCooke, B.Sc.Agr., CityWestIVF, 12 Caroline Street,Westmead, New SouthWales 2145, Australia (FAX:612-9633-3730; E-mail:[email protected]).a CityWest IVF.b SAGE BioPharma, SanClemente, California.
FERTILITY AND STERILITY�VOL. 78, NO. 6, DECEMBER 2002
Copyright ©2002 American Society for Reproductive MedicinePublished by Elsevier Science Inc.
Printed on acid-free paper in U.S.A.
0015-0282/02/$22.00PII S0015-0282(02)04343-1
1254

human embryo development in vitro (11). Alanyl-glutamineis a very stable form of glutamine that does not break downand release ammonium. Ammonium buildup in medium hasbeen shown to be embryotoxic (12).
Suboptimal culture conditions have also been noted as apossible cause of low embryo viability after transfer (13).Affected embryo variables included delayed cell division(14), less incorporation of amino acids (15), and a reductionin oxidative metabolism and increase in lactate production(16–18). More recent studies (2) have shown that the use ofsequential media that have components at the various sites inthe female reproductive tract at different stages of earlypregnancy has produced higher pregnancy and implantationrates.
Previous studies were retrospective and involved onedefined media (10), compared two different media (19, 20),or were done at different stages of embryo growth (19).There have been few prospective, comparative trials of im-proved formulations of a media series.
In our study, trial media were formulated based on recentinformation regarding the physiological environment for hu-man gametes and embryos and of other relevant mammalianspecies. We sought to prospectively compare a new series ofsequential culture media with the standard HTF formulationsthat were currently in use in our clinic for human IVFculture.
MATERIALS AND METHODS
Trial DesignThe experiment was designed as a sibling oocyte split, in
which oocytes were allocated to a particular medium byusing a random-numbers series. Patients undergoing IVF hadtheir oocytes allocated to each medium during oocyte re-trieval, whereas patients undergoing ICSI had their oocytesallocated to the culture media after the cumulus–coronacomplex had been removed and nuclear maturity had beenassessed.
Patients were excluded if they were �40 years of age atthe commencement of a treatment attempt; if at least oneembryo was not generated in each of the control and trialmedia; and if patients were having ICSI using testicularsurgically retrieved sperm, because these patients have areduced fertilization rate within our clinic.
Two hundred fifty-four patients had oocytes allocated tothe trial. Of these, 46 were excluded from analysis (15because of failed fertilization and 31 because less than oneembryo was generated in each media treatment), leaving 208patients eligible for inclusion in the media trial. These 208patients had an average age of 32.6 years (range, 20.0–39.9years) and their duration of infertility ranged from 0.7 to 6.0years.
Thirty-one of 254 patients were excluded from the trialbecause only one embryo was generated or the patient hadfew oocytes that failed to fertilize with IVF insemination (15of 254 patients). Our study required one embryo to begenerated in each media group. If any initial oocyte inclusionnumber had been chosen for inclusion in the trial, it wouldprove to be an unsatisfactory selection criterion because itwould select for better-performing patients and a better out-come. The broad inclusion criteria that we used permitted alarge cross-section and, therefore, generation of data that arerepresentative of a total ART patient population rather thana highly selected population.
Twenty-six patients had embryos generated but did notreceive an embryo transfer; instead, all utilizable embryoswere frozen because of medical concerns about overstimu-lated ovaries. Data from these patients were included be-cause the study was concerned with early embryonic devel-opment and not pregnancy outcome.
There were 107 ICSI cycles, mostly because of malefactor infertility, and 101 IVF cycles done because of tubalabnormalities, endometriosis, or unexplained infertility. Theinstitutional ethics committee approval was granted for thisstudy.
Culture MediaTwo sequential stage-specific culture media were used.
The control medium was commercially available and con-sisted of a fertilization medium (HTF; SAGE BioPharma,Bedminster, NJ) and a cleavage medium (Basal XI; SAGEBioPharma). The trial medium was a modification of thecontrol medium and consisted of a fertilization medium anda cleavage medium (SAGE BioPharma).
The antibiotic content, manufacturing process, storage,utilization, and osmolarity of the media were identical. Allmedia met standard manufacturing specifications, includinga quality control test with one-cell mouse embryos in whichat least 80% of zygotes cultured in the media developed tothe fully expanded blastocyst stage over 96 hours of culture.Table 1 shows alterations made to the trial medium.
Controlled Ovarian HyperstimulationControlled ovarian hyperstimulation was achieved by a
long down-regulation protocol involving GnRH agonist andFSH. The GnRH agonist was administered as a s.c. injection(Lucrin [leuprorelin acetate]; Abbott, Kurnell, New SouthWales, Australia) or a nasal spray (Syneral [nafarelin acetatesolution]; Searle, Rydalmere, New South Wales, Australia).Recombinant FSH (Gonal-F; Serono, Frenchs Forrest, NewSouth Wales, Australia, or Puregon; Organon, Lane Cove,New South Wales, Australia) was used to induce folliculargrowth. At least 5,000 IU of hCG (Profasi; Serono) wasadministered to achieve final maturation and trigger ovula-tion when at least two follicles were 18 mm in diameteraccording to transvaginal ultrasonography.
FERTILITY & STERILITY� 1255

Thirty-eight hours later, ultrasonography-guided oocyteretrieval was performed by using a single-lumen 16-gaugeovum pick-up set (COOK, Eight Mile Plains, Queensland,Australia). All oocytes were returned to the IVF laboratoryin Hepes-buffered HTF (SAGE BioPharma) at 37°C in aportable incubator (LEC-960, LEC Instruments, Scoresby,Victoria, Australia).
Culture ConditionsOocytes destined for microinjection had their cumulus
cells removed by 30 seconds of exposure to hyaluronidase(Sigma Type VIII Bovine Testis Enzyme; Sigma, CastleHill, New South Wales, Australia), 80 IU/mL, in Hepes-buffered HTF, followed by washing with Hepes-bufferedHTF containing human serum albumin (SAGE BioPharma),5 mg/mL. The coronal cells were mechanically removed byusing a finely drawn glass Pasteur pipette. Denuded oocyteswere then assessed for their structural integrity and meioticmaturity and placed into culture in 1.0 mL of the control ortrial fertilization medium with an oil overlay, in a 4-wellNunc dish (Nunclon; Medos, Lidcome, New South Wales,Australia) that had been pre-equilibrated at 37°C in 6%carbon dioxide in room air.
After randomization using a random-numbers table,groups of three or four oocytes intended for insemination byconventional IVF were placed directly in a culture dish andinseminated with approximately 100,000 motile sperm. Oo-cytes for ICSI were microinjected 42 hours after the ovula-tory hCG injection in a manner similar to that described byVan Steirteghem et al. (21) and then returned to culture. Alloocyte–embryo culture was performed in a mini incubator
(MINC-1000; COOK) supplied with a humidified triple gasmixture of 6% CO2, 5% O2, and 89% N2 at 37°C.
Fertilization was assessed 16 to 20 hours after microin-jection or addition of the spermatozoa. Oocytes that fertil-ized normally on day 1 were washed in their respectivecleavage medium. Five or fewer zygotes were grouped in50-�L droplets of medium for culture.
Embryos were graded 40 to 41 hours after addition ofsperm, and embryo transfer was performed transcervically 1to 3 hours later by using a double-lumen catheter (K-JETS7019; COOK). All patients received luteal phase supplemen-tation with progesterone (Orion Laboratories, Welshpool,Western Australia, Australia), given as a 200-mg vaginalpessary daily for 14 days starting at oocyte retrieval, or as a2,000-IU injection (Profasi; Serono) 2 days after oocyteretrieval, followed by three further injections at 72-hourintervals.
Early Embryonic DevelopmentFertilization rates were standardized and expressed on the
basis of meiotically mature oocytes because both ICSI andIVF insemination was performed. Oocyte maturity was as-sessed initially at the time of cumulus cell removal in pa-tients undergoing ICSI and during fertilization checking onday 1 in patients undergoing IVF. Oocytes that becamenecrotic after ICSI were excluded.
The fertilization rate was calculated as follows:
rate (%)� [(no. of fertilized oocytes)/((no. meiotically mature)� (no. necrotic after ICSI))]�(100).
Cleavage was defined as the presence of a two- or four-cellembryo approximately 40 to 41 hours after the addition ofspermatozoa. The size and regularity of blastomeres werecarefully assessed to allow determination of controlled anduncontrolled cleavage. Embryo cleavage and grading wereassessed within a 2-hour window to obtain a measurement ofthe speed of cleavage and embryo morphology.
Morphology grading was based on fragmentation andblastomere regularity (22–27). Each embryo was allocated ascore from 4 to 1 according to the regularity of the blas-tomeres and the amount of embryo fragmentation. Grade 4embryos had equal-sized blastomeres, were spherical inshape, and contained no extracellular fragmentation. Grade 3embryos had �10% extracellular fragmentation. Grade 2embryos had 10% to 50% extracellular fragmentation. Grade1 embryos were of poor quality and had �50% fragmenta-tion.
Uncontrolled division was defined as the complete frag-mentation of embryos. Unlike grade 1 embryos, which had atleast some clearly defined cells, uncontrolled division em-bryos have no visible normal blastomere structure andmasses of very small fragments, more similar to an apoptotic
T A B L E 1
Alterations to the ingredients of the trial fertilization andcleavage medium.
Culture medium stageIngredient alteration
(reference)
Fertilization medium 2.8 m/Mol Glucose (3)Fertilization and cleavage
mediumEDTA reduced to 0.01m/Mol
Fertilization and cleavagemedium
Magnesium increased to 2.0 mMol (5, 6)
Fertilization and cleavagemedium
Taurine added at 0.1 mMol (7, 8)
Fertilization and cleavagemedium
Citric acid added at 0.0005 mMol (7, 9, 10)
Fertilization and cleavagemedium
Alanyl-glutamine added at 1.0 mMol (11)
Fertilization and cleavagemedium
Essential amino acids added (34)
Cleavage medium Glucose reduced to 0.1 m/Mol (1, 4)Cleavage medium Phosphate removed (1, 4)
Note: EDTA � ethylenediamine tetraacetic acid
Cooke. Embryonic development in sequential media. Fertil Steril 2002.
1256 Cooke et al. Embryonic development in sequential media Vol. 78, No. 6, December 2002

event than normal cell division. Structures that had uncon-trolled division were not assessed for embryo morphologybecause they were not considered to be dividing normally asan embryo. The average embryo score reported here is anaccurate indication of the morphology of a dividing embryo.
The embryo score parameter per cleaved embryo wasdefined as the average morphology score for embryos gen-erated in a particular culture medium, multiplied by theaverage number of blastomeres produced in the embryos thatcleaved in a controlled manner. As an example, a morphol-ogy score of 4 in a four-cell embryo assessed early on day 2is 16: [4 � 4] (26). The embryo score parameter is anaverage score that incorporates both the morphology scoreand the cleavage speed of the embryos.
Statistical AnalysisThe Wilcoxon signed-rank test (SPSS software, version
0.7; SPSS, Inc., Chicago, IL) was used to analyze fertiliza-tion rates, cleavage rates, uncontrolled division, number ofblastomeres per embryo, average embryo morphology, andembryo score parameter between media and within patients.Differences in overall proportional data were analyzed byusing the �2 test. The patient population was analyzed as apooled group (IVF-generated and ICSI-generated embryos)and by individual treatments (IVF-generated embryos andICSI-generated embryos).
RESULTSEarly development parameters were assessed in 2,009
oocytes from 208 treatment cycles. This included 991 oo-cytes from 101 treatment cycles inseminated by IVF and1,018 oocytes from 107 treatment cycles inseminated byICSI.
Table 2 shows the results of early embryonic develop-ment as determined by pooled treatment data (IVF and ICSI)and by treatment type.
When the pooled treatment cycles were assessed, allvariables showed significant improvement when oocyteswere cultured in the trial medium compared with the controlmedium. When assessed by the type of treatment used forinsemination (IVF or ICSI), the trial medium also offeredsignificant improvements compared with the control me-dium. The average number of blastomeres per embryo wasthe only early embryonic developmental variable that did notdiffer statistically when analyzed by insemination method;however, with the larger embryo numbers in the pooled data,this variable was significantly increased in embryos culturedin trial medium.
The rates of cleavage and uncontrolled division differedgreatly between embryos cultured in control and trial media,regardless of whether they were derived by IVF or ICSI. Inthe control medium, cleavage rates (88.8%) were signifi-cantly lower (P�.0001) and rates of uncontrolled division
(7.8%) were significantly higher (P�.0001) than the corre-sponding values observed in the trial medium (94.5% and2.7%, respectively). When rates of cleavage and uncon-trolled division in each medium were compared betweenembryos produced by IVF or ICSI, rates of cleavage werealways significantly higher and rates of uncontrolled divisionalways significantly lower in embryos generated in trialmedium.
The increase in the early embryonic development vari-ables in embryos generated in trial medium also led to asignificant increase in the rate of utilization of these embryos(68.2%) compared with embryo generated in control me-dium (55.9%; �2 � 21.8; P�.005). Embryo utilization wasdefined as the total number of embryos transferred or frozendivided by the number of embryos that actually cleaved.
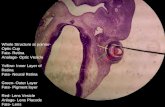
A phenomenon that became apparent during the trial wasthe presence of intrablastomere pitting in embryos culturedin control medium and the absence of this pitting in embryoscultured in trial medium (Fig. 1). This phenomenon occurredindependently of embryo fragmentation.
Pregnancy was not a measured outcome in this studybecause the best-quality embryos from either medium werereplaced. Embryo transfer occurred in 182 of the 208 cycles.Cryopreservation only occurred in the remaining 26 cycles.Ninety-six (53%) of the transfers resulted in a clinical preg-nancy confirmed by ultrasonography. On average, 1.99 em-bryos per patient were replaced, and the implantation ratewas 35%. Of the transferred embryos, 260 (71.6%) werederived from the trial medium and 103 (28.4%) from thecontrol medium. As a historical reference point, in 182patients matched for age before the start of the trial, theimplantation rate was 23.9% using embryos generated fromcontrol medium.
DISCUSSIONThe cleavage rate (27) and morphology (27, 28) of IVF-
generated embryos are reported to be more normal thanthose of ICSI-generated embryos (27). Our study confirmsthese findings.
Most previous studies did not compare the outcome ofimproved media formulations from the same manufactureror looked carefully at the cleavage or control of the cleavagein embryos. Regardless of the type of insemination, use ofour trial medium improved all early embryonic developmentvariables compared to the original control medium.
The definitions that we used for cleavage and uncon-trolled division provided an accurate indication of whatconstitutes controlled or uncontrolled division. One conse-quence of these definitions is that the zygote cleavage ratemay appear somewhat low between the two media (88.8%vs. 94.5%). However, this reflects our requirement that em-bryos with controlled cleavage have clearly defined blas-tomeres. An absence of defined blastomeres was considered
FERTILITY & STERILITY� 1257

uncontrolled division, which was significantly reduced in thetrial medium (7.8% vs. 2.7%). Our findings suggest that therate of uncontrolled division or complete fragmentation canbe mostly attributed to the culture media and their formula-tion and that the occurrence of severe fragmentation oruncontrolled division is not always the expression of thegenetic potential (or genotype) of the oocyte; rather, it maybe significantly influenced by the environment that the oo-cyte is exposed to during early culture. This observationsupports those of Munne et. al., (29) who found that certainculture conditions or hormonal stimulation may induce chro-mosomal abnormalities and partly explain differences inpregnancy rates among in vitro fertilization centers.
The embryo score parameter is an important tool thatcombines both the speed of embryo cleavage (cell number)and how well the embryo manages to divide (morphologyscore). The trial medium produced embryos with more rapidcleavage and a better average morphology score than theirsibling oocytes cultured in control medium. The significanceof the cell number was reduced when analyzed by insemi-nation method, which contrasts with the findings of Dumou-lin et al. (30). However, their average cell number perembryo of 3.22 for IVF and 3.48 for ICSI were well belowthe 3.98 and 4.15 that we found at similar developmentalstages.
A previous study (11) reported an increase in the numberof trophectoderm cells in blastocysts when glutamine wasadded to the culture media, but not the increase in totalblastomere numbers observed by day 2 of development.Similarly, when only glucose and phosphate are altered, theaverage number of blastomeres reported on day 2 was only3.2 (10), well below that rates that we report with multiplealteration to culture media. We cannot identify whichchanges to formulation in the trial medium were primarilyresponsible for each improvement in outcomes observed;however, it is probable that a combination of alterations hascontributed to the results obtained.
The improvement in embryo morphology scores and thereduction in uncontrolled division in the embryos generatedin the trial medium indicates that culture medium may affectthe internal processes of the oocyte during fertilization,syngamy, and early cleavage and influence the first cleavagealignment from the spindle poles. Previously, pronuclear andembryo grading systems have shown that embryos with ahigh development and pregnancy potential display a moreordered, predictable cleavage pattern and higher pregnancyrates with a lower incidence of aneuploidy (24, 25, 31–33).
The incidence of intrablastomere pitting seen in the em-bryos cultured in control medium and absent from those
T A B L E 2
Early embryonic development variables measured between pooled cycles (IVF and ICSI) and separately by theinsemination method.
VariableInsemination
treatmentControlmedium Trial medium Value P value
Fertilization rate (%) Pooled (IVF � ICSI) 74.1 � 22.6 80.1 � 21.0 Z � �2.995 .03IVF Generated 72.2 � 21.5 78.5 � 21.6 Z � �2.281 .023ICSI Generated 75.9 � 23.6 81.6 � 20.5 Z � �1.931 .05
Cleavage rate (%) Pooled (IVF � ICSI) 88.8 � 21.3 94.5 � 13.6 Z � �4.043 �.0001IVF Generated 90.1 � 18.7 96.4 � 11.6 Z � �3.099 .002ICSI Generated 87.6 � 23.4 92.7 � 15.2 Z � �2.584 .01
Rate of uncontrolled Pooled (IVF � ICSI) 7.8 � 18.6 2.7 � 9.9 Z � �4.563 �.0001division (%) IVF Generated 6.3 � 16.0 1.9 � 8.7 Z � �2.304 .03
ICSI Generated 9.2 � 20.8 3.3 � 10.9 Z � �3.491 �.0001Morphology scorea Pooled (IVF � ICSI) 3.25 � 0.52 3.45 � 0.63 Z ��4.950 �.0001
IVF Generated 3.27 � 0.73 3.45 � 0.62 Z � �2.693 .007ICSI Generated 3.20 � 0.60 3.42 � 0.52 Z � �4.342 �.001
Embryo score parameter Pooled (IVF � ICSI) 11.3 � 3.0 12.3 � 2.8 Z � �4.493 �.0001IVF Generated 11.5 � 3.7 12.4 � 3.0 Z � �2.304 .021ICSI Generated 11.0 � 3.0 12.1 � 2.8 Z � �4.036 �.0001
No. of blastomeres per embryo Pooled (IVF � ICSI) 3.92 � 1.35 4.06 � 1.37 Z � �2.256 .024IVF Generated 3.86 � 1.29 3.98 � 1.16 Z � �1.467 NSICSI Generated 3.97 � 1.41 4.15 � 1.55 Z � �1.604 NS
Embryo utilization rate (%) Pooled (IVF � ICSI) 55.9 68.2 �2 � 21.8 �.005IVF Generated 59.3 70.5 �2 � 9.4 �.005ICSI Generated 52.7 65.8 �2 � 12.4 �.0005
Note: Data after the plus/minus sign are SDs. NS � not significant.a Of a possible 1 (worst) to 4 (best).
Cooke. Embryonic development in sequential media. Fertil Steril 2002.
1258 Cooke et al. Embryonic development in sequential media Vol. 78, No. 6, December 2002

cultured in trial medium from the same patient (Fig. 1) areattributed to reduced EDTA concentration in trial medium.This finding confirms previously unpublished results (QuinnP). All embryos were subject to the same culture tempera-tures, and the media differed only in composition and not instorage or antibiotic content. This pitting of cultured humanembryos has been previously observed (34) but is not linkedto a specific ingredient of culture medium.
Embryo morphology grading protocols are largely based
on the volume of embryo fragmentation (22–25). Pitting isnot a parameter that would be excluded or selected for bythese grading protocols, since it occurs independently offragmentation. Pitted blastomeres were rarely observed inembryos cultured in the trial medium.
Our study shows that all aspects of early human preim-plantation embryo development can be substantially im-proved by using chemically defined sequential stage-specificculture medium rather than standard HTF-based formula-
F I G U R E 1
Four-cell embryos showing absence of intra-blastomere pitting when cultured in control medium or trial medium. (a), Anembryo with grade 4 morphology but extensive intracytoplasmic pitting cultured in control media. (b), An embryo from the samepatient, cultured in trial medium, having grade 4 morphology and no fragmentation or pitting. (c), An embryo of grade 3morphology cultured in control media. (d), Embryo of grade 3 morphology from the same patient, cultured in trial medium. Theintrablastomere pitting within a patient group occurs independently of embryo fragmentation. Bar � 50�m.
Cooke. Embryonic development in sequential media. Fertil Steril 2002.
FERTILITY & STERILITY� 1259

tions. This includes a significantly higher fertilization rate,cleavage rate, average embryo morphology score, embryoscore parameter, and embryo utilization rate and signifi-cantly less uncontrolled division, regardless of whether em-bryos were generated by IVF or ICSI. The higher rates ofembryo utilization and the overall pregnancy and implanta-tion rates obtained when fertilization and embryo develop-ment occurred in the trial medium have prompted us to usethis formulation exclusively.
References1. Quinn P, Kerin JF, Warnes GM. Improved pregnancy rate in human in
vitro fertilization with the use of a medium based on the composition ofhuman tubal fluid. Fertil Steril 1985;44:493–8.
2. Gardner DK, Lane M. Culture and selection of viable blastocysts: afeasible proposition for human IVF? Hum Reprod Update 1997;3:367–82.
3. Quinn P. Enhanced results in mouse and human embryo culture usinga modified human tubal fluid medium lacking glucose and phosphate. JAssist Reprod Genet 1985;12:97–105.
4. Seshagiri PB, Bavister BD. Glucose and phosphate inhibit respirationand oxidative metabolism in cultured hamster eight-cell embryos: ev-idence for the “Crabtree effect.” Mol Reprod Dev 1991;30:105–11.
5. Lane M, Boatman DE, Albrecht ERM, Bavister BD. Intracellulardivalent cation homeostasis and developmental competence in the ham-ster preimplantation embryo. Mol Reprod Dev 1998;50:443–50.
6. Lane M, Bavister BD. Calcium homeostasis in early hamster preim-plantation embryos. Biol Reprod 1998;59:1000–7.
7. Pool TB, Atiee SH, Martin JE. Oocyte and embryo culture: basicconcepts and recent advances. In: May J, (ed). Infertility and reproduc-tive medicine clinics of North America. Philadelphia: Saunders, 1998.
8. Dumoulin JCM, Evers JLH, Bras M, Pieters MHEC, Geraedts JPM.Positive effect of taurine on preimplantation development of mouseembryos in vitro. J Reprod Fert 1992;94:373–80.
9. Brinster RL, Thomson JL. Development of eight-cell mouse embryos invitro. Expl Cell Res 1966;42:308–15.
10. Carillo VJ, Lane B, Pridman DD, Risch PP, Pool TB, Silverman IH, etal. Improved clinical outcomes for in vitro fertilisation with delay ofembryo transfer from 48 to 72 hours after oocyte retrieval: use ofglucose and phosphate free media. Fertil Steril 1998;69:329–34.
11. Devreker F, Winston RML, Hardy K. Glutamine improves humanpreimplantation development in vitro. Fertil Steril 1998;69:293–9.
12. Lane M, Gardner DK. Increase in postimplantation development ofcultured mouse embryos by amino acids and induction of fetal retar-dation and exencephaly by ammonium ions. J Reprod Fertil 1994;102:305–12.
13. Bavister B. Culture of preimplantation embryos: facts and artifacts.Hum Reprod Update 1985;1:91–148.
14. Bowman P, McLaren A. Cleavage rate of mouse embryos in vivo andin vitro. J Embryol Exp Morphol 1970;54:203–7.
15. Jung T, Fischer B, Beier HM. Quantitative aspects of protein synthesisin non cultured and cultured rabbit blastocysts. Hum Reprod 1987;2:23–7.
16. Gardner DK, Leese HJ. Concentrations of nutrients in mouse oviductfluid and their effects on embryo development and metabolism in vitro.J Reprod Fertil 1990;88:361–8.
17. Gott AL, Hardy K, Winston RML, Leese HJ. The nutrition and envi-ronment of the early human embryos. Proc Nutr Sci 1990;49:Abstract2A.
18. Leese HJ, Conaghan J, Martin KL, Hardy K. Early human embryometabolism. Bioessays 1993;15:259–64.
19. Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, HeslaJ. A prospective randomized trial of blastocyst culture and transfer inin-vitro fertilisation. Hum Reprod 1998;13:3434–40.
20. Van Langendonckt A, Demylle D, Wyns C, Nisolle M, Donnez J.Comparison of G1.2/G2.2 and Sydney IVF cleavage/blastocyst sequen-tial media for the culture of human embryos: a prospective, random-ized, comparative study. Fertil Steril 2001;76:1023–31.
21. Van Steirteghem AC, Liu J, Joris H, Nagy Z, Janssenswillen C, Tour-naye H, et al. Higher success rate by intracytoplasmic sperm injectionthan by subzonal insemination. Report of a second series of 300consecutive treatment cycles. Hum Reprod 1993;8:1055–60.
22. Almeida PA, Bolton VN. The relationship between chromosomal ab-normality in the human preimplantation embryo and development invitro. Reprod Fertil Dev 1996;8:235–41.
23. Giorgetti C, Terriou P, Auquier P, Hans E, Spach JL, Salzmann J, et al.Embryo score to predict implantation after in-vitro fertilisation: basedon 957 single embryo transfers. Hum Reprod 1995;10:2427–31.
24. Pellestor F, Dufour MC, Arnal F, Humeau C. Direct assessment of therate of chromosomal abnormalities in grade IV human embryos pro-duced by in-vitro fertilization procedure. Hum Reprod 1994;9:293–302.
25. Erenus M, Zouves C, Rajamahendran P, Leung S, Fluker M, Gomel V.The effect of embryo quality on subsequent pregnancy rates after invitro fertilization. Fertil Steril 1991;56:707–10.
26. Steer CV, Mills CL, Tan SL, Campbell S, Edwards RG. The cumulativeembryo score: a predictive embryo scoring technique to select theoptimal number of embryos to transfer in an in-vitro fertilization andembryo transfer programme. Hum Reprod 1992;7:117–9.
27. Hsu MI, Mayer J, Aronshon M, Lanzendorf S, Muasher S, Kolm P, etal. Embryo implantation in in vitro fertilization and intracytoplasmicsperm injection: impact of cleavage status, morphology grade, andnumber of embryos transferred. Fertil Steril 1999;72:679–85.
28. Frattarelli JL, Leondires MP, Miller BT, Segars JH. Intracytoplasmicsperm injection increases embryo fragmentation without affecting clin-ical outcome. J Assist Reprod Genet 2000;17:207–12.
29. Munne S, Magli C, Adler A, Wright G, de Boer K, Mortimer D, et al.Treatment-related chromosome abnormalities in human embryos. HumReprod 1997;12:780–4.
30. Dumoulin JC, Coonen E, Bras M, van Wissen LC, Ignoul-VanvuchelenR, Bergers-Jansen JM, et al. Comparison of in-vitro development ofembryos originating from either conventional in-vitro fertilization orintracytoplasmic sperm injection. Hum Reprod 2000;15:402–9.
31. Tesarik J, Greco E. The probability of abnormal preimplantation de-velopment can be predicted by a single static observation on pronuclearstage morphology. Hum Reprod 1999;14:1318–23.
32. Tesarik J, Junca AM, Hazout A, Aubriot FX, Nathan C, Cohen-BacrieP, et al. Embryos with high implantation potential after intracytoplas-mic sperm injection can be recognized by a simple, non-invasiveexamination of pronuclear morphology. Hum Reprod 2000;15:1396–9.
33. Scott LA, Smith S. The successful use of pronuclear embryo transfersthe day following oocyte retrieval. Hum Reprod 1998;13:1003–13.
34. Desai NN, Goldstein J, Rowland DY, Goldfarb JM. Morphologicalevaluation of human embryos and derivation of an embryo qualityscoring system specific for day 3 embryos: a preliminary study. HumReprod 2000;15:2190–6.
1260 Cooke et al. Embryonic development in sequential media Vol. 78, No. 6, December 2002