IB Chemistry on Hydrogen Bonding, Polarity, Dipole Moment, Van Der Waals forces of attraction
-
Upload
lawrence-kok -
Category
Education
-
view
7.436 -
download
2
description
Transcript of IB Chemistry on Hydrogen Bonding, Polarity, Dipole Moment, Van Der Waals forces of attraction
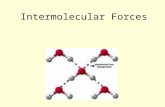
- 1. Video Tutorial on Polarity, HydrogenBonding and Van Der Waals forces ofattraction. Prepared by Lawrence Kok http://lawrencekok.blogspot.com
2. Polarity of Molecules Polarity depends on Electronegativity valuesElectronegativity (EN) of elements Difference in EN gives difference in polarity 3. Polarity of Molecules Polarity depends on Electronegativity valuesElectronegativity (EN) of elementsDifference in EN gives difference in polarityFluorine highest EN valueHF most polar difference in EN is HIGHEST HI least polar difference in EN is SMALLEST 4. Polarity of MoleculesPolarity also depends on shape (Symmetrical or Asymmetrical)Symmetrical Non Polar 5. Polarity of MoleculesPolarity also depends on shape (Symmetrical or Asymmetrical)Symmetrical Non Polar Asymmetrical - Polar 6. Polarity of MoleculesMolecule with a net dipole moment will have turn/spin if placed in an electric field 7. Bonding Forces - Intramolecular and Intermolecular1 Intramolecular forces of attraction 2 Intermolecular forces of attractionBonding within molecule Bonding between moleculesStrong bondingWeak BondingTHREE types THREE types1. Ionic Bonding 1 Temporary Dipole (VDF)2 Covalent Bonding 2. Permanent Dipole (VDF)3 Metallic Bonding 3 Hydrogen Bonding 8. Bonding Forces - Intramolecular and Intermolecular1 Intramolecular forces of attraction 2 Intermolecular forces of attractionBonding within molecule Bonding between moleculesStrong bondingWeak BondingTHREE types THREE types1. Ionic Bonding 1 Temporary Dipole (VDF)2 Covalent Bonding 2. Permanent Dipole (VDF)3 Metallic Bonding 3 Hydrogen Bonding1 9. Bonding Forces - Intramolecular and Intermolecular1 Intramolecular forces of attraction 2 Intermolecular forces of attractionBonding within molecule Bonding between moleculesStrong bondingWeak BondingTHREE types THREE types1. Ionic Bonding 1 Temporary Dipole (VDF)2 Covalent Bonding 2. Permanent Dipole (VDF)3 Metallic Bonding 3 Hydrogen Bonding Both are Van Der Waals forces1 2 10. 1Van Der Waals forces 11. 12Van Der Waals forces Van Der Waals forces 12. 312Hydrogen BondingVan Der Waals forces Van Der Waals forces Hydrogen Bonding 13. Factors affecting VDF forces of attractionRMM/Size1RMM up Van Der Waals up 14. Factors affecting VDF forces of attractionRMM/Size Surface Area1 2RMM up Van Der Waals up Surface area up Van Der Waals up 15. Factors affecting the boiling point of moleculesTemporary Dipole Moment (VDF)Permanent Dipole Moment (DP) Hydrogen Bonding (HB)Arrange the following Highest to Lowest Boiling Point12 16. Factors affecting the boiling point of moleculesTemporary Dipole Moment (VDF)Permanent Dipole Moment (DP) Hydrogen Bonding (HB)Arrange the following Highest to Lowest Boiling Point1Boiling Point Highest H2O > CI2 >N2 > H22Boiling Point Highest HF > HI > HBr > HCI 17. Factors affecting the boiling point of moleculesTemporary Dipole Moment (VDF) Permanent Dipole Moment (DP) Hydrogen Bonding (HB)Arrange the following Highest to Lowest Boiling Point34 18. Factors affecting the boiling point of moleculesTemporary Dipole Moment (VDF) Permanent Dipole Moment (DP)Hydrogen Bonding (HB)Arrange the following Highest to Lowest Boiling Point3Boiling Point Highest ICI > Br24Hydrogen Bonding in CH3CH2OHCH3CHO is polar has dipoleinteractionBoiling Point Highest CH3CH2OH > CH3CHO > C3H8 19. Factors affecting the boiling point of moleculesTemporary Dipole Moment (VDF) Permanent Dipole Moment (DP) Hydrogen Bonding (HB)Arrange the following Highest to Lowest Boiling Point5 20. Factors affecting the boiling point of moleculesTemporary Dipole Moment (VDF) Permanent Dipole Moment (DP) Hydrogen Bonding (HB)Arrange the following Highest to Lowest Boiling Point5Boiling Point Highest CH3COOH > CH3CH2CH2OH > CH3CH2OH > C2H5 -O-C2H5Why b/p CH3COOH > CH3CH2CH2OH 21. Factors affecting the boiling point of moleculesTemporary Dipole Moment (VDF) Permanent Dipole Moment (DP) Hydrogen Bonding (HB)Arrange the following Highest to Lowest Boiling Point5Boiling Point Highest CH3COOH > CH3CH2CH2OH > CH3CH2OH > C2H5 -O-C2H5Why b/p CH3COOH > CH3CH2CH2OH 123 22. Explain why NCI3 is polar while BCI3 in non polarNCI3 BCI3 VSPolarNon Polar 23. Explain why NCI3 is polar while BCI3 in non polarNCI3 BCI3 VSPolarNon PolarNCI3 is polarBCI3 in Non polar 24. Why 2 Nitrophenol has lower boiling point than 4 nitrophenol ?2 Nitrophenol 4 Nitrophenol1 1 25. Why 2 Nitrophenol has lower boiling point than 4 nitrophenol ?2 Nitrophenol 4 Nitrophenol1122 > Highest b/pLower than H2OLowest b/p 29. Which molecule is polar/non polar? 30. Which molecule is polar/non polar? 31. Which molecule has hydrogen bonding ? 32. Which molecule has hydrogen bonding ? 33. Polarity of Molecules for Cis/TransWHY? Cis b/p is HIGHER than Trans b/p Cis m/p is LOWER than Trans m/p 34. Cis b/p is HIGHER than Trans b/p Trans IsomerTrans Isomer bond polarity cancel No dipole 35. Cis b/p is HIGHER than Trans b/p Trans Isomer Cis IsomerTrans Isomer bond polarity cancel No dipole Cis Isomer bond polarity reinforce - Dipole 36. Cis m/p is LOWER than Trans m/p m/p = -50C m/p = -80CTrans IsomerTrans - linear structure, greater VDF interaction 37. Cis m/p is LOWER than Trans m/p m/p = -50C m/p = -80CTrans IsomerCis IsomerTrans - linear structure, greater VDF interaction Cis - bend structure, less VDF interaction 38. Video Tutorial on Hydrogen Bonding and PolarityH2 Bonding in HFH2 Bonding in H2OBond Polarity in detailed (Good tutorial)Bond Polarity in detailed (Good tutorial) 39. AcknowledgementsThanks to source of pictures and video used in this presentationThanks to Creative Commons for excellent contribution on licenseshttp://creativecommons.org/licenses/Prepared by Lawrence KokCheck out more video tutorials from my site and hope you enjoy this tutorialhttp://lawrencekok.blogspot.com