Fluorescence Microscopy Final
-
Upload
api-3845444 -
Category
Documents
-
view
129 -
download
3
Transcript of Fluorescence Microscopy Final

16 November 2007
By Chen Xiuli, Liu Yuchun, Ng Yen Shan, Cynthia Ong,
Adeline Sham & Eileen Yang
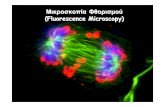
Fluorescence Microscopy

Overview Introduction
Principle of Fluorescence Microscopy Fluorescence Microscope Fluorescence Dyes
Experiment Objective Sample Preparation Experimental Procedures Results & Discussion
Comparison with AFM/NSOM Conclusion

Introduction
Fluorescence Microscopy
Source: White, N.S. & Errington, R.J. (2005). Fluorescence techniques for drug delivery research: theory and practice. Advanced drug delivery reviews 57 (2005) 17-42
•Increase in contrast•Chemical specificity•Dissect different functional aspects of biological systems

Imaging of specific regions of biological samples..
A cross section of cotton stained with Rhodamine B.
Source: http://nobelprize.org/educational_games/physics/microscopes/fluorescence/gallery/index.html
Fluorescence double-labeling of mammalian cells.

Pulmonary artery. Human cells.
Chinese hamster ovary cell. Rat tongue.

Principle of Fluorescence Microscopy
1. Introduction of fluorophore into sample
Source: 1. http:/nobelprize.org/educational_games/physics/microscopes/fluorescence/gallery/12.html

Principle 2. Excitation of Fluorophore
3. Stokes Shift
2. Shining Fluorescence Details. Basics of Light Microscopy and Imaging. Retrieved on Nov 10, 2007, from http://www.microscopy.olympus.eu/microscopes/images/4_ShiningFluorescencedetails.pdf

Fluorescence Microscopy
2 key aspectsFluorescence MicroscopeFluorescence Dyes

Olympus Fluorescent Microscope BX41

Olympus Fluorescent Microscope BX41
Internal Light Source
• Built-in transmitted Koehler illumination of 6V 30W halogen bulb
• For bright field imaging
External Light Source
• Mercury Arc Lamp
• For fluorescence imaging

Olympus Fluorescent Microscope BX41
Excitation & Emission Filters
• Band-pass filters
• Different filter sets are used for different dyes

Assembly of Fluorescence Imaging System

Fluorochromes
• Simultaneous/ multiple staining possible
• All fluorochromes show distinct spectral properties

Fluorochromes classification
1. Requires other molecules to bind to specific targets
2. Contains fluorescent proteins produced by organisms themselves
3. Have special properties• Inherent binding capacities• Fluorescent property developed by
enzymatic action

Fluorochromes with inherent binding capacity
Propidum iodide (PI)• An indicator for surface membrane
integrity• Binds to nuclei of dying or dead
cells• Excitation λ= 520 nm,
Emission λ= 620 nm (red)• Gives quantitative information

Fluorochromes with inherent binding capacity
4',6-Diamidino-2-phenylindole (DAPI)
• cell permeable• binds to the minor groove of double-
stranded DNA• Excitation λ= 350 nm,
Emission λ= 400 nm (blue)• often used as counter-stain

Fluorochromes developed by enzymatic action
Fluorescein diacetate (FDA)
• excitation λ = 480 nm, emission λ = 520 nm
• use to stain live cells
• quantitative information
FDA Fluorescein hydrolase
(colourless) (green fluorescence)

Experiments

Main experimentsUsing Pancreatic β-cells (β-TC-6) sample
To use fluorescence microscopy imaging technique to study cell viability of β-TC-6 cells
Additional experimentsUsing Human Umbilical Vein Endothelial Cells (HUVEC) sample – FDA and PI dyes
To reconfirm viability of dead and live HUVEC samples
Objectives

Sample Preparation
Samples β-TC-6 HUVEC
3 dyes for sample stainingPI and FDA to examine cell viability;DAPI acting as a counter stain.
Left to rightPI (PBS)– dark red 2mg/mlDAPI (DMF) – light yellowish 1mg/mlFDA (acetone) – colourless 5mg/ml
BTC 6
HUVEC
Dead (floating)
Live (adhered -> subcultured)

Sample cells cultured beforehand Extraction of dye – FDA/PI/DAPI
Adding dye to sample
Incubate
Wash with PBS
1 2
3 4
Experimental Procedures

Placed on microscope stage (bright field light source)
Adjusted to the correct filter
6 7
Focused, live previewed, imaged
Fluorescent light source
8
5

Results, Data Analysis & Processing

Results, Data Analysis and Results, Data Analysis and ProcessingProcessing
1. Cell viability
2. Rate of fluorescein efflux
3. Cell fixation
Experiments Conducted:Experiments Conducted:

1. Cell Viability
To determine which cells in a given sample are living and which are dead.

Bright FieldMicroscope
Image
DAPI: Stains all nuclei
FDA: Stains living cells
PI: Stains nuclei of dead cells
Viability Staining

Overlay
Red Subset (Dead Cells)Red Subset (Dead Cells) + + Green Subset (Living Cells)Green Subset (Living Cells)
= = Blue Set (All Cells)Blue Set (All Cells)
• To compare the relative distribution of cells of different colors
Red = dead cells
Green = living cells
Blue = all cells
Bright FieldBright Field OverlayOverlay
FDAFDAPIPI DAPIDAPI

Overlay
Overlapping Overlapping cellscells
Red = dead cells
Green = living cells
Bright FieldBright Field OverlayOverlay
• Generally, red and green do not overlap
• Patches of overlap due to multiple cell layers
FDAFDAPIPI

Experiment with HUVEC Cells
Sample ASample A
PIPI
FDAFDA
Sample BSample B
PIPI
FDAFDA
Trivia:Trivia:
Which sample is Which sample is living?living?

2. Rate of Fluorescein Efflux
Observation:Observation: Background Background
noise increased over time for noise increased over time for
fluorescein labeled cells.fluorescein labeled cells.
Hypothesis:Hypothesis: Fluorescein is Fluorescein is
constantly being transported constantly being transported
out of cells (active transport / out of cells (active transport /
passive diffusion).passive diffusion).
Experimental Aim:Experimental Aim: To observe To observe
the rate of efflux.the rate of efflux.
FDA Fluoresceinhydrolase
FDAFDA
Efflux
FluoresceinFluorescein

Fluorescein Efflux
00 11 44 77 TimeTime(min)(min)
1 min1 min 4 min4 min 7 min7 min
NoteNote: Not the same field of view: Not the same field of view

Fluorescein Efflux
7 min
7 min
• We observed fluorescein efflux but not PI or DAPI efflux.
• PI and DAPI are bound to the DNA in the nucleus.
• Cannot be moved out of the cells.

3. Cell Fixation
To determine the effect of fixation (using pure methanol) on fluorescent
staining of cells.

Cell Fixation
Steps:Steps:
After the usual After the usual staining procedure, staining procedure, immerse the slide in immerse the slide in cold methanol for 5 cold methanol for 5 min.min.
Take the slide out, let Take the slide out, let dry.dry.
Why it works:Why it works:
All the water in the sample (both inside and outside the cells) should be replaced by methanol, which evaporates during the drying step.
The cells are hence kept in a fixed position.
Also, without water as a medium, fluorescein efflux would not be observed.

Cell Fixation
FDAFDA PIPI OverlayOverlay
Green fluorescence is much weakerGreen fluorescence is much weakerthan red fluorescencethan red fluorescence
Green and red Green and red fluorescence fluorescence
overlap overlap completelycompletely

Cell Fixation
Unexpected observation:
all the cells in the sample
were dead. Probably because we left the
cells at room temperature for
too long.
Fluorescein efflux was
not observed even after
15 minutes.
OverlayOverlay
++FDAFDA PIPI

Ways to Improve Results
1. Avoid leaving cells outside the incubator for unnecessarily
long periods of time.
Prevents unnecessary cell death.
2. Culture cells for at least 3 days on the slide, so they have
sufficient time to adhere.
Alternatively, fix the cells on the slide.
3. Adjust exposure time according to objectives.
4. For more accurate experiments on viability, we suggest the
use of flow cytometry or other methods to count cells.

Comparison with AFM/NSOM images

AFM/NSOM vs. Fluorescence Microscopy
a) AFM
b) NSOM
c) Bright Field
d) Fluorescence

Figure : Stained H9C2 individual cell
Reference : Ianoul, Anatoli. Melissa Street and Donna Grant. "Near-Field Scanning Fluorescence Microscopy Study of Ion Channel." Biophysical Journal Volume 87 November 2004 3525–3535

Method Advantages Disadvantages
FluorescenceMicroscopy
High sensitivity High specificity in targeting Easy and fast
Out-of-focus flares Presence of auto-fluorescence Photobleaching effect of
fluorophores
Atomic ForceMicroscopy
High lateral and depth resolution Biological samples can be
studied in native state 3D surface profiling
Samples have to be very clean Sidewall angle induced artifacts
from inappropriate tip choice Long image acquisition time
Near-fieldScanningOpticalMicroscopy
High spatial resolution Minimal sample preparation Operated in ambient environment
Subjected to artifacts e.g. topography
Low depth of imaging Long image acquisition time

Conclusion
Principle of Fluorescence Microscopy
Experiment Preparation & Procedures
Fluorescence microscopy can be used to
quantify cell viability
Limitations & Comparison with AFM/NSOM
Fast and efficient method for biological
samples

Light path on a fluorescence microscope

Filter Nomenclature

Applications – Cell Staining
Dyes used Cell component stained
Color of stain
DiOC6(3) (3,3'-dihexyloxacarbocyanine
iodide)
Endoplasmic reticulum
Orange
Rhodamine 123 Mitochondria Green
Fura-2 Cytoplasm Depends on Ca concentration
Alexa Fluor 633 phallodin Actin filament Magenta