Experimental periodontitis induced in rats by streptococcal cell wall fragments
-
Upload
neil-hunter -
Category
Documents
-
view
213 -
download
0
Transcript of Experimental periodontitis induced in rats by streptococcal cell wall fragments

J. Periodontal Res. 14: 453-466, 1979
Experimental periodontitis inducedin rats by streptococcal cell
wall fragments
NEIL HUNTER, JOHN H. SCHWAB AND DAVID M. SIMPSON
Department of Bacteriology and Immunology, School of Medicine andDepartment of Periodontics, School of Dentistry, University of North Carolina,
Chapel Hill, North Carolina, U.S.A.
Bacterial cell wall fragments, consisting of peptidoglycan and associated polysaccharide orteichoic acid polymers were prepared from a group A streptococcus, a group D strepto-coccus, two Streptococcus mitis strains and Streptococcus sanguis. Injection of cell wallsinto rat gingival tissues resulted in self-limiting inflammatory lesions that reached a peakin 2 to 3 days and resolved by 4 to 7 days. An early polymorphonuclear leukocyte re-sponse was replaced by a mononuclear infiltrate characterized by a predominance ofmacrophages. Lymphocytes and plasma cells were not a common feature, even in animalsgiven repeated injections of cell walls into the same area. A wide range of changes wasseen in the alveolar bone, including loss of the periosteum with disappearance of osteo-blasts, osteoclastic bone resorption, hyperplasia of the periosteum with apparent osteo-blastic activity, and osteoid formation. Osteoclast-associated bone resorption was com-monly seen on the periodontal ligament aspect of the alveolar bone and less frequentlyon the periosteal surface. Neonatal thymectomy did not alter the response, and repeatedinjections did not increase the severity of the reactions.
(Accepted for publication February 12, 1979)
Introduction
It is recognized that the inflammatory pro-cess that characterizes gingivitis and pre-sumably also periodontitis in man is causallyassociated with the accumulation of micro-bial plaque in proximity to the gingival tis-sues (Page & Schroeder 1976, Socransky1977). Products or components of oralmicroorganisms are believed to be the prin-cipal etiologic agents in initiating and per-petuating the inflammatory lesions sinceviable microorganisms are not observedwithin the gingival tissue (Socransky 1970,Kelstrup & Theilade 1974).
sanguis are important components of earlysupragingival microbial plaque that formsabout the teeth in proximity to the gingivalmargin (van Houte, Gibbons & Pulkkinen1971, Socransky et al. 1977) and also havebeen demonstrated to be present in signifi-cant proportions in subgingival plaque inperiodontal pockets (Dwyer & Socransky1968, Darwish, Hyppa & Socransky 1978).These organisms comprise a high percentageof the total cultivatable flora in someplaques, depending on age of the plaque andcondition of the gingival tissue (Gibbons &van Houte 1973, Osterberg, Sudo & Folke1976, Loesche, Hockett & Syed 1977).
eisirup & lneuaae iy/4;. ly/o, l^uestuc, nocKcii OL oycu \.vi i).
Streptococcus mitis and Streptococcus Some reports demonstrate cellular anti-

454 H U N T E R , S C H W A B A N D S I M P S O N
gens of oral streptococci in inflamed perio-dontal tissues but not in clinically normaltissues (Tsutsui, Utsumi & Tsubakimoto1968, Wittwer, Toto & Dickler 1969, May-ron & LoiseUe 1973, Takeuchi et al. 1974).A recent study by Ranney (1978) using im-munofluorescent technique demonstratedsoluble plaque antigens within gingival tissuebiopsies from patients with periodontitis,although the bacterial identity of the anti-gens was not determined.
Previous reports from this laboratoryhave demonstrated the capacity of cell wallfragments isolated from group A strepto-cocci to produce a variety of inflammatorylesions in several experimental models: asingle intradermal injection of rabbits with asterile aqueous suspension of cell wallsinduces a recurrent, multinodular lesion ofthe skin (Schwab & Cromartie 1957); injec-tion into the knee joint of rabbits produces asynovitis (Schwab et al. 1967); intravenousor intraperitoneal injection of mice resultsin a pancarditis (Ohanian, Schwab & Cro-martie 1969), while systemic injection ofrats produces a remittent polyarthritis (Cro-martie et al. 1977). The essential cell wallstructure responsible for these models oftissue injury is the peptidoglycan-polysac-charide complex. The unique property ofgroup A streptococcal cell walls which ac-counts for the prolonged inflammation istheir resistance to biodegradation. Cell wallsfrom organisms such as group D strepto-cocci are readily degraded by lysozyme anddo not persist in tissue (Schwab & Ohanian
1967). These degradable cell wall prepara-tions can produce acute inflammatorychanges in tissue, but not chronic recurrentlesions. Oral streptococci have an inter-mediate susceptibility to lysozyme (Gins-burg & Sela 1976).
This paper reports on the inflammatorylesions produced in periodontal tissues bycell walls isolated from S. mitis and S.sanguis injected directly into the gingiva ofrats. The lesions are compared to those pro-duced by injection of cell walls from groupA and group D streptococci which havebeen studied extensively in tissues other thanperiodontium.
iUlateriais and iVIetiiods
Rat strains and injection technique. OutbredSprague-Dawley rats were purchased fromZivic-Miller, Allison Park, Pa. Inbred Buf-falo and Lewis rats were purchased fromSimonsen Laboratories, Bilroy, Ca. Females3 to 6 months old were used except in thethymectomy studies where both males andfemales were injected. Groups of rats wereinjected at one or more of three sites: thefacial aspects of the mandibular incisors, thefacial aspects of the first mandibular molarsand the palatal aspects of the first maxillarymolars. Cell wall fragments suspended inphosphate-buffered saline (PBS) were in-jected as follows: 20 ^g in 3 [il (dry wt/vol.)into the palate, 100 j.ig in 10 |xl in themandibular molar sites and 300 jxg in 50 |j,linto lower incisor sites. A Hamilton syringe
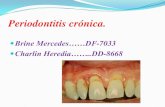
Fig. 1. Tissue response at 3 days following injection of S.]mitis (local insoiate) cell wall fragments into themandibular gingiva in a Sprague-Dawley rat. The pattern of osseous destruction along the lateral aspect ofthe alveolar bone suggests that the cell wail preparation has not localized entirely at the alveolar crest buthas extended apically along the periosteal surface of the bone. A. Destructive inflammatory process adjacentto alveolar bone which demonstrates advanced resorption below the crest (X25) HE. B. Infiammatory processhas eroded through alveolar bone and invaded the periodontal ligament (pi), which contains hyperemic vas-culature (X100) HE. C. Inflammation surrounding aiveoiar bone with resorptive lacunae on the ligament side.Apparent bony sequestrum(s) partially surrounded by epithelium is observable just beneath the sulcular epi-thelium. Clear spaces are artifacts of preparation (X100) HE. D. Higher power view of area outlined in C. In-flammatory cells consist of neutrophils and mononuclear cells. Osteoclasts are present adjacent to the bone(X450) HE.

E X P E R I M E N T A L P E R I O D O N T I T I S I N R A T S
wilh a 30 gauge needle was used. Usually, In addition, some control animals receivedthe opposite side of the jaw was Injected PBS only. The mandibular and palatal in-wilh an equal amount of PBS as a control, jections were given into the area of the at-

456 H U N T E R , S C H W A B A N D S I M P S O N
tached gingiva, as close to the crestalalveolar bone as possible. The incisal injec-tions were given into the alveolar mucosabelow the gingiva.
Neonatal thymectomy. Neonatal thymec-tomy was performed within 24 hr of birthby splitting the sternum vertically and re-moving each lobe of the thymus by a com-bination of blunt dissection and suction. Atsacrifice, the mediastimal contents wereprocessed for histology and sections wereexamined for thymus remnants. The animalsincluded in this study were completely thy-mectomized or had less than 10 % of thecontrol animal thymuses (Jankovic, Waks-man & Amason 1962).
Organisms and cell wall preparation.Group A streptococcus, type 3, strain D58;group D streptococcus, strain F24; 5. mitis,ATCC 6249; S. sanguis, ATCC 10556-1;and an S. mitis isolated by us from a humanwith periodontal disease, were grown inTrypticase Soy Broth (Baltimore Biologicals,Baltimore, Md.). Cell wall preparation, in-cluding digestion by ribonuclease and tryp-sin, has been previously described (Cro-martie et al. 1977). Aseptic technique wasobserved throughout the processing. Forinjections, lyophilized cell walls were sus-pended in PBS (20 mg/ml) and solubUizedby sonication in a Branson sonicator (Bran-son Ultrasonics Corp., Danbury, CT.) for70 min at maximum power. They were thenfiltered through a Millipore 0.45 [x filter.Sterility was confirmed by culture on sheepblood agar plates.
Histology and lesion evaluation. At inter-vals after injection animals were sacrificedand the jaws were formalin-fixed and decal-cified in formic acid - sodium citrate solu-tion. After embedding in paraffin, 6 ^ sec-tions were cut through each lesion at fourlevels, 500 ^ apart. Sections were stainedby haematoxylin and eosin (HE), periodicacid-Schiff reagent (PAS) and methyl green-pyronin (MGP) procedures. The PAS stain-
ing aided identification of polymorphonuc-lear leukocytes (PMN) and could be used toidentify cell wall material within macro-phages (Page, Davies & Allison 1974). Themethyl green-pyronin stain was used to aidin the identification of plasma cells andosteoblasts (Irving, Heeley & Socransky1975).
Grading of lesions. Lesions in the lowerincisor region were observed visually andgraded on the basis of degree of swelling:0—none, l + =up to 1.5 mm diameter,2 + = 1.5 to 3 mm, and 3+=greater than3 mm.
Histological preparations from each of thefour section levels were graded on a 0 to3+ scale for each parameter describedbelow. Lesions were scored with referenceto a section from a control site injectedwith PBS. All scoring was done by oneobserver.
Extent of the inflammatory process: 0=no inflammation, l + =inflammation local-ized to the immediate surface of the alveo-lar bone, 2 + = inflammation extendingabout half-way from the surface of thebone to the oral epithelium, 3 + = inflamma-tion which extended from the alveolar boneto the oral epithelium, completely fillingthe connective tissue space. Examples of a2+ response are illustrated in Figs. IA and2A.
Severity of the response: The extent ofhemorrhage, fibrin deposition and/or ne-crosis within the involved area of inflamma-tion was graded by the following scale: 0 =none, l + = < l / 3 , 2+--1/3 to 2/3, 3-f =>>2/3. An example of a 3+ response isseen in Fig. 2.
Presence of PAS-positive macrophages:This was assessed on a scale of 0 to 3 +based upon the relative abundance of thesecells within the inflammatory infiltrate,determined by their density and distribution.0 = no macrophages, 1 + = few scatteredcells; 2 + = scattered foci of macrophages;

E X P E R I M E N T A L P E R I O D O N T I T I S I N R A T S
Fig. 2. Buitaio rdi injected wilh S. mitis 6249 cell wall fiagmenis 3 days previously in mandibular molaf area.A. Survey view showing heavy inflammatory infiltrate along facial aspect of alveolar bone and osteoclastic re-sorption in the periodontal ligament space (X25) HE. B. View of alveolar crestal bone illustrating destructionof periosteum and adjacent connective tissue by an inflammatory process characterized by mononuclear cells,fibrin deposition and hemorrhage (XIOO) HE. C, Further down the alveolar bone the periosteum shows a hyper-plastic response in contrast to the invasion and destruction seen in B, The inflammatory infiltrate is locatedadjacent to the hyperplastic periosteum in this area [X100] HE. D. High magnification view from area outlinedin C. showing celluiarity of the periosteum with apparent deposition of osteoid (0) adjacent to bone surface1X450) HE.

458 H U N T E R , S O H W A B A N D S I M P S O N
3 4-= numerous macrophages foundthroughout the infiltrate.
Destruction of the periosteum: O=none,1 + = small foci of destruction, 2 + = u p to1/2 of the alveolar process, 3 + =greaterthan 1/2 of the alveolar process. Anexample of a 3-f response is shown in Fig.1 and a 2-f response in Fig. 2.
InfUtration of the periosteum by inflam-matory cells: This was scored as describedfor destruction. A 2+ response is illustratedin Fig. 2.
Hyperplasia of the periosteum: This wasassessed by MGP stain to confirm the pres-ence of large pyroninophilic osteoblasts,scored as described for destruction. A 3-+-response is illustrated in Fig. 2.
Osteoclast activity: Osteoclasts were con-sidered to be large, eosinophilic and usuallymultinucleated cells in proximity to bone orlying in lacunae at the bone surface. Scor-ing was done by counting the increase of thenumber of cells on both the periosteal andperiodontal ligament side of the bone fromthe alveolar crest to the apical extent of theinfiltrated area, compared to the same areaon the PBS control side. 1 + ^=2 to 4 ceUincrease, 2 -f = 5 to 8 cell increase, 3 -f —> 8 cell increase. Figures 1 and 2 repre-sent 3-f responses.
Effect of repeated injection of cell walls.Three groups of 20 Sprague-Dawley femalerats were injected 3 times with 300 [xg ofcell wall preparation into the tissue facialto the mandibular incisors. One group of20 rats received group A cell walls. A sec-ond group received group D cell walls anda third group received S. mitis (local isolate)cell walls. The 3 injections were given intothe same sites at intervals of 7 days. Thelesions were evaluated by visual inspectionat 24, 48, and 72 hr after each injection.Coinciding with the above injections, therats were injected in the mandibular molararea with the appropriate cell wall prep-aration. Two rats were killed for histo-
logical assessment at 24, 48, and 72 hr aftereach injection. Thus, 14 rats in each groupwere given 2 injections and 8 rats 3 injec-tions.
Comparison of cell wall preparations.Female Sprague-Dawley rats were injectedat incisal, palatal and mandibular molarsites as follows: 11 were injected with S.mitis 6249 cell walls, 5 were injected withS. sangius 10556-1 cell walls, 5 were in-jected with 5. mitis (local isolate) cell walls,11 were injected with group A cell walls.Incisal lesions were read at 24 hr, 48 hr and72 hr and the animals were then killed andprocessed for histological evaluation.
The effect of neonatal thymectomy andrat strain. Cell walls from S. mitis 6249were injected into the mandibular posterior,palatal and incisal sites of the followinggroups: 8 Sprague-Dawley thymectomizedand 6 sham-thymectomized, 6 Buffalo thy-mectomized and 7 sham-thymectomized,and 7 normal Lewis rats. The incisal lesionswere recorded on days 1, 2 and 3. All ratswere killed 3 days after cell wall injectionand tissue prepared for histological exam-ination.
Results
The study involved examination of lesionsin 165 rats. From the parameters of tissueinjury studied, it appeared that there wereno features unique to a particular cell wallpreparation or to a particular rat strain.
Histopathology of lesions. The followinggeneral description applies to observationsmade on all 5 cell wall preparations and tothe 3 rat strains following a single injection.In all animals, injection of cell wall frag-ments produced a lesion characterized bypolymorphonuclear leukocyte (PMN) infil-tration apparent by 3 hr and accompaniedin some cases by hemorrhage and fibrindeposition. By 48 to 72 hr after injection thepredominant feature was a diffuse infiltra-

E X P E R I M E N T A L P E R I O D O N T I T I S I N R A T S 459
Fig. 3. Inflammatory infiltrate with macrophages con-taining PAS-posltive granules in their cylopiasm 3days following injection. These cells were a prominentcomponent of the inflammatory process (X450) PAS.
tion of macrophages (Figs. 1, 2). NumerousPAS-positive granules were seen In thesecells, especially in the palatal injection sites(Fig. 3). Small and medium lymphocytescomprised only a small percentage of theinflammatory cells while plasma cells werenot seen in MGP-stained sections. Thecellular response reached a peak at 2 to 3days after injection with resolution occur-ring by 4 to 7 days. There was no evidence111' residual infiammation or relapse in ani-mals observed for 30 days post-injection.The onset of resolution was marked by theappearance of fibrobiasts at the inflamma-tory focus and a reduction in the number olmononuclear inflammatory cells.
In the alveolar bone adjacent to the in-flammatory sites a wide range of periostealchanges was seen, often within the same
section. In some cases the periosteum wasabsent or obliterated by massive inflamma-tory cell infiltration, and the alveolar bonecontained empty lacunae indicating loss ofosteocytcs, cften accompanied by saucer-like resorption along the outer surface ofbone (Fig. 1, A-D). This was especiallyevident In areas of necrosis consisting ofPMN debris and fibrin. Other periostealresponses to an intense adjacent inflamma-tion cansisted of heavy infiltration withmacrophages, or hyperplasia with evidenceof osteoblast activity leading to osteoidformation (Fig. 2). In some sections theperiosteum appeared normal in spite of theadjacent inflammation (Fig. 4).
In some lesions portions of the perio-dontal ligament were invaded by the in-flammatory infiltrate which eroded throughthe aiveoiar bone (P'ig. I}. Osteoclasts usu-ally were not found in areas of intense in-flammation but tended to be present at somedistance from or peripheral to such foci, orwithin the periodontal ligament opposite theinCiltrated area (Figs. 1, 2, 5). There was noobvious spatial relationship between granule-containing macrophages and osteoclasts.However, migration of attachment epithe-lium into the underlying connective tissuewas seen in some sections of palatal lesionsand heavy infiltrations of macrophageswere usually seen within this migrating epi-thelium (Fig. 6).
Comparison of cell wall preparations fol-lowing a single injection. There were nosignificant differences among the 5 cell wallpreparations by visual assessment of tissuereactions in the incisal mucosa.
The histological scores for palatal andmandibular lesions are shown in Table 1.Although there are quantitative differencesin responses produced by the cell wall prep-arations, there is no consistent pattern ofresponse which indicates meaningful differ-ences among the bacterial strains.
Effect of repeated injections of cell walls.

460 H U N T E R . S C H W A B A N D S I M P S O N
Fig. 4. Section Irom a Sprague-Dawley rat injected 3 iimes at 7 day intervals into the mandibulai molar areawith group A eel! wall fragments. A, A dense inflammatory mfiltrale is located adjacent to the alveolar crestbut no changes are observed in the bone or periosteum (X100) HE. B. An apparently normal periosteum (P) isobserved despite tha proximit/ of an intense mononuclBar infiltrate (X450) HE.
Following injection of Group A, Group Dor S. mitis ceil walls, visual assessments otthe incisai lesions were comparabie amongail three groups o! rats at the three time
intervals. Tlie results with group A cell wallsarc illustrated in Fig. 7. Repeated injectionsdid not protkitf more severe lesions; in fact,there was a trend for the second and third

E X P E R I M E N T A L P E R I O D O N T I T I S I N R A T S
Fig. 5. Palatal aspect of maxillary molar of Sprague- Dawley rat injected 3 days previously with Group A cellwall fragments, A. Osteoclastic resorption (arrows) of alveolar crestai bone adjacent to a minimal infiamma-lory infiltrate beneath tiie gingival sulcus (XIOO) HE. B, Higher magnification of osteoclasts at crestal boneadjacent to periodontai ligament (X450) HE.
injections to produce less severe reactions cant due to the large standard deviations ofand more rapid resolution. However, these the visual assessments.observations were not statistically signifi- Histoiogica! assessment of the mandibular
Table 1
Comparison of histologicai scores for palatal and mandibular lesions following a singleinjection in Sprague-Dawley rats
Bacterial Strain No. ol Extent of Severity PAS-rats Inflamma- of Positive
tion infiamma- Macto-tion phages
Periosteal Reaction OsteociastActivity
Destruc-tion
Infiltra-tion
Hyper.-plasia
S. mitis 6249
S. sanguis 10556-1
S. miiis (localisolate)
Group A
11
5
5
11
P-M
PM
PM
PM
1.13±D.922.09 + 0.84
0.60 + 0.761.40 ±0.91
0.1910.402.19±0.81
1.Q4±1,071.61 ±0.58
0,57 ±0.88
0.91±0,B7
0,24 + 0.440.68 ±0.69
•1.20 ±1.20
0.64 ±1.170.67 ±0.67
1.19 ±0.750.73 ±0,77
0.60 ±1.340.60 + 1.34
0.40 ±0.892,00±1.23
1.18 ±1.081.27 ±0.79
0.19±0.450.17±0.64
00.0810.28
00,30±0.66
0.3610.930.07 ±0.25
0.11 ±0,390.46 ±0.72
0.08 ±0.28O.G4±D.D2
00,05 ±0,22
0,09+0.290.26 ±0.44
0.16 + 0.570,37 ±0.71
00.21 ±0.51
0D.10±0.31
0.07 ±0.250.07 ±0.25
0.2510,53
0.7010.81
o.oa±o.2a0.2910.55
00.3010.47
0.7611.190.15 + 0.36
' = palate M = mandible

462 H U N T E R . S C H W A B A N D S I M P S O N
molar lesions confirmed the trend lor morerapid resolution in ail three groups. Becausehistological samples were obtained from only2 rats at eaeh time interval, statistical com-parison of scores was not possible. S. mitis(loeal isolate) — injected rats showed con-siderable destruction, infiltration and hyper-plasia of the periosteum together wilh in-creased numbers of osteoelasts. A feature ofthe group A and group D cell wall-injeetedrats was the finding of an intact periosteumadjaeent to intensely infiamed areas {Fig.4). There was iittle evidence of osteoclastactivity in preparations from group A andgroup D cell wali-injected rats foilowing
Fig. 6, Hyperplastic attachment epithelium Infiltratedby granuie-containing macrophages and neutrophits.Note that epithelium has migrated onto root surface(X450] HE,
Fig. 7. Visual assessment of incisal lesions in Spra-gue-Dawley female rats injected in the tissue anteriorto the lower incisor teath with cell wail fragmentsfrom Group A Btreptococci, Second and third injec-tions were made in the same site at 7 day intervals.
multiple injections. Maerophages with"foamy" cytoplasm were seen in prepara-tions from animals given repeated chal-lenges into the same site with group A cellwalls. Giant cells were seen in only oneanimal following repeated chailenge withgroup A cell walls.
Effect of thymectomy and rut strain. Neo-natai thymectomy of outbred Sprague-Daw-ley or inbred Buffalo rats did not influeneethe incisal lesion visual scores induced by asingie injection of S. mitis 6249 eeli wallswhen compared to sham-thymectoniizedcontrols or normal Lewis rats.
Tabie 2 shows the comparative histo-logical scores for paiatai and mandibulariesions. While there are some variations inthe scores for individual parameters. Iheoverall result does nofindicate a meaningfultrend for differences among the groups.From the tables it can be concluded thatthere are no response patterns unique toone partieuiar rat strain or to thymeeto-mized versus sham-operated animals. Theresults, however, point to a greater numberof PAS-positive macrophages in palatallesions and lo the consistent ability of S.

E X P E R I M E N T A L P E R I O D O N T I T I S I N R A T S 463
Table 2
The effect of thymectomy and rat strain on the response to S. tnitis 6249 cell walls
Rat strain
Sprague-Dawleysham
Sprague-Dawleythymectomized
Buffalo sham
Buffalothymectomized
Lewis normal
No. ofrats
6PM
8PM
7PM
6PM
7PM
Extent ofInflamma-
tion
1.3310.961.2110.79
2.1310.791.2610.77
2.39 + 0.991.61+0.92
2.8310.381.0 10.95
0.9611.111.8610.97
Severityof
Inflamma-tion
1.0011.381.1611.21
1.2211.390.8711.06
2.3911.131.5011.11
2.67 + 0.870.67 + 0.73
0.54 + 1.111.7111.27
PAS-Positive •Macro-phages
1.5011.380.83 + 0.75
2.0011.310.6310.74
1.7110.761.0 ±0.58
2.1310.700.1710.41
1.1411.470.14 + 0.38
Periosteal Reaction
Destruc-tion
0.0810.410
1.2511.370
2.00+1.250.18+0.61
2.4311.020
0.5011.040.2910.76
Infiltra-tion
0.54 + 0.660.6811.0
0.3810.760.2610.45
0.04 + 0.190.32 + 0.61
0.58 + 0.930.19 + 0.51
0.1110.420.64 + 1.13
Hyper-plasia
0.1310.340.0510.23
0.06+0.240.1310.34
0.29 + 0.460.82 + 0.72
0.25 + 0.440.91+0.94
0.21+0.500.8210.91
OsteoclastActivity
1.0411.080.5310.77
1.2511.140.1010.30
1.6411.130.93 + 0.94
2.17 + 0.870.24 + 0.54
0.50+0.960.5710.69
mitis 6249 cell walls to induce periostealreactivity in the form of inflammatory cellinfiltration and hyperplasia, as well as in-creased osteoclast activity, regardless of ratstrain or whether the animal was thymecto-mized.
Discussion
Several investigators (Gibbons et al. 1966,Sharawy & Socransky 1967) have shownthat alveolar bone loss could he induced ingnotobiotic or conventional rats by oral in-fection with streptococci of human origin.The present study demonstrates that perio-dontal inflammatory lesions which reflectcertain features of human periodontal dis-ease can be induced in rats by injectingstreptococcal cell wall fragments into thegingiva.
This experimental model did not attemptto reproduce the natural history of perio-dontal disease. We have studied only thetissue reaction to Gram-positive strepto-coccal cell walls by direct injection into thegingiva. Five bacterial strains were com-
pared to detect any unique association oflesion-inducing capacity with streptococciprominent in human dental plaque. Cell wallpreparations from all streptococcal strainstested induced similar types of inflammatoryresponses at injection sites.
The peptidoglycan and polysaccharidecomponents of cell walls are antigenic (Oha-nian et al. 1969) and the peptidoglycanmoiety has inherent toxic properties (Ab-dulla & Schwab 1966). Therefore, it is dif-ficult to distinguish between hypersensitiv-ity and toxic mechanisms of tissue injury.The following observations suggest that theimmune response is not essential for lesiondevelopment in this model: a) the inflam-matory changes do not become more severewith repeated injections of cell walls; b)neonatal thymectomy, which reduces cel-lular hypersensitivity and some antibody re-sponses in rats (Arnason et al. 1962, Janko-vic et al. 1962), has no measurable effect);c) the inflammatory cells consist largely ofdiffuse infiltrations of neutrophils and ma-crophages with few lymphoid celis and noplasma cells, which is not consistent with

464 H U N T E R , S C H W A B A N D S I M P S O N
cellular or humoral hypersensitivity in therat (Waksman 1970, Wilde, Cooper & Page1977).
A potential mechanism of tissue injurycould be provided by phagocytic cells whichhave been activated by ingestion of bacterialdebris. Several investigators have demon-strated chronic granuloma induction by in-jection of killed bacteria, the progression ofthe lesion correlating with persistence ofmicrobial components within macrophages(Ryan & Spector 1969, Spector, Reichold& Ryan 1970). Inability to degrade bacterialdebris can lead to extracellular release ofmacrophage enzymes or tissue-injuringagents (Page, Davies & Allison 1973, 1974).In addition, it has been shown that in vitrophagocytosis of group A streptococcal cellwalls activated macrophages to become cyto-toxic for target cells (Smialowicz & Schwab1977).
The rat periodontal lesions in this studyresolved within a few days regardless of thetype of streptococcal cell walls injected. Thecell wail debris was phagocytized by numer-ous macrophages, which contained exten-sive PAS-positive vacuoles within their cyto-plasm. The lesions did not persist or becomeremittent as they do in the rat arthritislesion following intraperitoneal or intra-venous injection of Group A streptococcalcell walls (Cromartie et al. 1977) and in therabbit dermal lesion (Schwab & Ohanian1967). The reason for lack of a remittentlesion following injection of group A cellwalls is not obvious. Resolution of thelesions suggests that the debris was removedfrom the periodontium by migration ofmacrophages to regional lymphatic tissue.Related to this, Adlersberg, Singer & Ende(1969) observed the apparent migration oflatex-bearing Kupffer cells from liver tolung and Ginsburg & Sela (1976) postulatedthat labeled cell wall fragments could betransferred between inflammatory foci bymacrophages.
Irving, Heely and Socransky (1975) in-jected cell walls from Actinomyces naes-lundii into rat gingival tissue. They notedan inflammatory response characterized bya predominantly lymphocytic infiltrationand early peak intensity, with resolutionbeginning by 24 to 48 hr post-injection.Macrophages were rare at the injection sitesand osteoblasts disappeared, although osteo-cytes remained intact. Their observationscontrast with the findings in the presentstudy where injection of streptococcal cellwall fragments induced a neutrophil andmacrophage infiltrate, with the bone re-sponse being more active and hyperplasia ofthe periosteum occurring concurrently withosteoclast-associated bone resorption.
Althotlgh osteoclastic bone resorption wasnot a feature following injection of A. naes-lundii cell walls by Irving et al. (1975), Ga-rant (1976) observed active alveolar crestalbone resorption by osteoclasts in rats mono-infected with A. naeslundii. The degree ofosteoclastic resorption appeared to correlatewith the amount of bacterial deposits onthe teeth and the extent of epithelial ulcer-ation, leading the author to speculate thatincreased penetration of bacterial productsinto the lamina propria resulted in increasedstimulation of osteoclasts.
The model used in the present study hasreproduced some of the features of humanperiodontitis, such as severe inflammatorydisruption of supporting connective tissues,epithelial migration onto the root surfaceand osteoclast-associated resorption of al-veolar bone. Destruction of the periosteum,periosteal hyperplasia, and inflammatory in-vasion of the periodontal ligament space areresponses not usually associated with humanperiodontal disease. However, direct injec-tion of bacterial cell walls adjacent to thealveolar bone is a severe insult compared tothe pathogenesis of human periodontitis,where it is likely that bacterial debris diffusesgradually into the connective tissue space in

E X P E R I M E N T A L P E R I O D O N T I T I S I N R A T S 465
relatively smaller quantities at any givensite. Macrophages and neutrophils accumu-late adjacent to and within the junctionaland pocket epithelium in human inflamma-tory periodontal disease, thereby phagocyt-izing some of the bacterial products beforethey reach deeper connective tissues or cre-stal bone. In addition, a constant source ofbacterial debris would be present over along period of time in human periodontitis,in contrast to one or several insults given inthe rat model in this study.
Acl(nowiedgnients
Supported by USPHS research grantDE3751 from the National Institute forDental Research and research grantAI13464 from the National Institute ofAllergy and Infectious Diseases.
References
Abdulla, E. M. & Schwab, J. H. 1966. Biolog-ical properties of streptococcal cell wall par-ticles. III. Dermonecrotic reaction to cellwall mucopeptides. /. Bacteriol. 91: 374—383.
Adlersberg, L., Singer, J. M. & Ende, E. 1969.Redistribution and elimination of intraven-ously injected latex particles in mice. /. Reti-culoendothel. Soc. 6: 536-560.
Arnason, G. G., Jankovic, B. D., Waksman,B. H. & Wennersten, C. 1962. Role of thethymus in immune reactions in rats. II. Sup-pressive effect of thymectomy at birth orreactions of delayed (cellular) hypersensitiv-ity and the circulating small lymphocyte. J.Exp. Med. 116: 177-186.
Cromartie, W. J., Craddock, J. G., Schwab, J.H., Anderle, S. K. & Yang, C.-H. 1977.Arthritis in rats after systemic injection ofstreptococcal cell walls. /. Exp. Med. 146:1585-1602.
Darwish, S., Hyppa, T. & Socransky, S. A.1978. Studies of the predominant cultivablemicrobiota of early periodontitis. /. Perio-dontai Res. 13: 1-16.
Dwyer, D. M. & Sccransky, S. S. 1968. Pre-dominant cultivable micro-organisms inhabit-ing periodontal pockets. Br. Dent. J. 124:560-564.
Garant, P. R. 1976. An electron microscopicstudy of the periodontal tissue of germfreerats and rats monoinfected with Actinomycesnaeslundii. J. Periodontal Res., Suppl. 15.
Gibbons, R. J., Berman, K. S., Knoettner, P.& Kapsimalis, B. 1966. Dental caries andalveolar bone loss in gnotobiotic rats infectedwith capsule forming streptococci of humanorigin. Arch. Oral Biol. 11: 549-560.
Gibbons, R. J. & van Houte, J. 1973. On theformation of dental plaques /. Periodontol.44: 347-360.
Ginsburg, I. & Sela, M. N. 1976. The role ofleukocytes and their hydrolases in the per-sistence, degradation and transport of bac-terial constituents in tissues: relation tochronic inflammatory processes in staphylo-coccal, streptococcal and myco-bacterial in-fections, and in chronic periodontal disease.Critical Reviews in Microbiology, vol. 4,issue 3: 249-332.
Irving, J. T., Heeley, J. D. & Socransky, S. S.1975. Cellular response to subgingival injec-tion of bacterial products in the rat. J. Perio-dontal Res. 10: 324-331.
Jankovic, B. D., Waksman, B. H. & Arnason,B. G. 1962. Role of the thymus in immunereactions in rats. I. The immunologic re-sponse to bovine serum albumin (antibodyformation, arthus reactivity, and delayed hy-persensitivity) in rats thymectomized or splen-ectomized at various times after birth. J. Exp.Med. 116: 159-176.
Kelstrup, J. & Theilade, E. 1974. Microbes andperiodontal disease. J. Clin. Periodontol. 1:15-35.
Loesche, W. J., Hockett, R. N. & Syed, S. A.1977. Reduction in proportions of dentalplaque streptococci following a 5 day topicalkanamycin treatment. /. Periodontal Res. 12:1-10.
Mayron, L. W. & Loiselle, R. J. 1973. Bac-terial antigens and antibodies in human perio-dontal tissue. J. Periodontol. 44: 164-166.
Ohanian, S. H., Schwab, J. H. & Cromartie, W.J. 1969. Relation of rheumatic-like cardiaclesions of the mouse to localization of groupA streptococcal cell walls. /. Exp. Med. 129:37.
Osterberg, S. K.-A., Sudo, S. Z. & Eolke, L. E.1976. Microbial succession in subgingivalplaque of man. /. Periodontal Res. 11: 243-255.
Page, R. C, Davies, P. & Allison, A. C. 1973.Effects of dental plaque on the productionand release of lysosomal hydrolases by macro-

466 H U N T E R , S C H W A B A N D S I M P S O N
phages in culture. Arch. Oral Biol. 18: 1481-1495.
Page, R. C , Davies, P. & Allison, A. C. 1974.Pathogen^is of the chronic inflammatorylesion induced by group A streptococcal cellwalls. Lab. Investigation 30: 568-581.
Page, R. C & Schroeder, H. E. 1976. Patho-genesis of inflammatory periodontal disease.A Nummary of current work. Lab. Invest. 33:235-249.
Ranney, R. R. 1978. Immunofluorescent local-ization of soluble dental plaque componentsin human gingiva affected by periodontitis./. Periodontal Res. 13: 99-108.
Ryan, G. B. & Spector, W. G. 1969. Naturalselection of long-lived macrophages in experi-mental granulomata. /. Pathol. 99; 139-151.
Schwab, J. H. & Cromartie, W. J. 1957. Stud-ies on a toxic cellular component of group Astreptococci. /. Bacteriol. 74: 673-679.
Schwab, J. H., Cromartie, W. J., Ohanian, S.H. & Craddock, J. G. 1967. Association ofexperimental chronic arthritis and the per-sistence of group A streptococcal cell walls inthe articular tissue. J. Bacteriol. 94: 1728-1735.
Schwab, J. H. & Ohanian, S. H. 1967. Degra-dation of streptococcal cell wall antigens invivo. /. Bacteriol. 94: 1346-1352.
Sharawy, A. M. & Socransky, S. S. 1967. Effectof human streptococcus strain GS-5 on cariesand alveolar bone loss in conventional miceand rats. /. Dent. Res. 46: 1385-1391.
Smialowicz, R. & Schwab, J. H. 1977. Cyto-toxicity of rat macrophages activated by per-sistent or biodegradable bacterial cell walls.Infect. Immun. 17: 599-606.
Socransky, S. S. 1970. Relationship of bacteriato the etiology of periodontal disease. J.Dent. Res. 49: 203-222.
Socransky, S. S. 1977. Microbiology of perio-dontal disease - present status and futureconsiderations. J. Periodontol. 48: 487-504.
Socransky, S. S., Manganiello, A. D., Propas,D., Oram, V. & van Houte, J. 1977. Bacteriological studies of developing supraging-iyal dental plaque. /. Periodontal Res. 12:•90-106.
Spector, W. G., Reichhold, N. & Ryan, G. B.1970. Degradation of granuloma-inducingmicro-organisms by macrophages. J. Pathol.101: 339-354.
Takeuchi, H., Sumitani, M., Tsubakimoto, K.& Tsutsui, M. 1974. Oral microorganisms inthe gingiva of individuals with periodontaldisease. /. Dent. Res. 53: 132-136.
Tsutsui, M., Utsumi, N. & Tsubakimoto, K.1968. Cellular components of staphylococciand streptococci in inflamed human gingiva.J. Dent. Res. 47: 663.
van Houte, J., Gibbons, R. J. & Pulkkinen, A.J. 1971. Adherence as an ecological deter-minant for streptococci in the human mouth.Arch. Oral Biol. 16: 1131-1141.
Waksman, B. H. 1970. Atlas of Experimentallmmunobiology and Immunopathology. NewHaven and London: Yale University Press.
Wilde, G., Cooper, M. & Page, R. C. 1977.Host tissue response in chronic periodontaldisease. VI. The role of cell-mediated hyper-sensitivity. 7. Periodontal Res. 12: 179-196.
Wittwer, J. W., Toto, P. D. &. Dickler, E. H.1969. Streptococcus mitis antigens in inflamedgingiva. J. Periodontol. 40: 639-640.
Address:
John H. SchwabDepartment of Bacteriology and ImmunologyUniversity of North CarolinaSchool of MedicineChapel Hill, North Carolina 27514U.S.A.