Evolution of magmas 1- Fractional crystallization: minerals formed.
-
Upload
nigel-hickcox -
Category
Documents
-
view
245 -
download
1
Transcript of Evolution of magmas 1- Fractional crystallization: minerals formed.

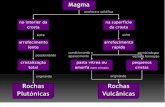
Evolution of magmas
1- Fractional crystallization: minerals formed.

Magmatic Differentiation• Two essential processes
1. Creates a compositional difference in one or more phases
2. Preserves the chemical difference by segregating (or fractionating) the chemically distinct portions

What processes allow magmas to differenciate?
• Fractionnal crystallization
• Liquid immiscibility
• Magma mixing
• Country-rock assimilation

1 - C Systems1 - C SystemsThe system SiOThe system SiO22
Stishovite
Coesite
- quartz
- quartz
Liquid
TridymiteCristobalite
600 1000 1400 1800 2200 2600
2
4
6
8
10P
ress
ure
(GP
a)
Temperature oC
After Swamy and Saxena (1994), J. Geophys. Res., 99, 11,787-11,794. AGU

2-C Eutectic Systems2-C Eutectic Systems Example: Diopside - AnorthiteExample: Diopside - Anorthite
No solid solutionNo solid solution
1274
Di 20 40 60 80 An
1200
1300
1400
1500
1600
T oC
Anorthite + Liquid
Liquid Liquidus
Diopside + Liquid
Diopside + Anorthite
1553
1392
Wt.% Anorthite
Isobaric T-X phase diagram at atmospheric pressure (After Bowen (1915), Amer. J. Sci. 40, 161-185.

a b
OrthocumulateAccumulated minerals in liquid

Amphibole (± Biotite) cumulatein a granite.

Augite forms before plagioclaseAugite forms before plagioclase
This forms on the This forms on the leftleft side of the eutectic side of the eutectic
Gabbro of Gabbro of the the Stillwater Stillwater Complex, Complex, MontanaMontana

Plagioclase forms before augitePlagioclase forms before augite
This forms on the This forms on the rightright side of the eutectic side of the eutectic
OphiticOphitic texture texture
Diabase dikeDiabase dike

Gravity settling
– Cool point a olivine layer at base of pluton if first olivine sinks
– Next get ol+cpx layer
– finally get ol+cpx+plag
Cumulate texture:Cumulate texture:Mutually touching Mutually touching phenocrysts with phenocrysts with interstitial crystallized interstitial crystallized residual meltresidual melt

Minerals that form during crystallizationMinerals that form during crystallization1250
1200
1150
1100
1050
1000
9500 0 0 010 10 20 10 102030 40 3050 40 50
Liquidus
Melt
Crust
Solidus
Olivine Clinopyroxene Plagioclase OpaqueT
emp
erat
ure
oC
Makaopuhi Lava LakeMakaopuhi Lava Lake
From Wright and Okamura, (1977) USGS Prof. Paper, 1004.


olivine Calcic plagioclase
Mg pyroxene
Mg-Ca pyroxene
amphibole
biotite(S
pin
el)
Te
mp
era
ture
potash feldspar muscovite quartz
alkalic plagioclase
Calci-alkalic plagioclase
alkali-calcic plagioclase
Bowen’s Reaction SeriesBowen’s Reaction Series
DiscontinuousDiscontinuousSeriesSeries
ContinuousContinuousSeriesSeries

Stoke’s Law
V = the settling velocity (cm/sec)
g = the acceleration due to gravity (980 cm/sec2)
r = the radius of a spherical particle (cm)
s = the density of the solid spherical particle (g/cm3)
l = the density of the liquid (g/cm3)
= the viscosity of the liquid (1 c/cm sec = 1 poise)
V2gr ( )
9
2
s l

Olivine in basalt
– Olivine (s = 3.3 g/cm3, r = 0.1 cm)
– Basaltic liquid (l = 2.65 g/cm3, = 1000 poise)
– V = 2·980·0.12 (3.3-2.65)/9·1000 = 0.0013 cm/sec

Rhyolitic melt = 107 poise and l = 2.3 g/cm3
– hornblende crystal (s = 3.2 g/cm3, r = 0.1 cm)
• V = 2 x 10-7 cm/sec, or 6 cm/year
– feldspars (l = 2.7 g/cm3)
• V = 2 cm/year• = 200 m in the 104 years that a stock might
cool• If 0.5 cm in radius (1 cm diameter) settle at
0.65 meters/year, or 6.5 km in 104 year cooling of stock

Two other mechanisms that facilitate the separation of crystals and liquid
1. Compaction

Two other mechanisms that facilitate the separation of crystals and liquid
2. Flow segregation






Ne Ab Q
1070 1060
1713
Ab + Tr
Tr + L
Ab + LNe + L
Liquid
Ab + L
Ne + Ab
ThermalDivide



Diopside-Albite-Anorthite
Di - An EutecticDi - An EutecticDi - Ab EutecticDi - Ab EutecticAb - An solid solutionAb - An solid solution
Figure 7-5. Figure 7-5. Isobaric Isobaric diagram illustrating diagram illustrating the liquidus the liquidus temperatures in the temperatures in the system diopside-system diopside-anorthite-albite at anorthite-albite at atmospheric pressure atmospheric pressure (0.1 MPa). After (0.1 MPa). After Morse (1994)Morse (1994), Basalts , Basalts and Phase Diagrams. and Phase Diagrams. Krieger PublushersKrieger Publushers

Isobaric polythermal projection
Figure 7-5. Isobaric diagram illustrating the liquidus temperatures in the system diopside-anorthite-albite at atmospheric pressure (0.1 MPa). After Morse (1994), Basalts and Phase Diagrams. Krieger Publishers.

> 4 Components
Figure 7-13. Pressure-temperature phase diagram for the melting of a Snake River (Idaho, USA) tholeiitic basalt under anhydrous conditions. After Thompson (1972). Carnegie Inst. Wash Yb. 71

olivine Calcic plagioclase
Mg pyroxene
Mg-Ca pyroxene
amphibole
biotite(S
pin
el)
Te
mp
era
ture
potash feldspar muscovite quartz
alkalic plagioclase
Calci-alkalic plagioclase
alkali-calcic plagioclase
Bowen’s Reaction SeriesBowen’s Reaction Series
DiscontinuousDiscontinuousSeriesSeries
ContinuousContinuousSeriesSeries