Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the...
-
Upload
ralph-heath -
Category
Documents
-
view
227 -
download
0
Transcript of Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the...
![Page 1: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/1.jpg)
Equilibrio
![Page 2: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/2.jpg)
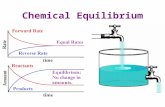
NOTE: [HI] at equilibrium is larger than the [H2] or [I2]; this means that the position of equilibrium favors products.
If the reactant concentrations decrease, then the forward reaction rate slows down.
![Page 3: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/3.jpg)
Express the equilibrium constant for the chemical equation.
Example 14.1 Expressing Equilibrium Constants for Chemical Equations
SOLUTION
For Practice 14.1
The equilibrium constant is the concentrations of the products raised to their stoichiometric coefficients divided by the concentrations of the reactants raised to their stoichiometric coefficients.
Express the equilibrium constant for the combustion of propane in the balanced chemical equation.
![Page 4: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/4.jpg)
Problem: Determine the Problem: Determine the KKcc value given the following concentrations at value given the following concentrations at equilibrium for the chemical reaction at room temperature: equilibrium for the chemical reaction at room temperature:
Given: 2 NOCl(g) 2 NO(g) + Cl2(g)
[NOCl]eq = 1.34
[NO]eq = 0.66
[Cl2]eq = 0.33
Solution: Kc = [NO]2 [Cl2] / [NOCl]2
Kc = {[0.66]2 [0.33]} / [1.34]2
Kc = (0.144)/(1.80)
KKcc = 0.0801 = 0.0801
Calculating Kc When Given Equilibrium Concentrations
![Page 5: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/5.jpg)
Example 14.8 Finding Equilibrium Concentrations When You Are Given the Equilibrium Constant and All but One of the Equilibrium Concentrations of the Reactants and Products
SOLUTION
Consider the reaction.
In an equilibrium mixture, the concentration of COF2 is 0.255 M and the concentration of CF4 is 0.118 M. What is the equilibrium concentration of CO2?
SORT You are given the equilibrium constant of a chemical reaction, together with the equilibrium concentrations of the reactant and one product. You are asked to find the equilibrium concentration of the other product.
STRATEGIZE Calculate the concentration of the product by using the given quantities and the expression for Kc.
![Page 6: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/6.jpg)
Example 14.8 Finding Equilibrium Concentrations When You Are Given the Equilibrium Constant and All but One of the Equilibrium Concentrations of the Reactants and ProductsContinued
For Practice 14.8Molecular iodine (I2) decomposes at high temperature to form I atoms according to the reaction:
In an equilibrium mixture, the concentration of I2 is 0.10 M. What is the equilibrium concentration of I?
SOLUTION
CHECK Check your answer by substituting the given values of 3COF2 4 and 3CF4 4 as well as the calculated value for 3CO2 4 back into the equilibrium expression.
SOLVE the equilibrium for [CO2] and then substitute in the appropriate values to calculate it.
[CO2] was found to be roughly equal to one. [COF2]2 ≈ 0.06 and [CF4] ≈ 0.12. Therefore Kc is approximately 2, as given
in the problem.
![Page 7: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/7.jpg)
Example 14.4 Writing Equilibrium Expressions for Reactions Involving a Solid or a Liquid
Write an expression for the equilibrium constant (Kc) for the chemical equation.
SOLUTIONSince CaCO3(s) and CaO(s) are both solids, you omit them from the equilibrium expression.
For Practice 14.4Write an expression for the equilibrium constant (Kc) for the chemical equation.
![Page 8: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/8.jpg)
K Value and Equilibrium
• K > 1: Product Favored • K < 1: Reactant Favored
![Page 9: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/9.jpg)
Q problem: Determining if a reaction is at equilibrium
Problem: Is the following reaction at equilibrium, and if not, in what direction must Problem: Is the following reaction at equilibrium, and if not, in what direction must the reaction proceed to obtain equilibrium?the reaction proceed to obtain equilibrium?
Given: 2 H2S(g) 2 H2(g) + S2(g)
Kp = 2.4 x 10-4 at 1073 K
0.112 atm = (H2)
0.055 atm = (S2)
0.455 atm = (H2S)
Strategy: 1. Write the equilibrium expression for the reaction.
2. Determine Q value.3. Compare calculated Q value to reaction’s Kc value.
![Page 10: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/10.jpg)
Answer: Is the following reaction at equilibrium, and if not, in what direction must : Is the following reaction at equilibrium, and if not, in what direction must the reaction proceed to obtain equilibrium?the reaction proceed to obtain equilibrium?
Given: 2 H2S(g) 2 H2(g) + S2(g) Kp = 2.4 x 10-4 at 1073 K
0.112 atm = (H2 )
0.055 atm = (S2)
0.455 atm = (H2S)
Solution: Q = {(H2)2 (S2)} / (H2S)2
Q = {(0.112)2 (0.055)} /( 0.455)2
Q = (6.90 x 10-4)/(0.455)2
Q = 3.33 x 10-3
Q value is GREATER than Kp value (Q > Kc).
Reaction value is NOT at equilibrium.Reaction must shift to the reactant side to achieve equilibrium.
![Page 11: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/11.jpg)
Example 14.2 Manipulating the Equilibrium Constant to Reflect Changes in the Chemical Equation
SOLUTIONYou want to manipulate the given reaction and value of K to obtain the desired reaction and value of K . You can see that the given reaction is the reverse of the desired reaction, and its coefficients are twice those of the desired reaction.
Begin by reversing the given reaction and taking the inverse of the value of K.
Calculate the value of K'.
Consider the chemical equation and equilibrium constant for the synthesis of ammonia at 25°C:
Calculate the equilibrium constant for the following reaction at 25°C:
Next, multiply the reaction bye ½ and raise the equilibrium constant to the ½ power.
![Page 12: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/12.jpg)
SORT You are given Kp for the reaction and asked to find Kc.
STRATEGIZE Use Equation 14.2 to relate Kp and Kc.
SOLVE Solve the equation for Kc.
Calculate Δn.
Substitute the required quantities to calculate Kc. The temperature must be in kelvins. The units are dropped when reporting Kc as described previously.
Example 14.3 Relating Kp and Kc
Nitrogen monoxide, a pollutant in automobile exhaust, is oxidized to nitrogen dioxide in the atmosphere according to the equation:
SOLUTION
Find Kc for this reaction.
![Page 13: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/13.jpg)
Continued
Example 14.3 Relating Kp and Kc
For Practice 14.3
CHECK The easiest way to check this answer is to substitute it back into Equation 14.2 and confirm that you get the original value for Kp.
Consider the following reaction and corresponding value of Kc:
What is the value of Kp at this temperature?
SOLUTION
![Page 14: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/14.jpg)
Example 14.5 Finding Equilibrium Constants from Experimental Concentration Measurements
1. Using the balanced equation as a guide, prepare an ICE table showing the known initial concentrations and equilibrium concentrations of the reactants and products. Leave space in the middle of the table for determining the changes in concentration that occur during the reaction.
2. Calculate the change in concentration that must have occurred for the reactant or product whose concentration is known both initially and at equilibrium.
A reaction mixture at 780°C initially contains [CO] = 0.500M and [H2] = 1.00 M. At equilibrium, the CO concentration is found to be 0.15 M. What is the value of the equilibrium constant?
![Page 15: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/15.jpg)
Example 14.5 Finding Equilibrium Constants from Experimental Concentration Measurements Continued
3. Use the change calculated in step 2 and the stoichiometric relationships from the balanced chemical equation to determine the changes in concentration of all other reactants and products. Since reactants are consumed during the reaction, the changes in their concentrations are negative. Since products are formed, the changes in their concentrations are positive.
4. Sum each column for each reactant and product to determine the equilibrium concentrations.
5. Use the balanced equation to write an expression for the equilibrium constant and substitute the equilibrium concentrations to calculate K.
![Page 16: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/16.jpg)
Example 14.5 Finding Equilibrium Constants from Experimental Concentration Measurements
1. Using the balanced equation as a guide, prepare an ICE table showing the known initial concentrations and equilibrium concentrations of the reactants and products. Leave space in the middle of the table for determining the changes in concentration that occur during the reaction.
2. Calculate the change in concentration that must have occurred for the reactant or product whose concentration is known both initially and at equilibrium.
A reaction mixture at 780°C initially contains [CO] = 0.500M and [H2] = 1.00 M. At equilibrium, the CO concentration is found to be 0.15 M. What is the value of the equilibrium constant?
![Page 17: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/17.jpg)
Example 14.5 Finding Equilibrium Constants from Experimental Concentration Measurements Continued
3. Use the change calculated in step 2 and the stoichiometric relationships from the balanced chemical equation to determine the changes in concentration of all other reactants and products. Since reactants are consumed during the reaction, the changes in their concentrations are negative. Since products are formed, the changes in their concentrations are positive.
4. Sum each column for each reactant and product to determine the equilibrium concentrations.
5. Use the balanced equation to write an expression for the equilibrium constant and substitute the equilibrium concentrations to calculate K.
![Page 18: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/18.jpg)
Example 14.9 Finding Equilibrium Concentrations from Initial Concentrations and the Equilibrium Constant
1. Using the balanced equation as a guide, prepare a table showing the known initial concentrations of the reactants and products. Leave room in the table for the changes in concentrations and for the equilibrium concentrations.
2. Use the initial concentrations to calculate the reaction quotient (Q) for the initial concentrations. Compare Q to K and predict the direction in which the reaction will proceed.
A reaction mixture (at 2000 °C) initially contains [N2] = 0.200 M and [O2] = 0.200 M. Find the equilibrium
concentrations of the reactants and products at this temperature.
![Page 19: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/19.jpg)
Continued
Example 14.9 Finding Equilibrium Concentrations from Initial Concentrations and the Equilibrium Constant
3. Represent the change in the concentration of one of the reactants or products with the variable x. Define the changes in the concentrations of the other reactants or products in terms of x. It is usually most convenient to let x represent the change in concentration of the reactant or product with the smallest stoichiometric coefficient.
4. Sum each column for each reactant and product to determine the equilibrium concentrations in terms of the initial concentrations and the variable x.
![Page 20: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/20.jpg)
Continued
Example 14.9 Finding Equilibrium Concentrations from Initial Concentrations and the Equilibrium Constant
5. Substitute the expressions for the equilibrium concentrations (from step 4) into the expression for the equilibrium constant. Using the given value of the equilibrium constant, solve the expression for the variable x. In some cases, such as Example 14.9 here, you can take the square root of both sides of the expression to solve for x . In other cases, such as the Example 14.10, you must solve a quadratic equation to find x.Remember the quadratic formula:
![Page 21: Equilibrio. NOTE: [HI] at equilibrium is larger than the [H 2 ] or [I 2 ]; this means that the position of equilibrium favors products. If the reactant.](https://reader036.fdocuments.net/reader036/viewer/2022081513/5697bff11a28abf838cbb7f0/html5/thumbnails/21.jpg)
Example 14.9 Finding Equilibrium Concentrations from Initial Concentrations and the Equilibrium ConstantContinued
6. Substitute x into the expressions for the equilibrium concentrations of the reactants and products (from step 4) and calculate the concentrations. In cases where you solved a quadratic and have two values for x, choose the value for x that gives a physically realistic answer. For example, reject the value of x that results in any negative concentrations.
7. Check your answer by substituting the calculated equilibrium values into the equilibrium expression. The calculated value of K should match the given value of K. Note that rounding errors could cause a difference in the least significant digit when comparing values of the equilibrium constant.
For Practice 14.9
Since the calculated value of Kc matches the given value (to within one digit in the least significant figure), the answer is valid.
The reaction in Example 14.9 is carried out at a different temperature at which Kc = 0.055. This time, however, the reaction mixture started with only the product, [NO] = 0.0100 M, and no reactants. Find the equilibrium concentrations of N2, O2 and NO at equilibrium.