Detection of monoclonal immunoglobulins ...immunoglobulins. Classically, thetechniqueinvolves the...
Transcript of Detection of monoclonal immunoglobulins ...immunoglobulins. Classically, thetechniqueinvolves the...

J Clin Pathol 1980; 33: 500-504
Detection of monoclonal immunoglobulins byimmunoelectrophoresis: a possible source of error
AM SMITH, RA THOMPSON, AND MR HAENEY
From the Supra Regional Protein Reference Unit, Department of Immunology, East Birmingham Hospital,Bordesley Green East, Birmingham, B9 5ST, UK
SUMMARY The technique of immunoelectrophoresis (IEP) is widely employed in the qualitativeanalysis of serum immunoglobulins. The most commonly used support media are agarose or agar
gels, but the mobility of immunoglobulins is different in these two media. The presence of a smallamount of a cathodal monoclonal immunoglobulin G may not be detected on IEP in agar if itis masked by larger amounts of polyclonal immunoglobulin of the same class. In these circum-stances the use of agarose imparts to the monoclonal protein a different mobility from that of thebulk of the serum IgG and allows its positive identification.
Since its introduction' immunoelectrophoresis (IEP)has proved a valuable tool in the qualitative investi-gation of proteins, particularly human serumimmunoglobulins. Classically, the technique involvesthe separation of proteins by electrophoresis in agel support medium, followed by visualisation of theseparated proteins using specific antisera. Initialseparation of the proteins depends on the chargecarried by each protein. Polyclonal immuno-globulins, being of varying amino-acid composition,will exhibit a spectrum of mobility, moving as abroad band on electrophoresis and producing apattern of smooth arcs with monospecific antiserato IgG, IgA, and IgM. A single clone of plasmacells synthesises immunoglobulin of one moleculartype only. A number of such molecules possessingidentical charge will travel as a discrete band onelectrophoresis, producing characteristic distortionof the polyclonal arc on visualisation with specificantisera.The diagnosis of a monoclonal immunoglobulin
is suggested by serum protein electrophoresis oncellulose acetate, agarose, or some other supportmedium. Its verification requires visual interpretationof precipitation patterns on IEP. If only a smallamount of monoclonal protein is present, there maybe insufficient distortion of the polyclonal arc toallow identification of the monoclonal protein,particularly in the presence of a polyclonal increaseof the same immunoglobulin class, and a faintgamma band seen on cellulose acetate electro-
Received for publication 3 September 1979
phoresis may then be interpreted as an artefact.This paper reports five cases in which the presenceof a monoclonal protein could not be shownconvincingly on IEP in agar, although all wereclearly visible after IEP in agarose.
Material and methods
IEP in agar was carried out using 1-5% agar in0-045 M barbitone buffer pH 8-6. The agar waspoured on to a glass plate (8 x 8 cm) to give a depthof agar of 1-7 mm. The pattern of sample wells andantiserum troughs was cut as required. Electro-phoresis of sera was carried out in an electrophoresistank (Shandon Southern Limited) containing0045 M barbitone buffer pH 8-6 at a current ofapproximately 20 mA per plate for 45 minutes. Thetroughs were then filled with the relevant antiseraand the plates incubated at room temperatureovernight.
In this laboratory, a mixture of three monospecificantisera (anti-IgG, anti-IgA, and anti-IgM) isemployed. These antisera were raised in sheep andabsorbed where necessary to render them mono-specific. Antisera to kappa or lambda light chainswere obtained from Kallestadt Laboratories.IEP in agarose was carried out using the Corning-
ACI agarose film cassette system. This systemincludes pre-poured gel plates of 1% agarose in0-065 M barbital buffer, containing 0-035 % disodiumEDTA pH 8 6 and 50 g/l sucrose. The pre-formedplates contain wells for the addition of test serumand troughs for the addition of antisera afterelectrophoresis. Electrophoresis is carried out in
500
on August 25, 2020 by guest. P
rotected by copyright.http://jcp.bm
j.com/
J Clin P
athol: first published as 10.1136/jcp.33.5.500 on 1 May 1980. D
ownloaded from

SerumNdrmaI
AniIgG
Normal
kapaAntikTest A chain_ _ = X {~~~~~~~ightchain
Test C
Normal
Anti lambdalight chain
Test
Test_...
=4.
Nlormal
Test
Anti wholehuman serum
Anti lgG
Anti IgANormat
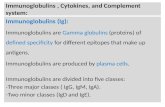
Fig. I Putient 1:(a) Electrophopre.si.sof serum in agartose.(b) Ininuno-electrophoresis of.serum in cigar.
(c) Inmmuno-electrophoresis of'.serumn in Jgar-ose.
Anti IgM
Test
NormalAnti kappalight chain
Anti lambddalight chain
Anti IgG,IgA, IgM
Test
Normtl
A Hpp iationwel
Cathode
P utientsserum
Anode
Normalserum
0Serum
.iiiill' "'
:A
on August 25, 2020 by guest. P
rotected by copyright.http://jcp.bm
j.com/
J Clin P
athol: first published as 10.1136/jcp.33.5.500 on 1 May 1980. D
ownloaded from

2D Applicationwel
Patientsserum
AnodeCathode
Normalserum
SerumTest
IX
............
Normal ;
Test
..- ...
Normal
Test
Serum
Normal
.:.=.......z ~~~~~~~~~A;'tL IgGTest
Normal
~Anti kappaTest light chain
Normal
*_4 "1111kAnti lambdaTe = _ light chain
Anti wholehuman serum
Anti IgO
Fig. 2 Patient 7:(a) Electrop/wresis
Anhi IgA of serum in agarose.(b) Immuzno-electrophlresis ofserum in cigar.(c) Iuniuno-
A nti 1g M electroplmresis ofserum in agcarose.
Anti kappalight chain
Anti lambda-light chain
Anti IgG.*^kt>*- <- ^IgA. IgM
Normal
Test
Norma l
on August 25, 2020 by guest. P
rotected by copyright.http://jcp.bm
j.com/
J Clin P
athol: first published as 10.1136/jcp.33.5.500 on 1 May 1980. D
ownloaded from

Detection of monoclonal immunoglobulins by immunoelectrophoresis; a possible source of error 503
barbital buffer 005 M containing 0.035% EDTAat pH 8-6. The cassette system comprises an electro-phoresis tank and pre-set power supply, the timefor an electrophoretic run being 30-40 minutes.After electrophoresis and the addition of antiserathe plates are incubated overnight. The antiserausually employed are provided by Corning Medicalas part of the above system and were found ontesting to be monospecific for the stated antigen.The precipitation lines so formed may be enhancedby staining with Amido Black.
Results
Figures 1 and 2 show comparisons of electro-phoresis and immunoelectrophoresis of two of thefive sera in agar and agarose. In all cases the mono-clonal protein in question is the IgG class. Theresults for IEP in agar show only the anti-IgG andanti-light chain plates. The Corning agarose systememploys a battery of antisera, and the full resultshave been shown.
In each case diagnosis of the monoclonal IgGwas difficult on the agar plates, but in the agarosesystem, the presence of a monoclonal IgG was quiteobvious. Relevant laboratory details for each of thefive cases are shown in the Table. IEP in agarosehas also been of value in the confirmation of thepresence of monoclonal IgM in sera.
Since the purpose of this study was to show avariation in results using different media, it wasnecessary to use the same antisera for both methods.The antisera usually employed in this laboratorywere used in the Corning system in place of theCorning antisera. (A previous comparison of ourin-house antisera and the Corning antisera had, infact, shown identical results.) Preincubation of thetest sera with agar or agarose did not affect themobility of the monoclonal immunoglobulin.
Discussion
All five sera cited in this report contained a mono-clonal IgG on IEP in agarose, which were difficultto visualise by IEP in agar. None of the patientshad multiple myelomata as defined by acceptedcriteria:2 all were probable examples of 'monoclonalgammopathy'. It is well recognised that paraproteinsmay be detected in as many as 3% of patients agedover 70 years.3The increasing use of cellulose acetate electro-
phoresis to screen serum samples undergoingbiochemical or immunological investigations hasled to the frequent accidental detection of manyasymptomatic monoclonal proteins.
Occasionally difficulty is found in the definitiveidentification of the nature of this monoclonal band.Positive identification of the immunoglobulin classinvolved is important in the long-term follow-up ofsuch patients since serum paraproteins may bedetected many years before clinical presentation withmalignant immunocytoma. Also, in patients receiv-ing chemotherapy for myeloma, assessment of thetumour size by serum paraprotein studies allowsimportant monitoring during treatment. It isimportant, therefore, to establish that the apparentfailure to detect a monoclonal protein on IEP is notan artefact due to the use of an inappropriatesupport medium. We have shown that, in some ofthese cases, the use of a different gel allows positiveidentification of the immunoglobulin class of theparaprotein.
References
Grabar P, Williams CA. Methode permettant l'etudeconjugee des proprietes electrophoretiques et im-munochimiques d'un melange de prot6ines. Applica-tion au serum sanguin. Biochem Biophys Acta1953;10:193-4.
Table Laboratory details offive cases
Case AgelSex Band on IEP findings in Immunoglobulin levels (g/litre)cellulose acetateelectrophoresis Agar Agarose IgG IgA IgM
1 85 M Normal Monoclonal 8.45 1 90 1-40IgG (A)
2 98 F y Normal Monoclonal 12-55 3.55 0 65IgG (A)
3 73 F y Normal Monoclonal 14-40 2.65 050IgG (K)
4 69 F y Equivocal Monoclonal 19-40 5.40 1-40IgG (A)
5 84 M y Equivocal Monoclonal 7-15 2 85 1-30IgG (K)
on August 25, 2020 by guest. P
rotected by copyright.http://jcp.bm
j.com/
J Clin P
athol: first published as 10.1136/jcp.33.5.500 on 1 May 1980. D
ownloaded from

504 Smith, Thompson, and Haeney
2 Medical Research Council. Report on the first myelo-matosis trial. Part I. Analysis of presenting featuresof prognostic importance. Brit J Haematol 1973;24:123-39.
3 Hallen J. Discrete gammaglobulin (M-) components inserum. Clinical study of 150 subjects without myelo-
matosis. Acta Med Scand 1966;Suppl :462.
Requests for reprints to: Mr AM Smith, Supra RegionalProtein Reference Unit, Department of Immunology,East Birmingham Hospital, Bordesley Green East,Birmingham B9 5ST, UK.
Reports and Bulletins prepared by the Association of Clinical BiochemistsThe following reports and bulletins are published by the Association of Clinical Biochemists. They may be obtainedfrom The Publishing Department, British Medical Journal (ACB Technical Bulletins), BMA House, TavistockSquare, London WC1H 9JR. Overseas readers should remit by British Postal or Money Order.
SCIENTIFIC REVIEWS (price £1 00/$2.00 each)
1 The assessment of thyroid function March 1971FV FLYNN and JR HOBBS
2 Renal function tests suitable for clinical practiceJanuary 1972 FL MITCHELL, N VEALL, and RWE WATTS
3 Biochemical tests for the assessment of fetoplacentalfunction May 1975 CE WILDE and RE OAKEY4 Test of exocrine pancreatic function March 1977AH GOWENLOCK
5 Assay of cholinesterase in clinical chemistry March1979 ELSIE SILK, J KING, and MARY WHITTAKER
TECHNICAL BULLETINS (price £1 00/$2.00 each)
22 Bilirubin standards and the determination of bilirubinby manual and technicon AutoAnalyzer methodsJanuary 1971 BARBARA BILLING, RUTH HASLAM, and NWALD
23 Interchangeable cells for spectrophotometers andfluorimeters September 1971 SS BROWN and AHGOWENLOCK
24 Simple tests to detect poisons March 1972 BWMEADE et al.
25 Blood gas analysers May 1972 K DIXON
26 Kits for enzyme activity determination September1972 SB ROSALKI and D TARLOW
27 Assessment of pumps suitable for incorporation intoexisting continuous flow analytical systems November1972 A FLECK et al.
28 Routine clinical measurements of transferrin inhuman serum September 1973 K DIXON
29 Control materials for clinical biochemistry (5thedition) September 1973 JF STEVENS
30 Notes on the quality of performance of serumcholesterol assays September 1973 SS BROWN
31 Determination of uric acid in blood and in urineJuly 1974 RWE WATTS
32 A survey of amino acid analysers readily available inthe United Kingdom September 1974 JE CARLYLEand P PURKISS
33 Definitions of some words and terms used in auto-mated analysis November 1974 A FLECK, R ROBINSON,SS BROWN, and JR HOBBS
34 Measurement of albumin in the sera of patientsJanuary 1975 LINDA SLATER, PM CARTER, and JR HOBBS
35 Investigation of the validity of temperature correctionfactors for serum aspartate and alanine transaminasesMarch 1975 SB ROSALKI et al.
36 Factors influencing the assay of creatinine November1975 JGH COOK
37 A survey of enzyme reaction rate analysers readilyavailable in the United Kingdom July 1977 RASAUNDERS and RF BURNS
38 Transport of specimens for clinical chemistry an-alysis November 1977 P WILDING, JF ZILVA, andCE WILDE
39 A scheme for the evaluation of diagnostic kits May1978 PH LLOYD
40 A practical guide to gamma-counting in radio-immunoassay January 1980 CE WILDE and D OTTEWELL
on August 25, 2020 by guest. P
rotected by copyright.http://jcp.bm
j.com/
J Clin P
athol: first published as 10.1136/jcp.33.5.500 on 1 May 1980. D
ownloaded from