desene diverticule
Transcript of desene diverticule

140 Indian Journal of Surgery 2004 Volume 66 Issue 3 (June)
Duodenal diverticulum: Review of literature
Sanjay K.Mahajan, Rajesh Kashyap, Upender K. Chandel*, Jatinder Mokta, Satinder S. Minhas*Department of Medicine and *Surgery, Indira Gandhi Medical College, Shimla - 171001, India.
ABSTRACTDuodenal Diverticulum was first reported by Chomell in 1710 and was regarded an anatomic curiosity until 1913when first radiological demonstration was done by JT Case. With modern radiological techniques and widespreaduse of endoscope it has been found that these diverticula occur more frequently than was formely supposed. Mostof these are asymptomatic, situated in second part of duodenam and are rarely associated with complication whichare usually cause of presentation.
KEY WORDSDuodenal Diverticulum, Extra luminal Duodenal Diverticulum, Periampullary Duodenal Diverticulum, Juxta papillaryDuodenal Diverticulum. Intra luminal Duodenal Diverticulum
How to cite this article: Mahajan SK, Kashyap R, Chandel UK, Mokta J, Minhas SS. Department of Medicine and Surgery, Indira GandhiMedical College, Shimla-171001, India. Duodenal Diverticulum: Review of literature. Indian J Surg 2004;66:140-5.
Review Article
Address for correspondence: Dr. Sanjay Mahajan, 31/3, US Club, Shimla-171001, India. E-mail: [email protected] Received: July 2003. Paper Accepted: October 2003. Source of Support: Nil.
INTRODUCTION
Duodenal diverticula was first reported by Chomall in
1710 and first well documented report was made by
Morgagni in 1762 and it was regarded an anatomic
curiosity until 1913 when radiological demonstration
was done by JT Case, who displayed roentgenograms
of 4 cases in the Scientific Exhibit of the American
Medical Association. With lengthening of life span,
diverticulosis has come to occupy a more important
position in the sphere of clinical gastroenterology.
Duodenum is second most common site of diverticula
in alimentary tract after colon followed by jejunum,
ileum and stomach.2,3 Duodenal diverticula are found
in up to 25% of patients, but rarely cause symptoms
when complications occur, early diagnosis is essential
if treatment is to be successful.3 Diverticula of
duodenum are classified as primary and secondary.
Majority of secondary or false diverticula are result of
chronic duodenal ulceration, so called prestenotic
diverticulum where as primary are true diverticula.4
These occur mainly in later decades of life with peak
incidence between 50 and 60 years of age and it
increases with age.1,2,4-6 There is no gender
predisposition but female preponderance has also been
reported.1-5 Incidences varies with diagnostic method
used.3 In UGI barium series it is 0.016 to 6%,2,4-8 in
autopsy series 22 to 23%,2,4,5,7 where as on ERCP studies
incidence is 9 to 23%.6,7 Over 95% of duodenal
diverticula project from inner or pancreatic border of
duodenal curve in second, third and fourth parts.
Second part is most common site with 85 to 90% of
total DD.1,2,4,9 Third and fourth parts of duodenum have
20 and 10% of diverticula respectively,2 but up to 30
to 40% of these may arise from third and fourth part of
duodenum.10 Diverticulum may be single or multiple
and as many as 6 or more has been reported. Incidence
of multiplicity at x-ray series is 1.4 to 23.5% and in
autopsy series it is 3.5 to 30%. These are usually
spherical or hemispherical and when filled with barium
usually oval in shape often resembling Erlenmeyer flask
showing narrow neck.2 These are classified into EDD
and IDD. EDD are more common3 and can be further
classified into PAD and JPDD. PADS are extra luminal
mucosal out pouching of duodenum arising adjacent
to or containing the ampulla of Vater or intraluminal
portion of CBD (common bile duct). JPDD are defined
as EDD located with in radius of 2 cm of major papilla
but not containing it. Some has referred to above
definition as ampullary and periampullary while others
refer both type of EDD collectively as JPDD. However,
consistent and precise terminology needs to establish.
About 75% of EDD occur with in 2 cm of ampulla of
Vater.2-6,10-12
© 2004 Indian Journal of Surgery www.indianjsurg.com

141
© 2003 Indian Journal of Surgery www.indianjsurg.com
Indian Journal of Surgery 2004 Volume 66 Issue 3 (June)
AETIOLOGY AND PATHOLOGYThese are true pouches or sacs protruding from
duodenal lumen not caused by any recognizable
intrinsic or extrinsic disease. EDD are acquired and
consist of a sac of mucosal or sub mucosal layers
herniated through a muscular defect in bowel wall but
precise manner of the development is not known.2,6,10
However, there exists a locus minoris resistentiae which
may be created by (i) passage of the biliary, pancreatic
duct and blood vessels through the wall of duodenum.
(ii) Congenital absence of an adequate muscle coat (iii)
heterotrophic pancreatic tissue. Once diverticulum is
formed it may increase in size through the years.2,6,10
The position of fundus and body of EDD is variable and
mostly lie in retro peritoneum with large portion lying
close to concave medial surface close to CBD and
pancreatic duct. Rarely, the diverticula may be localized
on the convex side of the second part of duodenum.
The sac lie along side, extend behind or even penetrate
into the pancreas and occasionally CBD and duct of
Wirsung may open into the diverticulum3,4,6 IDD or
windsock diverticula are not true diverticula and occur
as single saccular structure which are connected to the
entire circumference or only part of the wall of the
duodenum. Only less than 100 cases have been
reported in the literature. During early foetal life
duodenal lumen is initially occluded by proliferating
epithelial cells and recanalises later on. Abnormal
recanalising may lead to a duodenal diaphragm or web
which may not produce symptoms in child hood
however over the period of time peristaltic stretching
may transform it into an IDD. IDD may project as distally
as fourth part of duodenum and often a second opening
is located eccentrically in the sac and both sides of the
diverticulum are lined by mucosa.3,6,10
Pathologically the larger pouches usually lack a
muscular coat although often the smaller one do contain
some fibers. Attempts have been made to divide
duodenum diverticulum in to true or congenital
pouches and false or acquired pouches. If all muscle
coats are presents it was termed true or congenital
and if muscle layer was not present it was thought to
be acquired or false, such a division seems untenable
as there is nothing to prove that certain pouches are
basically congenital and others entirely acquired. The
amount of muscle fibers in the pouch depends more
upon its age and size. Histologically in majority, the
circular and longitudinal muscle coats of duodenum
are missing and the mucosa and muscularis mucosae
make up the wall of the diverticulum.2
CLINICAL FEATURESGreat majority of duodenal diverticula are
asymptomatic.2,4,6,7,9,10 Clinical presentation may be
characterized by non-specific abdominal symptoms4
and less than 5% of patients have abdominal
symptoms.2 Abdominal discomfort is usually located
in epigastrium, right upper abdomen or umbilical area
which is made worse or brought on by eating and
relieved by vomiting, belching or assuming certain
posture.2,4,6 There are no characteristic symptom
complex from which one may make a positive
diagnosis of DD. Many believe that there are three main
factors in production of symptoms.
A. Mechanical causes producing
1. Delayed empting of diverticula
2. Pressure on the common bile or pancreatic duct
3. Obstruction of the duodenum
B. Inflammatory causes producing
1. Symptoms simulating peptic ulcer, gall bladder
or pancreatic disease
2. Pyloro-spasm
3. Perforation
C. Neoplasia
Patients may complain of pain radiating to the back
between the scapulae and between costo-vertebral
angles. The pain caused by the diverticula in the third
part of the duodenum is situated near the mid line
where as that caused by the pressure upon the pancreas
may be situated to the left. The peptic ulcer like
syndrome has been noted in several patients, however
in the absence of ulcers, the symptoms may be due to
inflammation and stasis in the sac. The simulation of
peptic ulcer disease by DD may be close. Intermittent
diarrhea and constipation, weight loss because of the
fear of eating and steatorrhea may also occur in some
patients.2,3,10,11
The symptoms simulating primary biliary tract disease
may occur in DD located near ampulla of Vater and
some time CBD may terminate in the diverticulum. The
“perivaterian diverticulitis” may give rise to obstructive
jaundice. The greater propensity for the pigment stones
in the presence of EDD is not fully understood and
excess release of calcium bilirubinate by ascending
Beta-Lucuronidase colonization into CBD has been the
possible cause.2,3,5 Several authors have shown that
compression of CBD, dysfunctions of ampulla or a
poorly emptying diverticulum with a narrow neck can
lead to pancreatico-biliary disease and acute
Mahajan SK, et al

142 Indian Journal of Surgery 2004 Volume 66 Issue 3 (June)
pancreatitis. Some even suggested that PAD be
included in the aetiology of acute pancreatitis especially
in the elderly.13 JPDD are important causative factors in
the bile duct stone formation.14,15 Since
choledocholithasis leading to biliary obstruction is a
well known etiology of acute pancreatitis there is
debate whether the increased incidence of pancreatitis
with EDD is due primarily to the mechanical
complications or associated biliary stones. As most DD
occur on the concave border of second part of
duodenum in close contact with or embedded in to
the head of pancreas, perforation of diverticulum can
also cause pancreatitis.2,5,6 The majority of IDD, in
contrast to EDD, will become symptomatic and can
present at any age from the first through the eighth
decade of life. The most common symptoms are those
of incomplete duodenal obstruction by retention of
vegetable or foreign bodies. Pancreatitis and
hemorrhage has also been reported in IDD. In
childhood cases 20% can have symptoms and it is to
be dif ferentiated from duodenal intrinsic defect,
extrinsic defects, and anal rotation with congenital
bands.3,6,10,16
COMPLICATIONSAs diverticula are usually asymptomatic, complications
are responsible for presentation most of the times.11
The complications may be divided into two groups.
Those related to pressure on the adjacent structures
and those caused by inflammation. Jaundice,
cholangitis, acute and chronic pancreatitis and
duodenal obstruction are caused as a result of
mechanical compression by diverticula, whereas
diverticulitis and ulceration are caused by
inflammation.2-4,6,10,11
Diverticulitis and PerforationWhen drainage of sac is inadequate or neck is narrow
or ostium is buried, these conditions favour
inflammation and may lead to perforation which in turn
may lead to localized abscess formation, generalised
peritonitis. Ulceration, enteroliths and foreign body can
be other reasons for per foration. The on set of
perforation is acute in majority but can be gradual also.
The perforated diverticulum should be considered in
the presence of bile stained phlegmon in the para-
duodenal area, retroperitoneal edema or emphysema,
retroperitoneal yellow flaky exudates or pus or a right
sub-hepatic abscess.
Ulceration can occur because of presence of ectopic
gastric mucosa or inflammation. Diverticular ulceration
may give rise to massive bleeding or perforation into
aorta or a mesenteric vessel. The intake of NSAIDs has
also been attributed to ulcerations or
hemorrhage.2,3,10,11,17 Although it is extremely
uncommon nevertheless massive GI hemorrhage
leading to shock can be due to diverticula.2,9,11,12 Some
believe that bleeding from diverticula is more common
than thought, and index of suspicion should be raised
in UGI hemorrhage where causes have been excluded
by endoscopy.2,7 Duodeno-colic, gastro-jejuno colic
fistula formation may occur secondary to inflammation
in diverticula. Pancreatic and / or biliary fistula as a
complication during surgery has also been reported.
Intestinal obstruction as a result of blockage of the distal
part of the bowel by a large calculus (enteroliths) which
has been extruded from a DD has been reported.
Neoplastic changes including adenocarcinoma,
sarcoma, leiomyosarcoma or fibroma is also known.
Peridiverticulitis and bezoar formation are other
complication described with this condition.2,3,6,10
ASSOCIATED DISEASESDuodenal ulcerIt has been noted in large number of patients with DD.
A suspicion of duodenal ulcer has been principal reason
for subjecting many patients to investigation. Although
relationship of duodenal ulcer with DD is important
one, there does not seem to be any cause and effect
relationship.2,3,10
Hiatus HerniaThe relative prevalence of hiatus hernia and DD steadily
increases after the fifth decade so their frequent
association in later life is to be anticipated.
Diverticula elsewhereThe reported range of associated jejunal and ileal
diverticula is 4% to 13% of all patients with DD. The
incidence of colonic diverticula in patients with DD is
much higher (20-50%) than in general population over
age of 50 years (10%). A 40% prevalence of coexisting
congenital anomalies has been reported in IDD and its
predecessor, congenital DD. These anomalies include
annular pancreas, choledochocele, omphalocele,
hypoplastic kidney, situs inversus, malrotation, Ladd-
band, portal vein anomalies and Down syndrome.2,16
DIAGNOSISUpper GI barium studiesDuodenal diverticulum is an incidental finding of UGI
barium examination after ingestion of barium, study is
made in erect, recumbent and oblique positions. One
Duodenal Diverticulum

143
© 2003 Indian Journal of Surgery www.indianjsurg.com
Indian Journal of Surgery 2004 Volume 66 Issue 3 (June)
of most important feature is abnormal retention of
barium in the sac. Barium retention for 6 or more hours
is diagnostic. The use of a small amount of barium may
help to avoid the overlapping of duodenum by jejunal
loops and by the completely filled stomach. At times
sac is better visualized from 1-2 hours following the
opaque meals.2,4,6,10,18
Differential diagnosis barium upper GI seriesIn first part of duodenum, pseudodiverticula occur
proximally to stenosing duodenal ulcer. These have
either no neck or a very wide neck and are much longer,
narrower than true diverticula, these are invariably
associated with duodenal deformity distally. In second
part a small diverticulum may resemble the niche of a
post bulbar ulcer. The presence of a small neck
differentiate ulcer niche. In the third part diverticulum
from upper jejunal loops may be confused with DD.
Antero-posterior film exposed in patients with
diverticula of 4th part of duodenum may be confused
with ulcer or cancer of lesser curvature of the stomach.
But films taken with patient in lateral position will
demonstrate normal out line of the stomach.2,18
In upper GI series in patients with IDD, the “duodenal
Wind Sock sign” may be seen. It consists of barium
filled sac that lies entirely within duodenum and is
surrounded by a narrow radio-luscent line which is well
demonstrated as the barium in the duodenum passes
distal to the diverticulum.16
Upper G.I. Endoscopy is an important investigation for
diagnosis of DD. It is successful in diagnosing DD in
more than 75% of patients.19 Side viewing endoscopy
may further increase the success rate. The rate of failure
of endoscopy to diagnose DD may increase if DD is
situated in third or fourth part of the duodenum. At
endoscopy IDD appears as a sac like structure with
eccentric aperture lying with normal mucosa or as a
soft polypoidal mass.6,16 JPDD make endoscopic
examination as well as possible subsequent
intervention more difficult. Guide wires should be used
for complicated procedures. Diverticula may even be
the cause of failure or complications such as
hemorrhage or perforations. It can also diagnose
associated lesions which may be responsible for
symptoms. But it has also failed to identify bleeding
diverticula in up to 70%7 few other studies have also
highlighted the failure of endoscopy to diagnose
bleeding diverticulum and more so when it was situated
in third or fourth part of duodenum.7,9-11 ERCP
(Endoscopic Retrograde Cholangiopancreatography)
when done for investigating pancreatico-biliary system
can diagnose large number of diverticula.6,7,12,20
Whenever endoscopy can not determine the cause of
bleeding, angiography may be performed.7,21 Diagnosis
can also be established with combination of
arteriography and scanning with Technetium-99
lebelled red cells.7,9
CT scan and MRIWhen associated with diverticulitis or perforation the
CT appearance of DD include a mass like structure that
is interposed between the duodenum and the
pancreatic head containing air, an air fluid level, fluid,
contrast material, or debris. On perforation, duodenal
wall thickening and surrounding free air, fluid and fat
stranding may be noted.3,6,10,22,23 When diverticulum is
entirely filled with fluid it may mimic a cystic neoplasm
arising from the head of the pancreas. Location and
presence of small amount of intradiverticular gas when
present may aid in establishing the diagnosis. On MRI,
T2 weighted True FISP (Fast Imaging with Steady state
Precession) and HASTE (half Fourier Single Shot Fast
Spin ECHO) images demonstrate air fluid levels within
diverticula. Hypo intense oral contrast on T1 weighted
spoiled gradient ECHO images aid detection of the
smaller diverticula. MRCP images in coronal plane best
demonstrate the relationship of the diverticula to the
papilla. MRI facilitates precise delineation of the
complicated DD while MRCP allows assessment of the
effects of DD on biliary and pancreatic duct. IDD has
been reported to be evaluated with computed
tomography endoscopic Ultrasonography, intravenous
cholangiography, percutaneous transhepatic
cholangiography and ERCP.16,24,25
TREATMENTElective surgical treatment of asymptomatic
diverticulum is not justified as these are innocent.
However small percentage can cause problem ranging
from mild and slightly annoying symptoms to
dangerous and even fatal complications.
Diverticulectomy done for vague pain and abdominal
discomfort is dangerous and unrewarding2,6 and only
about 50% of patients treated by diverticulectomy
electively were entirely relieved of their symptoms.2-
6,26 Only 1-5% of diagnosed DD will require surgery
when complication has arisen having incapacitating
symptoms. In patients with symptoms due to distention
of diverticula, relief may be obtained by patient
assuming a position that allows dependent drainage
of diverticulum which may be determined by
fluoroscopy. Acute obstruction of diverticulum may be
Mahajan SK, et al

144 Indian Journal of Surgery 2004 Volume 66 Issue 3 (June)
relieved by endoscopic clearance. Operative procedure
for diverticula of second part of duodenum needs
mobilization of duodenum by Kochar maneuver.
Duodenum is turned medially and thin walled
diverticulum comes in view. Diverticulum is dissected
which is frequently near communication of the CBD
and main pancreatic duct. Dissected sac is split open
and position of papillae of Vater confirmed.
Diverticulum is excised and opening of duodenum
closed transversely. When duodenum is near the
common bile duct closure of duodenum may press on
it. Supra-duodenal part of CBD is opened and T-Tube
passed well into duodenum before closure of duodenal
wall after diverticulum excision. Diverticulum arising
from second and third part may be approached through
mesocolon. If the ampulla of Vater enters the
diverticulum in presence of CBD obstruction,
Scudamore et al reported that they per formed
choledochoduodenostomy than directly attack the
diverticulum.27 Choledochoduodenostomy,
Choledochojejunostomy alone do not relieve problems
arising from pancreatic duct. Resection however may
be difficult in cases where diverticulum is buried in the
head of pancreas or the papilla lies deep in the
diverticulum. Therefore in cases in which a direct
approach to the diverticulum seems to be hazardous,
division of duodenum 2 cm distal to the pylorus with
drainage by a Roux-en-Y duodenojejunostomy may be
performed. “Diverticulisation” of duodenum is another
method of bypassing diverticulum (Antrectomy,
Vagotomy, Choledochostomy and Billroth II
anastomosis).3,6
Surgical excision is not without complications and
mortality rate of 30% in post operative period has been
reported. A delay in diagnosis can lead to perforation
in DD which carries a mortality rate of 90%.10 Injury to
adjacent ampulla of Vater leading to interference with
drainage of bile or pancreatic secretions may lead to
jaundice, pancreatitis, or development of duodenal
fistula. An overaggressive attempt to dissect the
duodenum from pancreas may lead to pancreatitis and
hemorrhage. Laparoscopic duodenal diverticulectomy
can be done by using intraoperative endoscopic
guidance.27-30 All IDD require treatment as recurrence
of symptoms is certain. Curative treatment consists of
removal of diverticulum by laparotomy and
duodenotomy or by endoscopy. If diverticulum is not
circumferentially attached, it can be resected
endoscopically with an electrocautery snare. If it is
attached circumferentially it can be inverted with the
endoscope, partially resected to create an opening in
the blind end. When papilla, CBD, or pancreatic duct
cannot be clearly identified duodenostomy should be
performed. If at the time of duodenotomy the papilla
cannot be identified, choledochotomy and placement
of a bile duct probe to localize the papilla should be
per formed prior to the resection of the
diverticulum.3,4,10
Duodenal diverticulum present in about 25% of
patients. DD can be commoner than EDD, which are
acquired or IDD which are congenital and rare. Majority
are asymptomatic, only a small percentage of patients
will develop symptoms ranging from mild abdominal
discomfort to serious complications like diverticulitis,
perforation, ulceration and haemorrhage. Majority of
diverticula can be diagnosed by endoscopy, especially
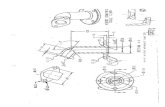
Figure 1: Barium follow through showing diverticulum insecond part of duodenum.
Figure 2: Schematic diagram showing surgical steps. (A & B):Exposure of the diverticulum of second part of duodenum. (C& D): Surgical removal of diverticulum of second part ofduodenum.
Duodenal Diverticulum

145
© 2003 Indian Journal of Surgery www.indianjsurg.com
Indian Journal of Surgery 2004 Volume 66 Issue 3 (June)
side viewing endoscopy and upper GI barium studies.
Asymptomatic diverticula do not require surgical
management. Surgical procedures in this area are
difficult to perform and are associated with high rate
of post-operative complications and mortality. Early
recognition is essential if treatment is to be successful.
REFERENCES
1. Lane JE, Ajjan M, Sedghi S. GI bleeding from duodenal diver-
ticula. Am J Gast; 2001:2799.
2. Pimparkar BD. Diverticulosis of the small intestine In: Bockus Henry
L, (Ed). Gastroenterology. 3rd (Ed). Philadelphia: WB Saunders
Co 1976:437-58.
3. Knoefel WT, Ratttner DW. Duodenal diverticula and duodenal tu-
mours In: Morris PJ, Malt RA, (Ed). Oxford Text Book of Surgery.
New York: Oxford University Press 1994;1:943-6.
4. Cheshire NJ, Glezer G. Diverticula, volvulus, superior mesenteric
artery syndrome and foreign bodies. In: Zinner MJ, Schwartz SI,
Ellis H, (Ed). Maingoats Abdominal operation 10th (Ed). London:
Prentice Hall International Ince (UK) Limited 916-21.
5. Lobo DN, Balfour TW, Iftikhar SY, Rowlands BJ. Periampullary di-
verticula and pancraeticobiliary disease. Br J Surg 1999;86:588-
97.
6. Harford WV. Diverticula of the hypopharynx and esophagus, the
stomach and small bowel. In: Feldman M, Scharschmidt BF,
Sleisenger MH, (Ed). Sleisenger and Fordtran’s Gastrointestinal
and Liver Diseases. 6th (Ed). Philadelphia: WB Saunders Co
1998;1:313-6.
7. Yin WY, Chen HT, Huang SM, Lin HH, Chang TM. Clinical analysis
and literature review of massive duodenal diverticular bleeding.
World J Surg 2001;25:848-55.
8. Balkissoon J, Balkissoon B, Leffall LD Jr, Posey DA Jr. Massive up-
per gastrointestinal bleeding in a patient with a duodenal diver-
ticulum: A case report and review of literature. J Natl Med Assoc
1992;84:365-7.
9. Rioux L, Des Groseilliers S, Fortin M, Mutch DO. Massive upper
gastrointestinal bleeding originating from a fourth stage duode-
nal diverticulum: A case report and review of the literature. Can J
Surg 1996;39:510-2.
10. Afridi SA, Fichenbaum CJ, Taubin H. Review of duodenal diver-
ticula. Am J Gastroenterology 1991;86:935-8.
11. Donald JW. Major complications of small bowel diverticula. Ann
Surg 1979;190:183-8.
12. Zoeof T, Zoepf DS, Arnold JC, Benz C, Riemann J. The relationship
between juxtapapillary and duodenal diverticula and disorders of
the biliopancreatic system. Analysis of 350 patients. Gastrointest
Endosc 2001;54:56-61.
13. Uomo G, Manes G, Ragozzino A, Cavallera A, Rabbiti PG.
Periampullary extra luminal duodenal diverticula and acute pan-
creatitis. An underestimated eitiological association. Am J Gas-
troenterology 1996;91:1186-8.
14. Christoforidis E, Goulimaris I, Kanellos I, Tsalis K, Dadoukis I. The
role of juxtapapillary duodenal diverticula in biliary stone disease.
Gastrointest. Endosc 2002;55:543-7.
15. Leivonen MK, Halthenen JA, Kivilaakso EO. Duodenal diverticu-
lum at endoscopic retrograde cholangiopancreaticography, analy-
sis of 123 patients. Hepatogastroenterology 1996;43:961-6.
16. Materne R. The duodenal windsock sign. Radiology
2001;218:749-50.
17. Mahajan SK, Vaidya P, Sood BR, Gupta D, Sharma A. Duodenal
diverticular hemorrhage in a patient taking NSAID. J Assoc Physi-
cians India 2003;51:416-8.
18. Eisenberg RL. Gastrointestinal Radiology. Philadelphia: Lippincott;
1990:529-34.
19. Zemlianoi AG, Gorbunov GM, Kerzikov AF. The role of duode-
noscopy in the diagnosis of duodenal diverticulosis. Khirurgiia
(Mosk) 1990;12:44-6.
20. Riebel O, Piskac P. Juxtapapillary diverticulum-the effect on en-
doscopic interventions and diagnosis. Rozhl Chir 1996;75:429-
32.
21. Mosimann F, Bronnimann B. The duodenal diverticulm: An ex-
ceptional site of massive bleeding. Hepatogastroentrology
1998;45:603-5.
22. Rao PM. Per forated duodenal diverticulitis. Radiology
1999;211:711-3.
23. Macari M, Lazarus D, Israel G, Megibow A. Duodenal diverticula
mimicking cystic neoplasms of the pancreas: CT and MRI imaging
findings in seven patients. AJR Am J Roentgenol 2003;180:195-
9.
24. Balci NC, Noone T, Akon E, Akinci A, Kler HV. Juxtapapillary di-
verticulum: findings on MRI. Journal of Magnetic Resonance
Imaging 2003;17:487-92.
25. Balci NC, Akinci A, Akon E, Kler HV. Juxtapapillary diverticulum:
Findings on CT and MRI. Clinical Imaging 2003;27:82-8.
26. Cattell RB, Mudge TJ. The surgical significance of duodenal diver-
ticula. N Engl J Med 1952;246:317-24.
27. Scudamore CH, Harrison RC. Management of duodenal diver-
ticula. Can J Surg 1982;25:311.
28. Solhang JH, Semb BK. Duodenal diverticulum with intermittent
biliary stasis. Acta Chir Scand 1974;140:670-3.
29. Fujii K, Fujioka S, Kato K, Machiki Y, Kutsuna Y, Ishikawa A, et al.
Recurrent bleeding from a duodenal diverticulum 8 years after
endoscopic treatment: Case report and review of the literature.
Hepatogastroenterology 2001;48:1058-60.
30. Callery MP, Aliperti G, Soper NJ. Laparoscopic duodenal diverti-
culectomy following heamorrhage. Surge Laparosc Endosc
1994;4:134-8.
Mahajan SK, et al