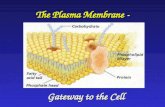
Department of Kinesiology and Applied Physiology WCR Chapter 3: Cells Plasma membrane: structure...
-
Upload
rosa-lindsey -
Category
Documents
-
view
228 -
download
0
Transcript of Department of Kinesiology and Applied Physiology WCR Chapter 3: Cells Plasma membrane: structure...

Department of Kinesiology and Applied Physiology WCR
Chapter 3: Cells• Plasma membrane: structure• Plasma membrane: transport• Resting membrane potential• Cell-environment interactions• Cytoplasm• Nucleus• Cell growth & reproduction• Extracellular materials, developmental aspects

Copyright © 2010 Pearson Education, Inc.
Cytoplasm
• Located between plasma membrane and nucleus
• Cytosol: water with solutes (protein, salts, sugars, etc.)
• Cytoplasmic organelles: metabolic machinery of cell
• Mitochondria, ER, Golgi, lysosomes, etc.
• Cytoskeleton, centrioles, ribosomes, etc.
• Inclusions
• Granules (glycogen, pigments), lipid droplets, etc.

Copyright © 2010 Pearson Education, Inc.
Mitochondria• Double-
membrane structure
• Provide most of cell’s ATP via aerobic cellular respiration
Figure 3.17Enzymes
Matrix
Cristae
Mitochondrial DNA
Ribosome
Outer mitochondrial membrane
Inner mitochondrial membrane
(b)
(a)
(c)

Copyright © 2010 Pearson Education, Inc. Figure 3.18a
Nuclearenvelope
Ribosomes
Rough ER
Smooth ER
(a) Diagrammatic view of smooth and rough ER

Copyright © 2010 Pearson Education, Inc. Figure 3.20
Protein-containing vesicles pinch off rough ERand migrate to fuse with membranes ofGolgi apparatus.
Proteins aremodified withinthe Golgi compartments.
Proteins arethen packagedwithin differentvesicle types, depending ontheir ultimatedestination.
Plasmamem-brane
Secretion byexocytosis
Vesicle becomeslysosome
Golgiapparatus
Rough ER ERmembrane
Phagosome
Proteins incisterna
Pathway B:Vesicle membraneto be incorporatedinto plasmamembranePathway A:
Vesicle contentsdestined for exocytosis Extracellular fluid
Secretoryvesicle
Pathway C:Lysosome containing acid hydrolaseenzymes
1
3
2

Copyright © 2010 Pearson Education, Inc. Figure 3.22
Golgiapparatus
Transportvesicle
Plasmamembrane
Vesicle
Smooth ER
Rough ER
Nuclear envelope
Lysosome
Nucleus

Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasisHe et al., Nature (2012) doi:10.1038/nature10758
Exercise has beneficial effects on human health, including protection against metabolic disorders such as diabetes1. However, the cellular mechanisms underlying these effects are incompletely understood. The lysosomal degradation pathway, autophagy, is an intracellular recycling system that functions during basal conditions in organelle and protein quality control2. During stress, increased levels of autophagy permit cells to adapt to changing nutritional and energy demands through protein catabolism3. Moreover, in animal models, autophagy protects against diseases such as cancer, neurodegenerative disorders, infections, inflammatory diseases, ageing and insulin resistance4, 5, 6. Here we show that acute exercise induces autophagy in skeletal and cardiac muscle of fed mice. To investigate the role of exercise-mediated autophagy in vivo, we generated mutant mice that show normal levels of basal autophagy but are deficient in stimulus (exercise- or starvation)-induced autophagy. These mice (termed BCL2 AAA mice) contain knock-in mutations in BCL2 phosphorylation sites (Thr69Ala, Ser70Ala and Ser84Ala) that prevent stimulus-induced disruption of the BCL2–beclin-1 complex and autophagy activation. BCL2 AAA mice show decreased endurance and altered glucose metabolism during acute exercise, as well as impaired chronic exercise-mediated protection against high-fat-diet-induced glucose intolerance. Thus, exercise induces autophagy, BCL2 is a crucial regulator of exercise- (and starvation)-induced autophagy in vivo, and autophagy induction may contribute to the beneficial metabolic effects of exercise.

Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis
He et al., Nature (2012) doi:10.1038/nature10758
This study shows that exercise can activate cellular recycling (autophagy). Other researchers have already shown that autophagy protects against cancer, diabetes, aging, etc. The authors also made genetically altered mice with normal “resting” autophagy but which did not “ramp up” autophagy in response to exercise. These mice had lower endurance and were more likely to develop a diabetic condition. This study is a strong step forward in understanding how and why exercise is good for us.

College or Department name here
Representative haematoxylin and eosin (H & E) and periodic acid-Schiff (PAS) staining in tibialis anterior muscle sections from mice of indicated genotype. Scale bar, 20 μm. He et al., Nature (2012) doi:10.1038/nature10758
This slide shows that normal (WT) and mutant (BCL2AAA) mice have normal looking muscle cells.
H&E stains nuclei blue and proteins pink and orange. PAS stain highlights glycogen and other polysaccharides and is useful for detecting abnormalities in glycogen storage, etc.

Copyright © 2010 Pearson Education, Inc.
Endomembrane System
PLAYPLAY Animation: Endomembrane System

Copyright © 2010 Pearson Education, Inc.
Cytoskeleton
Rods & struts throughout cytosol
• Microtubules – hollow tubes; tubulin; radiate from centrosomes; help regulate cell motility & shape; position intracellular structures
• Microfilaments – double helix; actin; help regulate cell motility & shape
• Intermediate filaments – various types including keratins; resist force; attach to desmosomes

Copyright © 2010 Pearson Education, Inc.
Motor Molecules
• Protein complexes that generate a pulling force
• Motility function: contraction, movement of organelles, etc.
• Powered by ATP
• Some types (substrate – what they “walk” on) (example)
• Myosin (actin) (muscle)
• Kinesin “walkers” (microtubules) (see video)
• Dynein (microtubules) (sperm flagellum)

College or Department name here
Molecular motors: A staggering giant, Walter WJ, Diez S, Nature 482, 44–45 (02 February 2012)Figure 1: Stepping patterns in motor proteins. a, Some motor proteins, such as kinesin-1, move in a highly coordinated way along microtubules (polymeric protein tubes; grey). The traces show the stepping of individual motor heads (each labelled with a different fluorescent marker, red or green). b, DeWitt et al.1 and Qiu et al.2 show that, by contrast, cytoplasmic dynein moves in a variable stepping pattern. Although the heads often move alternately, they only seldom pass each other, and the spacing between them varies strongly over time.
Molecular motors: A staggering giant, Walter WJ, Diez S, Nature 482, 44–45 (02 February 2012)Figure 1: Stepping patterns in motor proteins. a, Some motor proteins, such as kinesin-1, move in a highly coordinated way along microtubules (polymeric protein tubes; grey). The traces show the stepping of individual motor heads (each labelled with a different fluorescent marker, red or green). b, DeWitt et al.1 and Qiu et al.2 show that, by contrast, cytoplasmic dynein moves in a variable stepping pattern. Although the heads often move alternately, they only seldom pass each other, and the spacing between them varies strongly over time.

Copyright © 2010 Pearson Education, Inc. Figure 3.24
Cytoskeletal elements(microtubules or microfilaments)
Motor molecule (ATP powered)
ATP
(b) In some types of cell motility, motor molecules attached to oneelement of the cytoskeleton can cause it to slide over anotherelement, as in muscle contraction and cilia movement.
ATP
Vesicle
(a) Motor molecules can attach to receptors onvesicles or organelles, and “walk” the organellesalong the microtubules of the cytoskeleton.
Motor molecule (ATP powered)
Microtubule of cytoskeleton
Receptor for motor molecule

Copyright © 2010 Pearson Education, Inc. Figure 3.25a
Centrosome matrix
Centrioles
Microtubules
Centrosome
• “Cell center” near nucleus
• Generates microtubules; organizes mitotic spindle
• Contains centrioles: Small tubes formed by microtubules

Copyright © 2010 Pearson Education, Inc.
Cellular Extensions
• Cilia and flagella
• Whiplike, motile extensions on surfaces of certain cells
• Contain microtubules and motor molecules
• Cilia move substances across cell surfaces
• Longer flagella propel whole cells (tail of sperm)
• Microvilli
• Fingerlike extensions of plasma membrane
• Increase surface area for absorption
• Core of actin filaments for stiffening
PLAYPLAY Animation: Cilia and Flagella

Copyright © 2010 Pearson Education, Inc. Figure 3.26
Plasmamembrane
Outer microtubuledoublet
Dynein arms
Centralmicrotubule
Radial spoke
Radial spoke
TEM
TEM
Triplet
Basal body(centriole)
Cilium
Microtubules
Plasmamembrane
Basal body
Cross-linkingproteins insideouter doublets
Cross-linkingproteins insideouter doublets
A longitudinal section of acilium shows microtubules running the length of thestructure.
The doubletsalso have attached motor proteins, the dynein arms.
The outermicrotubule doublets and the two central microtubules are held together by cross-linking proteins and radial spokes.
A cross section through thebasal body. The nine outer doublets of a cilium extend into a basal body where each doublet joins another microtubule to form a ring of nine triplets.
A cross section through thecilium shows the “9 + 2”arrangement of microtubules.
TEM

Copyright © 2010 Pearson Education, Inc. Figure 3.28
Microvillus
Actinfilaments
Terminalweb

Copyright © 2010 Pearson Education, Inc.
Nucleus
• Genetic library with blueprints for nearly all cellular proteins
• Responds to signals; dictates kinds and amounts of proteins to be synthesized
• Most cells: 1
• RBCs: 0
• Skeletal muscle cells, osteoclasts, some hepatocytes: >1.
• Double-membrane barrier containing pores which regulate transport of large molecules in and out

Copyright © 2010 Pearson Education, Inc. Figure 3.29a
Chromatin (condensed)
Nuclear envelope Nucleus
Nuclear pores
Nucleolus
Cisternae of rough ER
(a)

Copyright © 2010 Pearson Education, Inc.
Nucleoli
• Dark-staining spherical bodies within nucleus
• Involved in rRNA synthesis, ribosome subunit assembly
Chromatin
• Threadlike strands of DNA (30%), histone proteins (60%), RNA (10%)
• Arranged in fundamental units called nucleosomes (8 histones plus 2 wraps of DNA)
• Condenses into chromosomes during cell division

Copyright © 2010 Pearson Education, Inc. Figure 3.30
Metaphasechromosome(at midpointof cell division)
Nucleosome (10-nm diameter; eight histone proteins wrapped by two winds of the DNA double helix)
Linker DNA
Histones
(a)
(b)
1 DNA doublehelix (2-nm diameter)
2 Chromatin(“beads on a string”) structurewith nucleosomes
3 Tight helical fiber(30-nm diameter)
5 Chromatid(700-nm diameter)
4 Looped domain structure (300-nm diameter)

Histones matterIn acute myeloid leukemia (AML, a blood cancer), protein BRD4 binds to acetylated tails of histone proteins and causes transcription of the MYC gene, leading to cancer progression (panel A). A drug, JQ1, interferes with BRD4 binding to histones, blocks MYC transcription, and slows the growth of cancerous cells in mice. Other drugs that affect histone acetylation are being tested, and one, vorinostat (Zolinza), is approved for treatment of lymphoma. (NEJM 2012)
Turning Off MYC Transcription in Acute Myeloid Leukemia. As shown in Panel A, histone proteins are highly modified by the addition of acetyl (Ac), methyl (Me), and phosphate (P) groups, and the BRD4 protein binds specifically to acetylated lysine residues within histone tails. In Panel B, altered reading of the histone code induced by the small molecule JQ1 results in suppression of MYC transcription and decreased cell growth in acute myeloid leukemia and implicates BRD4 as a key regulator of such growth. Recent work by Zuber et al. showed that inhibition of BRD4 function could slow progression in this disease — an effect that could be mimicked by blocking the ability of BRD4 to bind acetylated lysine histone tails.1 The resulting gene-expression program suggests that MYC is a key component of this pathway. Godley and Le Beau, N Engl J Med 2012; 366:960-961.



![Plasma Membrane [7.2] Goals: Understand the concept of homeostasis in relation to the plasma membrane Demonstrate and understand how the plasma membrane.](https://static.fdocuments.net/doc/165x107/5697c01d1a28abf838cd0a9a/plasma-membrane-72-goals-understand-the-concept-of-homeostasis-in-relation.jpg)