Crimean–congo hemorrhagic fever
-
Upload
tumalapalli-venkateswara-rao -
Category
Documents
-
view
4.800 -
download
2
description
Transcript of Crimean–congo hemorrhagic fever

Crimean–Congo Hemorrhagic fever
Dr.T.V.Rao MD

What is Viral
Hemorrhagic Fever?
•Severe multisystem syndrome
•Damage to overall vascular system
•Symptoms often accompanied by
hemorrhage• Rarely life threatening in itself
• Includes conjunctivitis, petechia, echymosis

Virology of Hemorrhagic fevers
• They are all RNA viruses
• They are all zoonotic (natural reservoir is an arthropod or other animal host)
• Disease is restricted to habitat of the host
• Humans become infected by contact with host
• Some viruses can be transmitted from human to human

Classification of Prominent
Hemorrhagic Fevers

Ce
nte
r fo
r Fo
od
Se
curi
ty a
nd
Pu
bli
c
He
alt
h I
ow
a S
tate
Un
ive
rsit
y -
20
04
Bunyaviridae History
• 1930: Rift Valley Fever – Egypt
• Epizootic in sheep
•1940s: CCHF - Crimean peninsula
•Hemorrhagic fever in agricultural workers
•1951: Hantavirus – Korea
•Hemorrhagic fever in UN troops
•5 genera with over 350 viruses

Ce
nte
r fo
r Fo
od
Se
curi
ty a
nd
Pu
bli
c
He
alt
h I
ow
a S
tate
Un
ive
rsit
y -
20
04
Bunyaviridae Transmission
• Arthropod vector
• Exception – Hantaviruses
• RVF – Aedes mosquito
•CCHF – Ixodid tick
• Hantavirus – Rodents
• Less common
• Aerosol
• Exposure to infected animal tissue

Bunyaviridae in Animals
• RVF• Abortion – 100%
• Mortality rate
• >90% in young
• 5-60% in older animals
• CCHF• Unapparent infection in
livestock
• Hantaviruses• Unapparent infection in
rodents

Transmission of Disease
• CCHFV usually circulates between asymptomatic
animals and ticks in an enzootic cycle. This virus has beenfound in at least 31 species of ticks, including sevengenera of the family Ixodidae (hard ticks). Members ofthe genus Hyalomma seem to be the principal vectors.Transovarial, transstadial and venereal transmission occurin this genus. Hyalomma marginatum marginatum isparticularly important as a vector in Europe, but CCHFVis also found in Hyalomma anatolicum anatolicum andother Hyalomma spp.

What is Crimean-Congo hemorrhagic fever?
• Crimean-Congo hemorrhagic
fever (CCHF) is caused by
infection with a tick-borne virus
(Nairovirus) in the family
Bunyaviridae. The disease was
first characterized in the Crimea
in 1944 and given the name
Crimean hemorrhagic fever. It
was then later recognized in
1969 as the cause of illness in
the Congo, thus resulting in the
current name of the disease.

What is Crimean-Congo
hemorrhagic fever• Crimean-Congo
hemorrhagic fever (CCHF)
is caused by infection with
a tick-borne virus
(Nairovirus) in the family
Bunyaviridae. The disease
was first characterized in
the Crimea in 1944 and
given the name Crimean
hemorrhagic fever. It was then
later recognized in 1969 as the cause of illness
in the Congo, thus resulting in the current
name of the disease.

Taxonomical character of
CCHF• The antigenic characterization of the virus is, on the
other hand, far better established than its position in
the International Committee on Taxonomy of Viruses
(ICTV) scheme. Exhaustive and continued efforts by
hemagglutination inhibition (HI), complement
fixation (CF) and agar gel diffusion and precipitation
(AGDP) tests have shown the virus to be antigenically
related to no other viruses except: to Hazara with
which it constitutes the CCHF group,

Crimean-Congo hemorrhagic fever caused by
Member of Nairovirus
• CCHFV is a member of the Nairovirus genus of the
family Bunyaviridae. Other genera within the family
include Orthobunyavirus, Hantavirus, Phlebovirus,
and Tospovirus. According to the most recent report
from the International Committee on the Taxonomy
of Viruses, there are seven recognized species in the
genus Nairovirus containing 34 viral strains. The most
important Serogroups are the CCHF group, which
includes CCHFV, and Hazara virus, which has not
been demonstrated to be pathogenic to human

CCHF is a Tick borne Disease
• CCHF spreads to humans either by tick-bites,
or through contact with viraemic animal
tissues during and immediately post-slaughter.
CCHF outbreaks constitute a threat to public
health services because of its epidemic
potential, its high case fatality ratio (10-40%),
its potential for nosocomial outbreaks and the
difficulties in treatment and prevention.

Crimean–Congo hemorrhagic fever
• Crimean–Congo hemorrhagic fever (CCHF) is a
widespread tick-borne viral disease, a zoonosis of
domestic animals and wild animals, that may affect
humans. The pathogenic virus, especially common in
East and West Africa, is a member of the
Bunyaviridae family of RNA viruses. Clinical disease is
rare in infected mammals, but commonly severe in
infected humans, with a 30% mortality rate.
Outbreaks of illness are usually attributable to
handling infected animals or people.

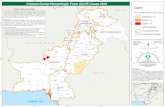
CCHF endemic in …..
• CCHF is endemic in all
of Africa, the Balkans,
the Middle East and in
Asia south of the 50°
parallel north, the
geographic limit of the
genus Hyalomma, the
principal tick vector.

Global distribution and phylogenetic relationships
of Crimean-Congo hemorrhagic fever virus
(CCHFV) strains

Taxonomical Name …
• The International Committee on Taxonomy of Viruses
proposed the name Congo-Crimean hemorrhagic
fever virus, but the Soviets insisted on Crimean–
Congo hemorrhagic fever virus. Against all principles
of scientific nomenclature based on priority of
publication, it was adopted as the official name in
1973 in possibly the first instance of a virus losing its
name to politics and the Cold War. However, since
then Congo-Crimean or just Congo virus


How is CCHF spread and how do
humans become infected?
Ixodid (hard) ticks, especially those of the genus, Hyalomma, are both a reservoir and a vector for the CCHF virus. Numerous wild and domestic animals, such as cattle, goats, sheep and hares, serve as amplifying hosts for the virus. Transmission to humans occurs through contact with infected animal blood or ticks.


HUMAN CONTACT CAN SPREAD THE
DISEASE • CCHF can be transmitted
from one infected human to another by contact with infectious blood or body fluids. Documented spread of CCHF has also occurred in hospitals due to improper sterilization of medical equipment, reuse of injection needles, and contamination of medical supplies.

Who are Who are at Risk
• People at most risk for CCHF include livestock workers, people who herd animals, and people who work in slaughterhouses in endemic areas. Additionally if health care workers in endemic areas have unprotected contact with infectious blood or body fluids, they are at high risk.

The disease starts with short
Incubation period
• Incubation period – 3-7 days
•Hemorrhagic Fever - 3–6 days following clinical signs

Pathophysiology • The target organ is the vascular bed.
• Dominant clinical features are due to micro
vascular damage and changes in vascular
permeability
• In most cases of viral hemorrhagic fever, the
coagulopathy is multifactorial, including:
• hepatic damage
• disseminated intravascular coagulation
• primary marrow injury to megakaryocytes

How the disease manifests
• Typically, after a 1–3 day incubation period following
a tick bite (5–6 days after exposure to infected blood
or tissues), flu-like symptoms appear, which may
resolve after one week. In up to 75% of cases,
however, signs of hemorrhage appear within 3–5
days of the onset of illness in case of bad
containment of the first symptoms: first mood
instability, agitation, mental confusion and throat
petechia, then soon nosebleeds, bloody urine and
vomiting, and black stools.

Symptoms
• Fever, fatigue, dizziness, myalgia's, and prostration
• Signs of bleeding range from only conjunctival hemorrhage, mild hypotension, flushing, and petechiae to shock and generalized mucous membrane hemorrhage and evidence of pulmonary, hematopoietic, and neurologic dysfunction
• Renal insufficiency is proportional to cardiovascular compromise except in Hemorrhagic Fever and Renal Syndrome in which it is an integral part of the disease

Hemorrhagic disorders are cause of
Morbidity and Mortality• As the illness
progresses, large areas
of severe bruising,
severe nosebleeds, and
uncontrolled bleeding at
injection sites can be
seen, beginning on about
the fourth day of illness
and lasting for about two weeks

Can lead to Major Organ Failure
• The liver becomes swollen and painful. Disseminated intravascular coagulation may occur as well as acute kidney failure and shock, and sometimes acute respiratory distress syndrome.

How is Crimean-Congo hemorrhagic
fever diagnosed?• Laboratory diagnosis of
CCHF can be made by finding a positive serological test result, evidence of viral antigen in tissue by immunohistochemical staining and microscopic examination, or identification of viral RNA sequence in blood or tissue, in a patient with a clinical history compatible with CCH

Virological Diagnosis
• Crimean-Congo hemorrhagic fever can be diagnosed by isolating CCHFV from blood, plasma or tissues. At autopsy, the virus is most likely to be found in the lung, liver, spleen, bone marrow, kidney and brain. CCHFV can be isolated in a variety of cell lines including SW-13, Vero, LLC-MK2 and BHK-21 cells.

RT- PCR is a sensitive tool in
Diagnosis• Crimean-Congo
hemorrhagic fever is often diagnosed by RT-PCR on blood samples. This technique is highly sensitive. However, due to the genetic variability in CCHFV strains, a single set of primers cannot detect all virus variants, and most RT-PCR assays are either designed to detect local variants or lack sensitivity.

Serological Tests are easier to
use• Crimean-Congo
hemorrhagic fever can also be diagnosed by serology. Tests detect CCHFV-specific IgM, or a rise in IgG titers in paired acute and convalescent sera. IgG and IgM can usually be found with indirect immunofluorescence or ELISA after 7-9 days of illness

Outbreaks leads to Mortality
• In documented
outbreaks of CCHF,
fatality rates in
hospitalized patients
have ranged from 9%
to as high as 50%.
• CCHF - Africa, Eastern
Europe, Asia
• 30% case fatality rate

Prevention and Control
• Protective clothing
• Disposable gowns, gloves, masks and shoe covers, protective eyewear when splashing might occur, or if patient is disoriented or uncooperative
• WHO and CDC developed manual
• “Infection Control for Viral Hemorrhagic Fevers In the African Health Care Setting”

Prevention and Control
• Anyone suspected of
having a VHF must use
a chemical toilet
• Disinfect and dispose of
instruments
• Use a 0.5% solution of
sodium hypochlorite
(1:10 dilution of bleach)

How to Prevent CCHF
• Agricultural workers and others working with animals
should use insect repellent on exposed skin and clothing.
Insect repellants containing DEET (N, N-diethyl-m-
toluamide) are the most effective in warding off ticks.
Wearing gloves and other protective clothing is
recommended. Individuals should also avoid contact with
the blood and body fluids of livestock or humans who
show symptoms of infection. It is important for healthcare
workers to use proper infection control precautions to prevent occupational exposure.

Health care workers can be at risk
• Avoiding contact with blood and body fluids of humans or animals, especially those who have symptoms of infection is also important. Health care workers need to be sure to use proper sanitation methods

Ribavirin in experimental use
• Prevalence needs to be measured in animals and in at-risk humans in endemic areas; and a useful animal model needs to be developed. Further research is needed to determine the efficacy of specific treatment with ribavirin and other antiviral drugs, and develop a safe and effective vaccine for human use.

Prophylactic Methods to
prevent include• Prophylactic treatment
with ribavirin has
occasionally been used
after high-risk exposures.
Safe burial practices,
including the use of 1:10
liquid bleach solution as
a disinfectant, have been
published

Control of Tick Bites is priority
in Prevention • Measures to avoid tick bites include tick repellents,
environmental modification (brush removal, insecticides),
avoidance of tick habitat and regular examination of clothing
and skin for ticks. Clothing should be chosen to prevent tick
attachment; long pants tucked into boots and long-sleeved
shirts are recommended. Acaricides can be used on livestock
and other domesticated animals to control ticks, particularly
before slaughter or export.

How to Prevent Outbreaks
• Agricultural workers and others working with animals
should use insect repellent on exposed skin and
clothing. Insect repellants containing DEET (N, N-
diethyl-m-toluamide) are the most effective in warding
off ticks. Wearing gloves and other protective clothing
is recommended. Individuals should also avoid contact
with the blood and body fluids of livestock or humans
who show symptoms of infection. It is important for
healthcare workers to use proper infection control precautions to prevent occupational exposure.

Follow the Universal Precautions
while caring Patients• Strict universal
precautions are
necessary when caring
for human patients.
These recommendations
include barrier nursing,
isolation and the use of
gloves, gowns, face-
shields and goggles with
side shields.

Laboratory Workers to be
cautious …
•Laboratory
workers must
follow
stringent
biosafety
precautions.

Yet no Safe and Effective Vaccine
• An inactivated, mouse-brain derived vaccine against CCHF has been developed and is used on a small scale in Eastern Europe. However, there is no safe and effective vaccine widely available for human use.

•Programme created by Dr.T.V.Rao
MD for e-learning for awareness to
Medical and Public Health personal
in the developing world