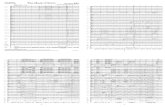
Counting Atoms Worksheet. NaCl – Na = 1 – Cl = 1 BaCl 2 – Ba = 1 – Cl = 2 BaSO 4 – Ba = 1...
-
Upload
dora-white -
Category
Documents
-
view
214 -
download
0
Transcript of Counting Atoms Worksheet. NaCl – Na = 1 – Cl = 1 BaCl 2 – Ba = 1 – Cl = 2 BaSO 4 – Ba = 1...

Counting Atoms Worksheet

• NaCl–Na = 1–Cl = 1
• BaCl2
–Ba = 1–Cl = 2
• BaSO4
–Ba = 1– S = 1–O = 4
• CaSO4
–Ca = 1– S = 1–O = 4
• K2Cr2O7
–K = 2–Cr = 2–O = 7

• C6H4Cl2
– C = 6–H = 4– Cl = 2
• C2H4O2
– C = 2–H = 4–O = 2
• CH3OH– C = 1–H = 4–O = 1
• Mg (OH)2
–Mg = 1–O = 2–H = 2
• C7H5(NO2)3
– C = 7–H = 5–N = 3–O = 6
• Ca (H2PO4)2
– Ca = 1–H = 4– P = 2–O = 8

• (CH3)2CO–C = 3–H = 6–O = 1
• 2HBr–H = 2–Br = 2
• 2CHC3OOH–C = 8–H = 4–O = 4
• 6C6H4BrOH–C = 36–H = 30–Br = 6–O = 6

E, C, M, Worksheet
1. Element– Any substance that contains only one kind of atom– Cannot be broken down
2. Compound– Consists of atoms of two or more different elements– Can be broken down
3. Mixture– Consists of two or more different
elements/compounds– Can be separated

E, C, M, Worksheet
4. Homogeneous – Evenly mixed/distributed Hetergeneous – Not evenly mixed

E, C, M, Worksheet
6. Element7. Compound8. Homogeneous Mixture9. Heterogeneous Mixture10. Homogeneous Mixture11. Compound

E, C, M, Worksheet
12. Homogeneous / Heterogeneous Mixture13. Heterogeneous Mixture14. Element15. Compound16. Compound17. Element18. Homogeneous Mixture

E, C, M, Worksheet
19. Heterogeneous Mixture20. Homogeneous Mixture21. Heterogeneous Mixture22. Homogeneous/Heterogeneous Mixture23. Element24. Heterogeneous Mixture25. Heterogeneous Mixture26. Element

E, C, M, Worksheet
27. Compound28. Homogeneous Mixture29. Heterogeneous Mixture







![CLICK ON 4 CL[1].X L2_09281430](https://static.fdocuments.net/doc/165x107/5571f2e449795947648d356d/click-on-4-cl1x-l209281430.jpg)