CONTENTS Brønsted-Lowry theory of acids and bases Lewis theory of acids and bases Strong acids and...
-
Upload
annabelle-carson -
Category
Documents
-
view
239 -
download
0
Transcript of CONTENTS Brønsted-Lowry theory of acids and bases Lewis theory of acids and bases Strong acids and...
CONTENTS• Brønsted-Lowry theory of acids and bases
• Lewis theory of acids and bases
• Strong acids and bases
• Weak acids
• Weak bases
• Hydrogen ion concentration and pH
• Ionic product of water Kw
• Relation between pH and pOH
• Introduction to buffer solutions
• Check list
Acid & BasesAcid & Bases
Before you start it would be helpful to…
• know the simple properties of acids, bases and alkalis
Acid & BasesAcid & Bases
BRØNSTED-LOWRY THEORY
ACID proton donor HCl ——> H+(aq) + Cl¯(aq)
BASE proton acceptor NH3 (aq) + H+(aq) ——> NH4+(aq)
ACIDS AND BASESACIDS AND BASES
BRØNSTED-LOWRY THEORY
ACID proton donor HCl ——> H+(aq) + Cl¯(aq)
BASE proton acceptor NH3 (aq) + H+(aq) ——> NH4+(aq)
Conjugate systemsAcids are related to bases ACID PROTON + CONJUGATE BASE
Bases are related to acids BASE + PROTON CONJUGATE ACID
ACIDS AND BASESACIDS AND BASES
BRØNSTED-LOWRY THEORY
ACID proton donor HCl ——> H+(aq) + Cl¯(aq)
BASE proton acceptor NH3 (aq) + H+(aq) ——> NH4+(aq)
Conjugate systemsAcids are related to bases ACID PROTON + CONJUGATE BASE
Bases are related to acids BASE + PROTON CONJUGATE ACID
For an acid to behave as an acid, it must have a base present to accept a proton...
HA + B BH+ + A¯acid base conjugate conjugate
acid base
example CH3COO¯ + H2O CH3COOH + OH¯
base acid acid base
ACIDS AND BASESACIDS AND BASES
Conjugate Pairs
HCl (aq)
+ H2O (aq) H3O+(aq) + Cl-
(aq)
Acid Acid BaseBase
Conjugate pair
Conjugate pair
The negative ion is referred to as the conjugate base of the acid
HCl (aq)
+ H2O (aq) H3O+(aq) + Cl-
(aq)
Acid Conjugate Acid
Conjugate Base
Base
The positive ion is referred to as the conjugate acid of the base
• Water can act as an acid and a base – it can be described as amphoteric
HCOOH (aq)
+ H2O (aq) H3O+(aq) + HCOO-
(aq)
H2O (aq)
+ NH3 (aq) NH4+
(aq) + OH-(aq)
Pure water will always contain some H+ & OH- ions it is described as self protonating
2H2O (aq) H3O+
(aq) + OH-(aq)
LEWIS THEORY
ACID lone pair acceptor BF3 H+ AlCl3
BASE lone pair donor NH3 H2O
ACIDS AND BASESACIDS AND BASES
LONE PAIR DONOR LONE PAIRACCEPTOR
LONE PAIR DONOR LONE PAIR ACCEPTOR
STRONGACIDS completely dissociate (split up) into ions in aqueous solution
e.g. HCl ——> H+(aq) + Cl¯(aq) MONOPROTIC 1 replaceable H
HNO3 ——> H+(aq) + NO3¯(aq)
H2SO4 ——> 2H+(aq) + SO42-(aq) DIPROTIC 2 replaceable H’s
STRONG ACIDS AND BASESSTRONG ACIDS AND BASES
STRONGACIDS completely dissociate (split up) into ions in aqueous solution
e.g. HCl ——> H+(aq) + Cl¯(aq) MONOPROTIC 1 replaceable H
HNO3 ——> H+(aq) + NO3¯(aq)
H2SO4 ——> 2H+(aq) + SO42-(aq) DIPROTIC 2 replaceable H’s
STRONGBASES completely dissociate into ions in aqueous solution
e.g. NaOH ——> Na+(aq) + OH¯(aq)
STRONG ACIDS AND BASESSTRONG ACIDS AND BASES
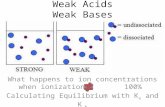
Weak acids partially dissociate into ions in aqueous solution
e.g. ethanoic acid CH3COOH(aq) CH3COO¯(aq) + H+(aq)
When a weak acid dissolves inwater an equilibrium is set up HA(aq) + H2O(l) A¯(aq) + H3O+(aq)
The water stabilises the ions
To make calculations easier the dissociation can be written... HA(aq) A¯(aq) + H+(aq)
WEAK ACIDSWEAK ACIDS
Weak acids partially dissociate into ions in aqueous solution
e.g. ethanoic acid CH3COOH(aq) CH3COO¯(aq) + H+(aq)
When a weak acid dissolves inwater an equilibrium is set up HA(aq) + H2O(l) A¯(aq) + H3O+(aq)
The water stabilises the ions
To make calculations easier the dissociation can be written... HA(aq) A¯(aq) + H+(aq)
The weaker the acid the less it dissociates the more the equilibrium lies to the left.
The relative strengths of acids can be expressed as Ka or pKa values
The dissociation constant for the weak acid HA is Ka = [H+(aq)] [A¯(aq)] mol dm-3
[HA(aq)]
WEAK ACIDSWEAK ACIDS
Partially react with water to give ions in aqueous solution e.g. ammonia
When a weak base dissolves in water an equilibrium is set up
NH3 (aq) + H2O (l) NH4+ (aq) + OH¯ (aq)
as in the case of acids it is more simply written
NH3 (aq) + H+ (aq) NH4+ (aq)
WEAK BASESWEAK BASES
Partially react with water to give ions in aqueous solution e.g. ammonia
When a weak base dissolves in water an equilibrium is set up
NH3 (aq) + H2O (l) NH4+ (aq) + OH¯ (aq)
as in the case of acids it is more simply written
NH3 (aq) + H+ (aq) NH4+ (aq)
The weaker the base the less it dissociatesthe more the equilibrium lies to the left
The relative strengths of bases can be expressed as Kb or pKb values.
WEAK BASESWEAK BASES
Hydrogen ion concentration [HHydrogen ion concentration [H++(aq)(aq)]]
Introduction hydrogen ion concentration determines the acidity of a solutionhydroxide ion concentration determines the alkalinity
for strong acids and bases the concentration of ions is very muchlarger than their weaker counterparts which only partially dissociate.
Hydrogen ion concentration [HHydrogen ion concentration [H++(aq)(aq)]]
pH hydrogen ion concentration can be converted to pH pH = - log10 [H+(aq)]
to convert pH into hydrogen ion concentration [H+(aq)] = antilog (-pH)
pOH An equivalent calculation for bases convertsthe hydroxide ion concentration to pOH pOH = - log10 [OH¯(aq)]
in both the above, [ ] represents the concentration in mol dm-3
STRONGLY ACIDIC
100 10-1 10-2 10-3 10-4 10-5 10-6 10-7 10-8 10-9 10-10 10-11 10-12 10-13 10-14
10-14 10-13 10-12 10-11 10-10 10-9 10-8 10-7 10-6 10-5 10-4 10-3 10-2 10-1 10-0
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14pH
OH¯
[H+]
WEAKLY ACIDIC
NEUTRAL STRONGLY ALKALINE
WEAKLY ALKALINE
Ionic product of water - KIonic product of water - Kww
Despite being covalent, water conducts electricity to a very small extent.
This is due to the slight ionisation ... H2O(l) + H2O(l) H3O+(aq) + OH¯(aq)
or, more simply H2O(l) H+(aq) + OH¯(aq)
Ionic product of water - KIonic product of water - Kww
Despite being covalent, water conducts electricity to a very small extent.
This is due to the slight ionisation ... H2O(l) + H2O(l) H3O+(aq) + OH¯(aq)
or, more simply H2O(l) H+(aq) + OH¯(aq)
Applying the equilibrium lawto the second equation gives Kc = [H+(aq)] [OH¯(aq)]
[ ] is the equilibrium concentration in mol dm-3 [H2O(l)]
Ionic product of water - KIonic product of water - Kww
Despite being covalent, water conducts electricity to a very small extent.
This is due to the slight ionisation ... H2O(l) + H2O(l) H3O+(aq) + OH¯(aq)
or, more simply H2O(l) H+(aq) + OH¯(aq)
Applying the equilibrium lawto the second equation gives Kc = [H+(aq)] [OH¯(aq)]
[ ] is the equilibrium concentration in mol dm-3 [H2O(l)]
As the dissociation is small, the water concentration is very large compared with the dissociated ions and any changes to its value are insignificant; its concentration can be regarded as constant. This “constant” is combined with(Kc) to get a new constant (Kw). Kw = [H+(aq)] [OH¯(aq)] mol2 dm-6
= 1 x 10-14 mol2 dm-6 (at 25°C)
Because the constant is based on an equilibrium, Kw VARIES WITH TEMPERATURE
Ionic product of water - KIonic product of water - Kww
The value of Kw varies with temperature because it is based on an equilibrium.
Temperature / °C 0 20 25 30 60
Kw / 1 x 10-14 mol2 dm-6 0.11 0.68 1.0 1.47 5.6
H+ / x 10-7 mol dm-3 0.33 0.82 1.0 1.27 2.37 pH 7.48 7.08 7 6.92 6.63
What does this tell you about the equation H2O(l) H+(aq) + OH¯(aq) ?
Ionic product of water - KIonic product of water - Kww
The value of Kw varies with temperature because it is based on an equilibrium.
Temperature / °C 0 20 25 30 60
Kw / 1 x 10-14 mol2 dm-6 0.11 0.68 1.0 1.47 5.6
H+ / x 10-7 mol dm-3 0.33 0.82 1.0 1.27 2.37 pH 7.48 7.08 7 6.92 6.63
What does this tell you about the equation H2O(l) H+(aq) + OH¯(aq) ?
• Kw gets larger as the temperature increases
• this means the concentration of H+ and OH¯ ions gets greater
• this means the equilibrium has moved to the right
• if the concentration of H+ increases then the pH decreases
• pH decreases as the temperature increases
Ionic product of water - KIonic product of water - Kww
The value of Kw varies with temperature because it is based on an equilibrium.
Temperature / °C 0 20 25 30 60
Kw / 1 x 10-14 mol2 dm-6 0.11 0.68 1.0 1.47 5.6
H+ / x 10-7 mol dm-3 0.33 0.82 1.0 1.27 2.37 pH 7.48 7.08 7 6.92 6.63
What does this tell you about the equation H2O(l) H+(aq) + OH¯(aq) ?
• Kw gets larger as the temperature increases
• this means the concentration of H+ and OH¯ ions gets greater
• this means the equilibrium has moved to the right
• if the concentration of H+ increases then the pH decreases
• pH decreases as the temperature increases
Because the equation moves to the right as thetemperature goes up, it must be an ENDOTHERMIC process
Relationship between pH and pOHRelationship between pH and pOH
Because H+ and OH¯ ions are producedin equal amounts when water dissociates [H+] = [OH¯] = 1 x 10-7 mol dm-3
their concentrations will be the same.
Kw = [H+] [OH¯] = 1 x 10-14 mol2 dm-6
take logs of both sides log[H+] + log[OH¯] = -14
multiply by minus - log[H+] - log[OH¯] = 14
change to pH and pOH pH + pOH = 14 (at 25°C)
Relationship between pH and pOHRelationship between pH and pOH
Because H+ and OH¯ ions are producedin equal amounts when water dissociates [H+] = [OH¯] = 1 x 10-7 mol dm-3
their concentrations will be the same.
Kw = [H+] [OH¯] = 1 x 10-14 mol2 dm-6
take logs of both sides log[H+] + log[OH¯] = -14
multiply by minus - log[H+] - log[OH¯] = 14
change to pH and pOH pH + pOH = 14 (at 25°C)
N.B. As they are based on the position of equilibrium and that varies withtemperature, the above values are only true if the temperature is 25°C (298K)
Neutral solutions may be regarded as those where [H+] = [OH¯]. Therefore a neutral solution is pH 7 only at a temperature of 25°C (298K)
Kw is constant for any aqueous solution at the stated temperature
Buffer solutions - Buffer solutions - BriefBrief introductionintroduction
Definition “Solutions which resist changes in pH when small quantities of acid or alkali are added.”
Acidic Buffer (pH < 7) made from a weak acid + its sodium or potassium salt ethanoic acid sodium ethanoate
Alkaline Buffer (pH > 7) made from a weak base + its chloride ammonia ammonium chloride
Uses Standardising pH metersBuffering biological systems (eg in blood)Maintaining the pH of shampoos
CONTENTS
• What is pH? - a reminder
• Calculating the pH of strong acids and bases
• Calculating the pH of weak acids
• Calculating the pH of mixtures - strong acid and strong alkali
pH calculationspH calculations
Before you start it would be helpful to…
• know the differences between strong and weak acid and bases
• be able to calculate pH from hydrogen ion concentration
• be able to calculate hydrogen ion concentration from pH
• know the formula for the ionic product of water and its value at 25°C
pH calculationspH calculations
What is pH or pOH?What is pH or pOH?
pH = - log10 [H+(aq)]
pOH = - log10 [OH-(aq)]
where [H+] is the concentration of hydrogen ions in mol dm-3
where [OH-] is the concentration of hydroxide ions in mol dm-3
to convert pH intohydrogen ion concentration [H+(aq)] = antilog (-pH) to convert pOH into [OH-(aq)] = antilog (-pOH)OH- concentration
IONIC PRODUCT OF WATER Kw = [H+(aq)] [OH¯(aq)] mol2 dm-6
= 1 x 10-14 mol2 dm-6 (at 25°C)
pKw = 14 = -log(1 x 10-14 )
pKw = pH + pOH
pH + pOH = 14
Calculating pH - Calculating pH - strong acids and alkalisstrong acids and alkalis
Strong acids and alkalis completely dissociate in aqueous solution
It is easy to calculate the pH or pOH ; you only need to know the concentrationonly need to know the concentration.
Calculate the pH of 0.02M HCl
HCl completely dissociates in aqueous solution HCl H+ + Cl¯
One H+ is produced for each HCl dissociating so [H+] = 0.02M = 2 x 10-2 mol dm-3
WORKEDEXAMPLEWORKEDEXAMPLE
pH = - log [H+]
= - log [0.02]
= 1.7
Note that negative pH vales are also possibleFor example : The pH of 1.5 mol dm–3 HCl is -0.18
Calculating pH - Calculating pH - strong acids and alkalisstrong acids and alkalis
Strong acids and alkalis completely dissociate in aqueous solution
It is easy to calculate the pH or pOH ; you only need to know the concentrationonly need to know the concentration.
Calculate the pH of 0.1M NaOH
NaOH completely dissociates in aqueous solution NaOH Na+ + OH¯
One OH¯ is produced for each NaOH dissociating [OH¯] = 0.1M = 1 x 10-1 mol dm-3
WORKEDEXAMPLEWORKEDEXAMPLE
pH + pOH = 14
pH + 1 = 14
pH = 13
To find pH of a strong base use pH + pOH = 14
pOH = -log [OH¯]
= -log [ 1 ] =
1
Calculating pH - Calculating pH - weak acidsweak acids
A weak acid, HA, dissociates as follows HA(aq) H+(aq) + A¯(aq)(1)
A weak acid is one which only partially dissociates in aqueous solution
Calculating pH - Calculating pH - weak acidsweak acids
A weak acid, HA, dissociates as follows HA(aq) H+(aq) + A¯(aq)(1)
Applying the Equilibrium Law Ka = [H+(aq)] [A¯(aq)] mol dm-3 (2)
[HA(aq)]
A weak acid is one which only partially dissociates in aqueous solution
Calculating pH - Calculating pH - weak acidsweak acids
A weak acid, HA, dissociates as follows HA(aq) H+(aq) + A¯(aq)(1)
Applying the Equilibrium Law Ka = [H+(aq)] [A¯(aq)] mol dm-3 (2)
[HA(aq)]
The ions are formed in equal amounts, so [H+(aq)] = [A¯(aq)]
therefore Ka = [H+(aq)]2 (3)
[HA(aq)]
A weak acid is one which only partially dissociates in aqueous solution
Calculating pH - Calculating pH - weak acidsweak acids
A weak acid, HA, dissociates as follows HA(aq) H+(aq) + A¯(aq)(1)
Applying the Equilibrium Law Ka = [H+(aq)] [A¯(aq)] mol dm-3 (2)
[HA(aq)]
The ions are formed in equal amounts, so [H+(aq)] = [A¯(aq)]
therefore Ka = [H+(aq)]2 (3)
[HA(aq)]
Rearranging (3) gives [H+(aq)]2
= [HA(aq)] Ka
therefore [H+(aq)] = [HA(aq)] Ka
A weak acid is one which only partially dissociates in aqueous solution
Calculating pH - Calculating pH - weak acidsweak acids
A weak acid, HA, dissociates as follows HA(aq) H+(aq) + A¯(aq)(1)
Applying the Equilibrium Law Ka = [H+(aq)] [A¯(aq)] mol dm-3 (2)
[HA(aq)]
The ions are formed in equal amounts, so [H+(aq)] = [A¯(aq)]
therefore Ka = [H+(aq)]2 (3)
[HA(aq)]
Rearranging (3) gives [H+(aq)]2
= [HA(aq)] Ka
therefore [H+(aq)] = [HA(aq)] Ka
pH = [H+(aq)]
A weak acid is one which only partially dissociates in aqueous solution
Calculating pH - Calculating pH - weak acidsweak acids
A weak acid, HA, dissociates as follows HA(aq) H+(aq) + A¯(aq)(1)
Applying the Equilibrium Law Ka = [H+(aq)] [A¯(aq)] mol dm-3 (2)
[HA(aq)]
The ions are formed in equal amounts, so [H+(aq)] = [A¯(aq)]
therefore Ka = [H+(aq)]2 (3)
[HA(aq)]
Rearranging (3) gives [H+(aq)]2
= [HA(aq)] Ka
therefore [H+(aq)] = [HA(aq)] Ka
pH = [H+(aq)]
ASSUMPTION HA is a weak acid so it will not have dissociated very much. You can assume that its equilibrium concentration is approximately that of the original concentration.
A weak acid is one which only partially dissociates in aqueous solution
Calculating pH - Calculating pH - weak acidsweak acids
Calculate the pH of a weak acid HX of concentration 0.1M ( Ka = 4x10-5 mol dm-3 )
HX dissociates as follows HX(aq) H+(aq) + X¯(aq)
WORKEDEXAMPLEWORKEDEXAMPLE
Calculating pH - Calculating pH - weak acidsweak acids
Calculate the pH of a weak acid HX of concentration 0.1M ( Ka = 4x10-5 mol dm-3 )
HX dissociates as follows HX(aq) H+(aq) + X¯(aq)
Dissociation constant for a weak acid Ka = [H+(aq)] [X¯(aq)] mol dm-3
[HX(aq)]
WORKEDEXAMPLEWORKEDEXAMPLE
Calculating pH - Calculating pH - weak acidsweak acids
Calculate the pH of a weak acid HX of concentration 0.1M ( Ka = 4x10-5 mol dm-3 )
HX dissociates as follows HX(aq) H+(aq) + X¯(aq)
Dissociation constant for a weak acid Ka = [H+(aq)] [X¯(aq)] mol dm-3
[HX(aq)]
Substitute for X¯ as ions are formed in [H+(aq)] = [HX(aq)] Ka mol dm-3
equal amounts and then rearrange equation
WORKEDEXAMPLEWORKEDEXAMPLE
Calculating pH - Calculating pH - weak acidsweak acids
Calculate the pH of a weak acid HX of concentration 0.1M ( Ka = 4x10-5 mol dm-3 )
HX dissociates as follows HX(aq) H+(aq) + X¯(aq)
Dissociation constant for a weak acid Ka = [H+(aq)] [X¯(aq)] mol dm-3
[HX(aq)]
Substitute for X¯ as ions are formed in [H+(aq)] = [HX(aq)] Ka mol dm-3
equal amounts and the rearrange equation
ASSUMPTIONHA is a weak acid so it will not have dissociated very much. You can assume that its equilibrium concentration is approximately that of the original concentration
WORKEDEXAMPLEWORKEDEXAMPLE
Calculating pH - Calculating pH - weak acidsweak acids
Calculate the pH of a weak acid HX of concentration 0.1M ( Ka = 4x10-5 mol dm-3 )
HX dissociates as follows HX(aq) H+(aq) + X¯(aq)
Dissociation constant for a weak acid Ka = [H+(aq)] [X¯(aq)] mol dm-3
[HX(aq)]
Substitute for X¯ as ions are formed in [H+(aq)] = [HX(aq)] Ka mol dm-3
equal amounts and the rearrange equation
ASSUMPTIONHA is a weak acid so it will not have dissociated very much. You can assume that its equilibrium concentration is approximately that of the original concentration
[H+(aq)] = 0.1 x 4 x 10-5 mol dm-3
= 4.00 x 10-6 mol dm-3
= 2.00 x 10-3 mol dm-3
ANSWER pH = - log [H+(aq)] = 2.699
WORKEDEXAMPLEWORKEDEXAMPLE
CALCULATING THE pH OF MIXTURESCALCULATING THE pH OF MIXTURES
The method used to calculate the pH of a mixture of an acid and an alkali depends on...
• whether the acids and alkalis are STRONG or WEAK
• which substance is present in excess
STRONG ACID and STRONG BASE - EITHER IN EXCESSSTRONG ACID and STRONG BASE - EITHER IN EXCESS
pH of mixturespH of mixtures
Strong acids and strong alkalis (either in excess)Strong acids and strong alkalis (either in excess)
1. Calculate the initial number of moles of H+ and OH¯ ions in the solutions
2. As H+ and OH¯ ions react in a 1:1 ratio; calculate unreacted moles species in excess
3. Calculate the volume of solution by adding the two original volumes
4. Convert volume to dm3 (divide cm3 by 1000)
5. Divide moles by volume to find concentration of excess the ion in mol dm-3
6. Convert concentration to pH
If the excess is H+ pH = - log[H+]
If the excess is OH¯ pOH = - log[OH¯] then
pH + pOH = 14
or use Kw = [H+] [OH¯] = 1 x 10-14 at 25°C therefore
[H+] = Kw / [OH¯] then
pH = - log[H+]
Calculate the pH of a mixture of 25cm3 of 0.1M NaOH is added to 20cm3 of 0.1M HCl
pH of mixturespH of mixtures
Strong acids and alkalis (either in excess)Strong acids and alkalis (either in excess)
WORKEDEXAMPLEWORKEDEXAMPLE
Calculate the pH of a mixture of 25cm3 of 0.1M NaOH is added to 20cm3 of 0.1M HCl
1. Calculate the number of moles of H+ and OH¯ ions present
pH of mixturespH of mixtures
Strong acids and alkalis (either in excess)Strong acids and alkalis (either in excess)
25cm3 of
0.1M NaOH
20cm3 of
0.1M HCl
2.5 x 10-3 moles
2.0 x 10-3 moles
moles of OH ¯
= 0.1 x 25/1000= 2.5 x 10-3
moles of H+
= 20 x 20/1000= 2.0 x 10-3
WORKEDEXAMPLEWORKEDEXAMPLE
Calculate the pH of a mixture of 25cm3 of 0.1M NaOH is added to 20cm3 of 0.1M HCl
1. Calculate the number of moles of H+ and OH¯ ions present
2. As the ions react in a 1:1 ratio, calculate the unreacted moles of the excess species
pH of mixturespH of mixtures
Strong acids and alkalis (either in excess)Strong acids and alkalis (either in excess)
The reaction taking place is… HCl + NaOH NaCl + H2O
or in its ionic form H+ + OH¯ H2O (1:1 molar ratio)
25cm3 of
0.1M NaOH
20cm3 of
0.1M HCl
2.5 x 10-3 moles
2.0 x 10-3 moles
WORKEDEXAMPLEWORKEDEXAMPLE
Calculate the pH of a mixture of 25cm3 of 0.1M NaOH is added to 20cm3 of 0.1M HCl
1. Calculate the number of moles of H+ and OH¯ ions present
2. As the ions react in a 1:1 ratio, calculate the unreacted moles of the excess species
pH of mixturespH of mixtures
Strong acids and alkalis (either in excess)Strong acids and alkalis (either in excess)
The reaction taking place is… HCl + NaOH NaCl + H2O
or in its ionic form H+ + OH¯ H2O (1:1 molar ratio)
2.0 x 10-3 moles of H+ will react with the same number of moles of OH¯
this leaves 2.5 x 10-3 - 2.0 x 10-3 = 5.0 x 10-4 moles of OH¯ in excess
5.0 x 10-4
moles of OH¯
UNREACTED
25cm3 of
0.1M NaOH
20cm3 of
0.1M HCl
2.5 x 10-3 moles
2.0 x 10-3 moles
WORKEDEXAMPLEWORKEDEXAMPLE
Calculate the pH of a mixture of 25cm3 of 0.1M NaOH is added to 20cm3 of 0.1M HCl
1. Calculate the number of moles of H+ and OH¯ ions present
2. As the ions react in a 1:1 ratio, calculate the unreacted moles of the excess species
3. Calculate the volume of the solution by adding the two individual volumes
pH of mixturespH of mixtures
Strong acids and alkalis (either in excess)Strong acids and alkalis (either in excess)
the volume of the solution is 25 + 20 = 45cm3
WORKEDEXAMPLEWORKEDEXAMPLE
Calculate the pH of a mixture of 25cm3 of 0.1M NaOH is added to 20cm3 of 0.1M HCl
1. Calculate the number of moles of H+ and OH¯ ions present
2. As the ions react in a 1:1 ratio, calculate the unreacted moles of the excess species
3. Calculate the volume of the solution by adding the two individual volumes
4. Convert volume to dm3 (divide cm3 by 1000)
pH of mixturespH of mixtures
Strong acids and alkalis (either in excess)Strong acids and alkalis (either in excess)
the volume of the solution is 25 + 20 = 45cm3
there are 1000 cm3 in 1 dm3
volume = 45/1000 = 0.045dm3
WORKEDEXAMPLEWORKEDEXAMPLE
Calculate the pH of a mixture of 25cm3 of 0.1M NaOH is added to 20cm3 of 0.1M HCl
1. Calculate the number of moles of H+ and OH¯ ions present
2. As the ions react in a 1:1 ratio, calculate the unreacted moles of the excess species
3. Calculate the volume of the solution by adding the two individual volumes
4. Convert volume to dm3 (divide cm3 by 1000)
5. Divide moles by volume to find concentration of excess ion in mol dm-3
pH of mixturespH of mixtures
Strong acids and alkalis (either in excess)Strong acids and alkalis (either in excess)
WORKEDEXAMPLEWORKEDEXAMPLE
[OH¯] = 5.0 x 10-4 / 0.045 = 1.11 x 10-2 mol dm-3
Calculate the pH of a mixture of 25cm3 of 0.1M NaOH is added to 20cm3 of 0.1M HCl
1. Calculate the number of moles of H+ and OH¯ ions present
2. As the ions react in a 1:1 ratio, calculate the unreacted moles of the excess species
3. Calculate the volume of the solution by adding the two individual volumes
4. Convert volume to dm3 (divide cm3 by 1000)
5. Divide moles by volume to find concentration of excess ion in mol dm-3
6. As the excess is OH¯ use pOH = - log[OH¯] then pH + pOH = 14
or Kw = [H+][OH¯] so [H+] = Kw / [OH¯]
pH of mixturespH of mixtures
Strong acids and alkalis (either in excess)Strong acids and alkalis (either in excess)
Kw = 1 x 10-14
mol2 dm-6 (at 25°C)
Kw = 1 x 10-14
mol2 dm-6 (at 25°C)
WORKEDEXAMPLEWORKEDEXAMPLE
[OH¯] = 5.0 x 10-4 / 0.045 = 1.11 x 10-2 mol dm-3
[H+] = Kw / [OH¯] = 9.00 x 10-13 mol dm-3
pH = - log[H+] = 12.05
CONTENTS
• What is a buffer solution?
• Uses of buffer solutions
• Acidic buffer solutions
• Alkaline buffer solutions
• Buffer solutions - ideal concentration
• Calculating the pH of a buffer solution
• Salt hydrolysis
• Check list
Buffer solutionsBuffer solutions
Before you start it would be helpful to…
• know that weak acids and bases are only partly ionised in solution
• be able to calculate pH from hydrogen ion concentration
• be able to construct an equation for the dissociation constant of a weak acid
Buffer solutionsBuffer solutions
Buffers
• A buffer is a solution whose pH is resistant to change on the addition of relatively small quantities of an acid or base.
• Buffers have the ability to absorb added H+
or OH- ions.
Buffer solutions - usesBuffer solutions - uses
Definition “Solutions which resist changes in pH when small quantities of acid or alkali are added.”
Biological Uses
In biological systems (saliva, stomach, and blood) it is essential thatthe pH stays ‘constant’ in order for any processes to work properly.e.g. If the pH of blood varies by 0.5 it can lead to unconsciousness and coma
Most enzymes work best at particular pH values.
Other Uses Many household and cosmetic products need to control their pH values.
Shampoo Buffer solutions counteract the alkalinity of the soap and prevent irritation
Baby lotion Buffer solutions maintain a pH of about 6 to prevent bacteria multiplying
Others Washing powder, eye drops, fizzy lemonade
Acidic Buffers
• Contain a weak acid and its salt formed with a strong base
• Ethanoic acid & Sodium ethanoate
CH3COOH & CH3COO-Na+
Buffer Action
• The important species present in the buffer solution are the
• undissociated weak acid CH3COOH
• and its conjugate base CH3COO-
• Undissociated ethanoic acid can remove any added OH- ions.
• CH3COOH + OH- → CH3COO- + H2O
• The conjugate base can remove any added H+ ions
• CH3COO- + H+ → CH3COOH
• For a buffer to work effectively it must contain a large reservoir of the weak acid and its conjugate base.
• CH3COOH + H2O CH3COO- + H3O+
• CH3COOH CH3COO- + H+
• Write an expression for Ka state units
Ka = [CH3COO-] [H+] [CH3COOH]
CH3COOH CH3COO- + H+
[H+] = Ka x [CH3COOH] [CH3COO-]
If both [CH3COOH] and [CH3COO-] are large………..
[H+] = Ka x [CH3COOH] [CH3COO-]
• If both [CH3COOH] and [CH3COO-] are large
• small changes in their concentrations will not affect the overall ratio significantly
• so [H+] remains almost constant
• So very small change in pH
Basic Buffers
• Contain a weak base and its salt formed with a strong acid
• Ammonia & Ammonium chloride
NH3 & NH4+
Cl-
Buffer Action
• The important species present in the buffer solution are the
• undissociated weak base NH3
• and its conjugate acid NH4+
• Undissociated ammonia can remove any added H+ ions.
• NH3 + H+ → NH4+
• The conjugate acid can remove any added OH- ions
• NH4+ + OH- → NH3 + H2O
Calculating the pH of a Buffer
• When performing acidic buffer calculations it is assumed that
• The acid is completely undissociated
• The A- ions are formed solely from the salt
Calculate the pH of a buffer solution produced by adding 3.28g of sodium ethanoate to 1dm3 of 0.01M ethanoic acid. The Ka of ethanoic acid is 1.84 x 10-5 at 300K
moles = mass/Mr
1. Find the number of moles of sodium ethanoate
Moles = 3.28/82 = 0.04
2. Find the [CH3COOH] and [CH3COO-]
[CH3COOH] = 0.01M [CH3COO-] = 0.04M
3. Find [H+]
[H+] = Ka x [CH3COOH] [CH3COO-]
[H+] = 1.84 x 10-5 x 0.01 0.04
= 4.6 x 10-6
3. Find pH
pH = -log[H+]
pH = -log 4.6 x 10-6 = 5.34
Calculate the pH of this buffer if 10cm3 of 0.1M HCl are now added
1. Find the number of moles of acid added
moles = conc x volume
moles of acid = 0.1 x 10/1000 = 1 x 10-3
CH3COO- + H+ → CH3COOH
1 x 10-3 + 1 x 10-3 → 1 x 10-3
Buffer solutions - Buffer solutions - ideal concentrationideal concentration
The concentration of a buffer solution is also important
If the concentration is too low, there won’t be enough CH3COOH and CH3COO¯
to cope with the ions added.
SummaryFor an acidic buffer solution one needs ...
large [CH3COOH(aq)] - for dissociating into H+(aq) when alkali is added
large [CH3COO¯(aq)] - for removing H+(aq) as it is added
This situation can’t exist if only acid is present; a mixture of the acid and salt is used.
The weak acid provides the equilibrium and the large CH3COOH(aq) concentration.
The sodium salt provides the large CH3COO¯(aq) concentration.
One uses a WEAK ACID + its SODIUM OR POTASSIUM SALT
Calculating the pH of an acidic buffer solutionCalculating the pH of an acidic buffer solution
Ka * concentration of acid
concentration of salt[H+] =
Ka * concentration of acidconcentration of salt
Log [H+] = Log
= Log Ka + Log concentration of acidconcentration of salt
=> (both sides put log)
=>(multiply all by -1) = - Log Ka - Log concentration of acidconcentration of salt
- Log [H+]
pKa - Log concentration of acidconcentration of salt
pH =
Calculating the pH of an acidic buffer solutionCalculating the pH of an acidic buffer solution
Calculate the pH of a buffer whose concentration of acid is 0.40 mol dm-3
Methanoic acid and is 1 mol dm-3 sodium ethanoate
Ka of ethaoic acid = 1.6*10-4
Ka * concentration of acid
concentration of salt[H+] =
1.6*10-4 *
0.40
1=
= 6.4*10-5
pH = -log (6.4*10-5 ) = 4.19
SALT HYDROLYSISSALT HYDROLYSIS
Many salts dissolve in water to produce solutions which are not neutral. This is because the ions formed react with the hydroxide and hydrogen ions formed when water dissociates. There are four distinct systems.
All dissociated ions are aqueous ions. When mixed, the ions of strong acids and bases remain apart. Ions of weak acids and bases associate.
SALT HYDROLYSISSALT HYDROLYSIS
Many salts dissolve in water to produce solutions which are not neutral. This is because the ions formed react with the hydroxide and hydrogen ions formed when water dissociates. There are four distinct systems.
All dissociated ions are aqueous ions. When mixed, the ions of strong acids and bases remain apart. Ions of weak acids and bases associate.
Salts of strong acids and strong bases - SODIUM CHLORIDE
NaCl dissociates completely in water Na+ Cl¯ ——> Na+ + Cl¯ Water only ionises to a very small extent H2O OH¯ + H+
Na+ and OH¯ are ions of a strong base so remain apart H+and Cl¯ are ions of a strong acid so remain apart
all the OH¯ and H+ ions remain in solution
therefore [H+] = [OH¯] and the solution will be NEUTRAL
SALT HYDROLYSISSALT HYDROLYSIS
Many salts dissolve in water to produce solutions which are not neutral. This is because the ions formed react with the hydroxide and hydrogen ions formed when water dissociates. There are four distinct systems.
All dissociated ions are aqueous ions. When mixed, the ions of strong acids and bases remain apart. Ions of weak acids and bases associate.
Salts of strong acids and weak bases - AMMONIUM CHLORIDE
NH4Cl dissociates completely in waterNH4+ Cl¯ ——> NH4
+ + Cl¯
Water only ionises to a very small extent H2O OH¯ + H+
NH3 + H2O
Na+ and OH¯ are ions of a strong base so tend to be associatedH+and Cl¯ are ions of a strong acid so remain apart
all the H+ ions remain in solution
therefore [H+] > [OH¯] and the solution will be ACIDIC
SALT HYDROLYSISSALT HYDROLYSIS
Many salts dissolve in water to produce solutions which are not neutral. This is because the ions formed react with the hydroxide and hydrogen ions formed when water dissociates. There are four distinct systems.
All dissociated ions are aqueous ions. When mixed, the ions of strong acids and bases remain apart. Ions of weak acids and bases associate.
Salts of weak acids and strong bases - SODIUM ETHANOATE
CH3COONa dissociates completely in water CH3COO ¯ Na + ——> Na+ + CH3COO¯
Water only ionises to a very small extent H2O OH¯ + H+
CH3COOH
Na+ and OH¯ are ions of a strong base so remain apartH+and CH3OO¯ are ions of a weak acid so tend to be associated
all the OH¯ ions remain in solution
therefore [OH¯] > [H+] and the solution will be ALKALINE
SALT HYDROLYSISSALT HYDROLYSIS
Many salts dissolve in water to produce solutions which are not neutral. This is because the ions formed react with the hydroxide and hydrogen ions formed when water dissociates. There are four distinct systems.
All dissociated ions are aqueous ions. When mixed, the ions of strong acids and bases remain apart. Ions of weak acids and bases associate.
Salts of weak acids and weak bases - AMMONIUM ETHANOATE
CH3COONH4 dissociates completely in water CH3COO ¯ NH4 + ——> NH4+ + CH3COO¯
Water only ionises to a very small extent H2O OH¯ + H+
NH3 + H2O CH3COOH
Na+ and OH¯ are ions of a weak base so tend to be associatedH+and CH3OO¯ are ions of a weak acid so tend to be associated
the solution might be alkaline or acidic i.e. APPROXIMATELY NEUTRAL
REVISION CHECKREVISION CHECK
What should you be able to do?
Recall the definition of a buffer solution
Recall the difference between an acidic and an alkaline buffer solution
Recall the uses of buffer solutions
Understand the action of buffer solutions
Calculate the pH of an acidic buffer solution
Recall and understand the reactions due to salt hydrolysis
CAN YOU DO ALL OF THESE? CAN YOU DO ALL OF THESE? YES YES NONO
You need to go over the You need to go over the relevant topic(s) againrelevant topic(s) again
Click on the button toClick on the button toreturn to the menureturn to the menu
REVISION CHECKREVISION CHECK
What should you be able to do?
Calculate pH from hydrogen ion concentration
Calculate hydrogen ion concentration from pH
Write equations to show the ionisation in strong and weak acids
Calculate the pH of strong acids and bases knowing their molar concentration
Calculate the pH of weak acids knowing their Ka and molar concentration
Calculate the pH of mixtures of strong acids and strong bases
CAN YOU DO ALL OF THESE? CAN YOU DO ALL OF THESE? YES YES NONO
You need to go over the You need to go over the relevant topic(s) againrelevant topic(s) again
Click on the button toClick on the button toreturn to the menureturn to the menu