Contents · As heart failure evolves, ATP synthesis from oxidation of metabolic substrates by...
Transcript of Contents · As heart failure evolves, ATP synthesis from oxidation of metabolic substrates by...


Contents
Conte
nts
EDITORIAL .....................................................................................................................................................................................3Energetics in heart diseaseD. Feuvray
BASIC ARTICLE .........................................................................................................................................................................5Myocardial energetics and efficiencyE. Aasum
MAIN CLINICAL ARTICLE ...............................................................................................................................................9Modulation of cardiac energetics as a target in ischemic heart diseaseH. Ashrafian
METABOLIC IMAGING ....................................................................................................................................................14Glucose metabolism as a marker of myocardial ischemiaP. G. Camici
NEW THERAPEUTIC APPROACHES .................................................................................................................19An expanded role for AMP kinase: self-renewal of the cardiomyocyte K. K. Baskin and H. Taegtmeyer
FOCUS ON TRIMETAZIDINE (Vastarel®MR) ..............................................................................................25Beneficial effects of trimetazidine (Vastarel®MR) in patients with chronic heart failureG. Fragasso, L. Alberti and L. Lauretta
CASE REPORT ........................................................................................................................................................................29Myocardial power delivery is impaired in progressive left ventricular pump failure: a case reportF. L. Dini
REFRESHER CORNER ....................................................................................................................................................33Impact of fatty acid oxidation on cardiac efficiencyN. Fillmore and G. D. Lopaschuk
HOT TOPICS ..............................................................................................................................................................................38Statin therapy: reduction in cardiovascular events still pays in new-onset diabetesA. Huqi
GLOSSARY ..................................................................................................................................................................................40G. D. Lopaschuk
Heart Metab. (2011) 53:1

Energetics in heart diseaseDanielle Feuvray, CNRS UMR 8162, Marie Lannelongue Hospital,
Le Plessis Robinson, France
Correspondence: Danielle Feuvray, UPS & CNRS UMR 8162,Marie Lannelongue Hospital, 92350 Le Plessis Robinson, France.
e-mail: [email protected]
Even subtle variations in the efficiency of energy generation or utilization may have a pro-found impact on cellular energy levels. Different cardiac pathologies can alter cardiac effi-
ciency, both as a result of a decrease efficiency of producing ATP or alterations in the efficiencyof using ATP to produce contractile work. Given that the requirement for ATP for all metabolicprocesses and for cell viability is absolute, a renewed interest in metabolism has led to identifi-cation of the molecular links between physiological and metabolic stimuli and the regulation ofgene expression in the heart. Metabolism remodels in the failing heart leading to the inability toincrease ATP supply. Ultimately, this may lead to a fall in ATP. The likely time line is decreasedenergy production via the phosphotransferase reactions (creatine kinase and adenylate kinase)leading to increases in ADP and AMP. As heart failure evolves, ATP synthesis from oxidation ofmetabolic substrates by mitochondria, the major source of ATP in the heart, falls. Remodelingof the failing myocardium is controlled by energy sensors, such as AMP-activated proteinkinase (AMPK) that regulates energy substrate metabolism and regulates transcription ofmetabolic genes. This issue of Heart and Metabolism addresses the important topic of ener-getics in heart disease.
In the Basic Article, Dr. Aasum offers a concise review of myocardial energetic mechanisms,focusing on alterations in processes related to energy production and energy utilization in thefailing heart. The Main Clinical Article by Dr. Ashrafian gives a clear and elegant overview ofsuccessful metabolic therapies presently available, especially for chronic ischemic heartdisease. While considering the likely future directions for metabolic therapy, Dr. Ashrafian alsopoints out the need for greater experience with the existing metabolic therapies, which couldbenefit most to those patients with concomitant metabolic disease, such as metabolicsyndrome or diabetes mellitus.
In this context the Refresher Corner article by Drs. Fillmore and Lopaschuk provides adidactic summary of the state of the art, showing notably how metabolic substrates competeat myocardial cell level for energy production and how they may affect cardiac efficiency.Alterations in the balance between fatty acid and glucose use are known to occur in certainheart pathologies such as during ischemia and in the failing heart. This leads to decreased car-diac efficiency through a number of mechanisms that are reviewed and discussed herein,among which are intracellular ionic (H+, Na+, Ca2+) disturbances and their deleterious conse-quences. Measuring metabolic substrate utilization in humans has been difficult. The MetabolicImaging article by Dr. Camici underlines that although the quantification of glucose utilizationrates in patients encounters many difficulties, positron emission tomography with the glucose
Editoria
lEDITORIAL - DANIELLE FEUVRAY
Heart Metab. (2011) 53:3–4 3

analogue 18F-fluorodeoxyglucose (FDG) may help toestablish values of the metabolic rates of glucose utili-zation in normal and pathologic conditions.
Furthermore, Drs. Baskin and Taegtmeyer pro-vide an authoritative New Therapeutic Approachesarticle that broadens the role of energy substratemetabolism from a provider of ATP to a regulator ofself-renewal of cardiac myocytes. They highlight theexciting new concept of how heart muscle cells canrenew themselves from within by the identification ofcertain metabolic signals as a root cause for alteredrates of intracellular protein turnover and, hence,self-renewal of cardiac myocytes. Metabolic remodel-ing precedes, triggers and sustains structural andfunctional remodeling of the heart. As mentionedbefore, AMPK supports energy provision in thecell by sensing changes in the ratio [ATP]/[AMP]. In
addition, decrease in [ATP]/[AMP] and the subsequentactivation of AMPK regulate protein degradation.Since individual proteins are degraded through theubiquitin proteasome system Drs. Baskin and Taegt-meyer investigated the role of AMPK in proteasome-mediated protein degradation. They found thatproteasome-mediated protein degradation in theheart is indeed increased with AMPK activation.They therefore speculate that the activation ofAMPK results in enhanced availability of intracellularamino acids for either ATP production or the syn-thesis of new proteins as the heart adapts to a newphysiological state. These most recent data advancea new understanding of cardiac metabolism. Theyshould also set us on the path to develop novel strat-egies aimed at optimizing metabolic therapy in heartdisease. ●
EDITORIAL - DANIELLE FEUVRAY
4 Heart Metab. (2011) 53:3–4

Myocardial energetics and efficiencyEllen Aasum, Cardiovascular Research Group Faculty of Health Sciences, University of Tromsø, Norway
Correspondence: Ellen Aasum, Cardiovascular Research Group,Institute of Medical Biology, Faculty of Health Sciences,
University of Tromsø, N-9037 Tromsø, Norway.Tel: +47 77646486 fax: +47 77645440, e-mail: [email protected]
AbstractIn addition to structural and functional abnormalities, it is well established that energy homeostasis isimpaired in the failing heart. As the heart requires large amounts of energy to sustain its continuouspumping activity, it is highly dependent on an optimal substrate metabolism with efficient ATP genera-tion and utilization. Alterations in processes related to energy production as well as energy utilization inthe failing heart may lead to energetic imbalance, an inefficient hearts with impaired contractile reserve.
Keywords: cardiac energy metabolism; oxygen consumption; mechano energetics; heart failure
■ Heart Metab. (2011) 53:5–8
Introduction
The heart maintains its pumping action by converting chemical energy in metabolic substratesinto mechanical energy, and because of its high energy requirement and relatively low contentof high energy compounds (ATP and creatine phosphate [PCr]) ATP must be continuouslygenerated at a high rate. Thus, the heart must adjust energy production to energy utilization,and at the same time secure an efficient energy transfer. These processes involve substrateuptake, breakdown and entry into the Krebs cycle, as well as mitochondrial oxidative phos-phorylation, ATP transfer (e.g., the creatine kinase energy shuttle), and hydrolysis at the energyconsuming sites. The metabolically healthy heart has the capacity to switch between lipids andcarbohydrates as energy substrates, and the fuel selection is to a large extent governed by theavailability of plasma substrates, as well as the levels of hormones (insulin and catecholamines)in the circulation. Since the majority (>90%) of ATP production is derived from mitochondrialoxidative phosphorylation, myocardial oxygen consumption (mVO2) in the normoxic heart canbe used as a measure of the total myocardial energy utilization. Mechanical efficiency, whichconnote the ability of the heart to perform its functions, is the ratio between external (stroke)work and mVO2 [1]. Decreased mechanical efficiency has been suggested to play a leadingrole in the pathogenesis of a number of cardiovascular conditions leading to heart failure. Theimbalance between energy demand and availability will ultimately lead to an energetically com-promised heart with reduced working capacity. As decreased efficiency will be particularly dis-advantageous under conditions of reduced oxygen availability, it will also contribute to theincreased susceptibility of the failing myocardium to acute ischemia or hypoxia.
The failing heart is energetically compromised
In accordance with this notion, clinical and experimental studies on heart failure, using 31Pmagnetic resonance spectroscopy (MRS), have revealed a decreased cardiac PCr:ATP ratio.
Basic
Article
BASIC ARTICLE - ELLEN AASUM
Heart Metab. (2011) 53:5–8 5

Decreased PCr:ATP ratio has been shown to corre-late with the severity of heart failure in patients with idi-opathic dilated cardiomyopathy [2] and to be a pre-dictor of mortality in these patients [3]. DecreasedPCr:ATP ratio is also found in hearts from type 2 dia-betic patients [4], which show increased prevalenceand worsened prognosis of heart failure [5].
Impaired ATP homeostasis in the failing heart isobviously multifactorial and complex, includingreduced ATP production, loss of the total adeninenucleotide pool and changes in the creatine kinasesystem, which in turn affect the energy transport tothe energy consuming sites, such as myofibrilsand sarcoplasmatic reticulum (SR) [2,6–8]. The failingheart is also characterized by altered energy sub-strate utilization. The mechanisms behind thesemetabolic changes are complex due to the hetero-geneous etiology of heart failure, as well as to differ-ences in the progression of the disease. Experimentalmodels of heart failure generally report decreasedfatty acid oxidation and increased reliance on glucoseoxidation and glycolysis, with a depressed overalloxidative metabolism in end-state failure [9]. Changesin human hearts show less consistency with respectto fuel selection, likely due to the complexity anddiversity of the metabolic status of these patients.Patients with heart failure often have increased plasmanoradrenalin and free fatty acid concentrations reflect-ing stress hormone-induced lipolysis [10]. In addition,co-morbidities such as obesity, insulin-resistanceand type 2 diabetes will influence myocardial substrateutilization. In the uncompensated state, the fattyacid oxidation pathway is generally down-regulated(metabolic remodeling due to a decline in the activationof the transcription factors PPARα), and glucoseuptake and oxidation is insufficient to secure an ade-quate energy production. Altered mitochondrial mass,structure, and functional capacity have also beendemonstrated in failing myocardium. Whether inade-quate oxygen availability or metabolic substrate supplyare limiting factors for oxidative capacity is not clear.Several studies have, however, shown decreasedactivity of the complexes of the respiratory chain,Krebs’ cycle enzymes and the ATP synthase (F0F1)[8], and functional studies also suggest that mitochon-dria from failing hearts are less coupled [11]. As there isa clear correlation between oxidative ATP productionand heart work, decreased mitochondrial oxidative
capacity and/or loss of functional coupling withsites of energy utilization, can limit the heart’s abilityto generate work and thus contribute to cardiacdysfunction.
Myocardial efficiency and mechano-energetics
Decreased myocardial mechanical efficiency in the fail-ing heart is a consistent and early finding both clinicallyand in experimental models. Assessment of myo-cardial mechanical efficiency is an important clinicaltool for evaluation of the outcome of therapies. As illus-trated in the Fig. 1, energy is used for both mechanicaland non-mechanical processes in the heart. The latterdeals with energy used in excitation-contraction cou-pling (ECC), i.e., cardiac sarcoplasmic reticulum func-tion, notably calcium pumping, and basal metabolism(BM), and is consequently referred to as the work-independent mVO2. Energy for the mechanicalprocesses (total mechanical energy, TME), includesgeneration of myocardial force and pressure in the ven-tricular wall (potential energy) and for ejection of bloodagainst an afterload pressure (external work, EW).Oxygen cost for mechanical work is therefore work-dependent and correlates closely with TME of theheart (panel B). This principle implies that each stepco-determines the overall mechanical efficiency, andthat mechanical efficiency not only depends on intrin-sic properties of the heart, but also strongly on hemo-dynamic conditions (loading conditions) [12]. Assess-ment of the relationship between mVO2 and TME inexperimental models of heart failure can reveal theunderlying mechanisms leading to mechanical ineffi-ciency by identifying mechano-energetic changes inthe heart. Failing hearts in different experimental mod-els (pressure/volume overload, coronary microemboli-sation, rapid ventricular pacing, diabetes, infarctedhearts) have generally reveal unchanged or decreasedslope of this relationship, which suggest unchangedor improved efficiency of the chemo-mechanical energytransduction (contractile efficiency) [12]. These changesmay reflect an adaptive response to the impairedenergy balance, and has been associated with a shiftfrom the myosin heavy chain (MHC) α isoform to theslower, but more economical, β isoform in rodentmodels. The functional role of such a shift in MHC iso-form in larger mammals (including human) is, however,less clear.
BASIC ARTICLE - ELLEN AASUM
6 Heart Metab. (2011) 53:5–8

The failing heart shows increased oxygen costfor non-mechanical processes
The y-intercept of the work-mVO2 relationship reflectsthe oxygen cost for non-mechanical processes(unloadedmVO2) [12], which is reported to be increasedin several models of heart failure. Altered unloadedmVO2 may be related to altered myocardial Ca2+
handling, altered substrate utilization and/or inductionof oxygen wasting processes. Decreased sarcoplas-mic reticulum (SR) Ca2+ATPase (called SERCA2 in theheart) expression and activity are generally acceptedas important mediators of cardiac dysfunction in thefailing heart. As a compensatory mechanism, sarco-lemmal Na+-Ca2+ exchange activity is increased,which energetically will lead to a less efficient Ca2+
transport during excitation-contraction coupling. Inaddition, increased sarcoplasmic reticulum Ca2+ leak,as well as desynchronised Ca2+ release via SR calciumchannels may also contribute to increased oxygencost for Ca2+ handling in these hearts. The pivotalrole of SERCA2 in ventricular dysfunction is sup-ported by studies demonstrating enhanced contractilefunction via either transgenic approaches or adenoviralgene transfer. Hence, supportive SERCA2 gene ther-apy is a potential treatment strategy for heart failure.There are, however, controversies with regard to theenergetic consequence of such interventions [13].
Since fatty acids is an energetically less efficientenergy substrate compared to glucose (i.e. require a
higher oxygen consuming due to a lower ATP:oxygenratio), the switch towards glucose in the failing heart isgenerally regarded an adaptive mechanism. On theother hand, the predominant myocardial fatty acid oxi-dation in diabetes has been associated with increasedmVO2 and decreased mechanical efficiency [9]. Basedon this, reduction of fatty acid oxidation by inhibitingfatty acid transport into the mitochondria, or fatty acidβ-oxidation, has proven beneficial in animal models ofheart failure and in clinical trials [9,10,14,15]. Mjøs andcoworkers demonstrated more that 40 year ago, thatelevation of circulating fatty acids induced oxygenwastage and decreased mechanical efficiency in anopen chest dog model [16]. This fatty acid-inducedincrease in mVO2 is due to increased oxygen cost fornon-mechanical purposes [17], and cannot solely beascribed to increased fatty acid oxidation rate, as ashift from glucose to fatty acid oxidation can onlyaccount for approximately 1/3 of the increasedmVO2. Fatty acids are ligands for PPARα that regulatethe expression of uncoupling proteins (UCPs), andalthough the role of mitochondrial uncoupling in heartfailure is not definitively established, UCP expressionhas been shown to correlate to circulating fatty acidconcentrations in human and animal samples [11,18].In experimental studies the presence of fatty acids hasalso been shown to decrease cardiac mechanical effi-ciency, not only in the normal heart [17] but also in thechronically infarcted rat heart [11]. Finally there are
A B
MVO2
mV
O2ATP
ECC
ECC
TME
TME
BM
BMEW
heat
heat
heat
heat
Contractille Effic
iency
(1/slope)
work-dependent mVO2
work-independent mVO2PE
Fig. 1 Panel A. Energy flow diagram from oxygen consumption to external work (EW) via ATP. ATP in the heart is used for non-mechanical activity (excitation-contraction coupling, ECC) and basal metabolism (BM), and for generating total mechanical energy (TME)which includes generation of myocardial tension in the ventricular wall (potential energy, PE) within the ventricular wall and pressure in the leftventricle for ejection of blood against an afterload pressure (external work, EW). Panel B. A linear relationship exists between total mechani-cal energy (TME) and mVO2, where the y-intercept defines the work-independent mVO2, and the inverse of the slope of the relationship willindicate the efficiency of oxygen to TME (contractile efficiency). Adapted from Suga (1990) [12].
BASIC ARTICLE - ELLEN AASUM
Heart Metab. (2011) 53:5–8 7

evidence linking fatty acids and oxidative stress tomitochondrial uncoupling in several models of heartfailure [10,19].
Despite favorable effects of current therapies, thehigh mortality rate in heart failure indicates the needfor developing new and more targeted therapeuticstrategies [20]. While the current treatments of heartfailure (ACE inhibitors, cardiac β-blockers and resyn-chronization therapy) aim to decrease energy demand,future strategies could focus on re-establishing theenergetic balance by also improving energy produc-tion and/or reducing processes leading to themechano-energetic uncoupling in the failing heart. ●
References
1. Bing RJ, Hammind MM, Handelsman JC, Powers SR, Spen-cer FC, Eckenhoff JE, Goodal MD, Hafkenschiel JH, Kety SS(1949) The measurement of coronary blood flow, oxygen con-sumption, and efficiency of the left ventricle in man. Am HeartJ 38(1):1–24
2. Neubauer S (2007) The failing heart--an engine out of fuel.N Engl J Med 356(11):1140–1151
3. Neubauer S, Horn M, Cramer M, Harre K, Newell JB, PetersW, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K (1997)Myocardial phosphocreatine-to-ATP ratio is a predictor ofmortality in patients with dilated cardiomyopathy. Circulation96(7):2190–2196
4. Scheuermann Freestone M, Madsen PL, Manners D, BlamireAM, Buckingham RE, Styles P, Radda GK, Neubauer S,Clarke K (2003) Abnormal cardiac and skeletal muscle energymetabolism in patients with type 2 diabetes. Circulation 107(24):3040–3046
5. Kannel WB, McGee DL (1979) Diabetes and cardiovasculardisease. The Framingham study. JAMA 241(19):2035–2038
6. Ventura-Clapier R, Garnier A, Veksler V, Joubert F (2011)Bioenergetics of the failing heart. Biochim Biophys Acta1813(7):1360–1372
7. Ingwall JS, Weiss RG (2004) Is the failing heart energystarved? On using chemical energy to support cardiac func-tion. Circ Res 95(2):135–145
8. Ingwall JS (2009) Energy metabolism in heart failure and remo-delling. Cardiovasc Res 81(3):412–419
9. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, StanleyWC (2010) Myocardial fatty acid metabolism in health and dis-ease. Physiol Rev 90(1):207–258
10. Opie LH, Knuuti J (2009) The adrenergic-fatty acid load inheart failure. J Am Coll Cardiol 54(18):1637–1646
11. Murray AJ, Cole MA, Lygate CA, Carr CA, Stuckey DJ, LittleSE, Neubauer S, Clarke K (2008) Increased mitochondrialuncoupling proteins, respiratory uncoupling and decreasedefficiency in the chronically infarcted rat heart. J Mol Cell Car-diol 44(4):694–700
12. Suga H (1990) Ventricular energetics. Physiol Rev 70(2):247–277
13. Pinz I, Tian R, Belke D, Swanson E, Dillmann W, Ingwall JS(2011) Compromised myocardial energetics in hypertrophiedmouse hearts diminish the beneficial effect of overexpressingSERCA2a. J Biol Chem 286(12):10163–10168
14. Abozguia K, Elliott P, McKenna W, Phan TT, Nallur-Shivu G,Ahmed I, Maher AR, Kaur K, Taylor J, Henning A, Ashrafian H,Watkins H, Frenneaux M (2010) Metabolic modulator perhexi-line corrects energy deficiency and improves exercise capacityin symptomatic hypertrophic cardiomyopathy. Circulation122(16):1562–1569
15. Fragasso G, Perseghin G, De CF, Esposito A, Palloshi A,Lattuada G, Scifo P, Calori G, Del MA, Margonato A (2006)Effects of metabolic modulation by trimetazidine on left ventric-ular function and phosphocreatine/adenosine triphosphateratio in patients with heart failure. Eur Heart J 27(8):942–948
16. Mjøs OD (1971) Effect of fatty acids on myocardial functionand oxygen consumtion in intact dogs. J Clin Invest50:1386–1389
17. Korvald C, Elvenes OP, Myrmel T (2000) Myocardial substratemetabolism influences left ventricular energetics in vivo. Am JPhysiol Heart Circ Physiol 278(4):H1345–H1351
18. Murray AJ, Anderson RE, Watson GC, Radda GK, Clarke K(2004) Uncoupling proteins in human heart. Lancet364(9447):1786–1788
19. Echtay KS, Pakay JL, Esteves TC, Brand MD (2005) Hydroxy-nonenal and uncoupling proteins: a model for protectionagainst oxidative damage. Biofactors 24(1–4):119–130
20. Mudd JO, Kass DA (2008) Tackling heart failure in the twenty-first century. Nature 451(7181):919–928
BASIC ARTICLE - ELLEN AASUM
8 Heart Metab. (2011) 53:5–8

Modulation of cardiac energeticsas a target in ischemic heart disease
Houman Ashrafian, Department of Cardiovascular Medicine, Oxford University, Oxford, United Kingdom
Correspondence: Houman Ashrafian, Department of Cardiovascular Medicine, University of Oxford,John Radcliffe Hospital, Oxford, OX3 9DU, United Kingdom.
e-mail: [email protected]
AbstractSubstantial advances in mechanical and adjunctive pharmacological therapies have reduced theconsequences of ischemic heart disease. Despite these advances, cardiovascular disease and itsmajor contributor coronary artery disease continue to accrue substantial morbidity and mortality.Metabolic therapies (ranging from insulin to fatty acid oxidation inhibitors and late sodium channelcurrent inhibitors) represent a novel and immediately clinical relevant class of therapies that cancontribute to improving patient outcomes. In the current article, I will discuss the basic biology ofcardiac metabolism and the clinical efficacy of agents, some of which have direct clinical applicability.As well as outlining the considerations that may culminate in the effective deployment of these agentsin the care of patients, I also consider the likely future directions for metabolic therapy in cardiovas-cular disease.
Keywords: cardiac energetics; ischemic heart disease; metabolic therapies; cardiac metabolism
■ Heart Metab. (2011) 53:9–13
Introduction
Cardiovascular disease remains the leading cause of mortality in the developed world and hasemerged as a major cause of morbidity and mortality in the developing world [1]. Ischemicheart disease resulting from coronary artery disease, along with hypertensive heart disease,represents the engine of cardiovascular mortality and is consequent upon the lifestyle changesoccurring with increasing “development” (e.g., smoking, obesity, psychosocial factors andsedentary lifestyle) [2]. Advances in mechanical interventions (e.g., percutaneous coronaryintervention and coronary artery bypass surgery) that augment the oxygen supply to the myo-cardium coupled with therapies that reduce cardiac oxygen demand (e.g., β-blockers andivabradine, which reduces heart rate exclusively by acting on the sinus node-If current) are themainstay of current therapy. However, despite the use of these therapies, many patientsremain symptomatic, expanding the reservoir of aging patients who, having undergone thedemographic transition, ail with angina and chronic heart failure. To combat the morbidity andmortality associated with these conditions, novel therapies are urgently required—therapiesthat modify cardiac metabolism represent a hitherto practically unexplored group of treatmentsthat are increasingly recognized to have promise in addressing the chronic consequences ofcardiac disease [3].Main
Clinic
alArticle
MAIN CLINICAL ARTICLE - HOUMAN ASHRAFIAN
Heart Metab. (2011) 53:9–13 9

Metabolic changes in the ischemic myocardium
It is frequently stated that the heart is capable ofmetabolizing a range of substrates, including freefatty acids (FFA), glucose and other carbohydrates(e.g. pyruvate and lactate) and even amino acids. Theconsequence of changing this substrate is less fre-quently considered.
A striking exemplification of the influence of metabo-lism on myocardial structure, albeit in the reptile hearts,can be adduced from the influence of metabolism onBurmese python hearts. These reptile hearts hypertro-phy by >40% after the snakes’ monthly meal [4]. Thisentirely physiological—and likely reversible—structuralchange derives from the metabolism of a combinationof three fatty acids: myristic, palmitic, and palmitoleicacids. Importantly, mice hearts also undergo cardiachypertrophy (~10%) when exposed to the same cock-tail of FFA. Thus while the mammalian heart is thereforea metabolic omnivore [5], the choice of substrate hasprofound consequences on cardiac structure, energet-ics and function. For example, theoretically a completeswitch from FFA metabolism as an energy source toglucose can save 11–13% of myocardial oxygen usebased on stoichiometric considerations (and by ~50%as measured experimentally in pig and canine hearts).
The healthy adult myocardium, especially duringfasting, preferentially metabolizes FFA and their deriva-tives (60–100%) [3]. In hypertrophy and heart failure, itis believed (though not unanimously accepted) that adownregulation of fatty acid metabolism is compen-sated for by increased carbohydrate metabolism inan attempt to spare oxygen. Although far from experi-mentally confirmed, during myocardial ischemia arapid activation of AMP-activated protein kinase(AMPK) occurs, resulting in an activation of both glu-cose uptake and an increase in fatty acid oxidation [6].It is therefore presumed that the ischemic myocardiumcontinues to rely on FFA metabolism with the attend-ant inefficiency of this substrate. Not only do FFAconfer an oxygen utilization penalty on the heart at atime of blood/oxygen deficiency, but inappropriatelyhigh FFA metabolism may, as Randle proposed,compromise coupled glucose metabolism and haveespecially adverse sequelae on ischemic hearts (e.g.,due to the influence of excess FFA on calcium transi-ents in ischemia).
Accordingly, substantial emphasis has been placedon physiological or therapeutic strategies designed to
suppress FFA uptake and/or oxidation in order to stim-ulate coupled myocardial glucose utilization. Althoughthis substrate switch continues to be the primary focusfor metabolic therapies, it is increasingly recognizedthat redox and aldehyde-induced stress responsescan effect a shift in glucose metabolism from glycolysisto the pentose phosphate pathway [7]. Such studiesprovide a rationale for investigating other such signalingpathways with a broader view to elaborating resistanceagainst acute oxidative stress induced by ischemia/reperfusion through metabolism.
Metabolic therapies
Glucose-insulin
In an attempt to recapitulate and exaggerate “physio-logical” glucose uptake into myocardium, Sodi-Pallareset al., in 1962, successfully applied “polarizing solution”,i.e., glucose-insulin-potassium infusion (GIK), for treat-ment of acute myocardial infarction. GIK infusion wasinitially thought to confer benefit primarily by increasingglycolysis and by reducing in FFA uptake and meta-bolism. More recently, we have demonstrated thatGIK treatment also engenders a number of signalingchanges (e.g., increased signaling protein phosphoryla-tion and O-GlcNAcylation) likely to contribute to myo-cardial protection [8].
A number of early studies supported this earlypromise, for example the ECLA (Estudios Cardiológi-cos Latinoamérica) Collaborative Group were able toshow a dramatic reduction of death rate of acute myo-cardial infarction from 11.5% in the control group to6.7% in patients treated with GIK. However the nega-tive results of large trials such as the CREATE-ECLAtrial, which studied 20,201 patients with ST-elevationacute myocardial infarction, mostly in India and China,have questioned the role of GIK in the context of mod-ern reperfusion therapy [9]. The conclusions of the lat-ter study are moderated by the observation that GIKmay have been given too late to be effective [10], itsefficacy may have depend on the dose, its efficacymay have been limited by pharmacokinetics and phar-macodynamics and may depend on the exact popula-tion studied (including the nature of the adjunct phar-macology/coronary revascularisation).
Nevertheless, the current evidence suggests thatGIK provision as performed in existing trials does notreduce mortality in patients with AMI but that tight
MAIN CLINICAL ARTICLE - HOUMAN ASHRAFIAN
10 Heart Metab. (2011) 53:9–13

glycemic control is beneficial [11]. One way in whichthese limitations of insulin have been overcome is bythe use of the incretin glucagon-like peptide-1 (GLP-1),which has demonstrable cardioprotective properties inexperimental models and patients with cardiac ische-mia [12]. Indeed, there is accumulating evidence sug-gests that albiglutide, a novel longer lasting GLP-1,rather akin to the early GIK studies, may protect theheart against from ischemic injury by altering substrateuse and ameliorating cardiac energetics [13].
Partial fatty oxidation (pFOX) inhibitors
In order to achieve a more enduring and practicableswitch between FFA and carbohydrate metabolism,a number of inhibitors of fatty acid oxidation havebeen sought and executed. CPT-1 is the rate-limitingenzyme that transports FFA into the mitochondria.Pharmacological inhibition of CPT-1 by etomoxir,oxfenecine and perhexeline and experimental malonylCoA decarboxylase inhibitors (which augments thenative CPT-1 inhibitor - malonyl CoA) have beeninvestigated in pre-clinical models and human stud-ies of cardiac ischemia. Similarly, the β-oxidationenzymes downstream of CPT-1, such as mitochon-drial 3-ketoacyl-CoA thiolase inhibited by trimetazi-dine, are recognized to be therapeutic targets in thetreatment of ischemic heart disease. Notably, despitethe challenges of dosemonitoring and intellectual prop-erty issues, both perhexiline and trimetazidine continueto be used successfully in the clinical setting [14].
It is important to recognize that the more potentpFOX inhibitors (inhibitors) tend to be limited by theirside effect of cardiac lipotoxicity arising from excesscardiac lipid accumulation (etomoxir, oxfenecine). Incontrast, competitive inhibitors of these enzymessuch as perhexiline and trimetazidine, which allowexcess endogenous FFA to break through the inhibi-tion, do not show such effects. It is likely, therefore,that any successful cardiac metabolic therapy shouldbe carefully moderated in order to prevent extremeinhibition of any single metabolic pathway, highly likelyto be harmful to an organ.
Late sodium channel current
In the ischemic myocardium, inhibition of the energy-requiring Na+/K+ ATPase and other ATP dependentcurrents results in excess of myocellular sodium load-ing through failure of sodium efflux. The late sodiumcurrent, as a result of its persistent flow throughout
the action potential, may make a substantial contribu-tion to this ischemic sodium loading [15]. Excesssodium loading increases oxidative stress, increasesmyocellular calcium loading perhaps through the influ-ence of sodium on calcium countertransport throughNCX and depletes mitochondria of their calcium (whichreduces the mitochondrial Kreb’s cycle activity andexacerbates energy deficiency) [16]. The mechanismthrough which blocking late inward sodium currents,leads to a reduction in angina remains the subjectof active investigation but ranolazine, a late inwardsodium current blocker with pFOX activity [17] doesexhibit some anti-anginal properties [18].
The Future
A number of successful metabolic therapies are there-fore presently available for clinical therapy, especiallyfor chronic ischemic heart disease. Two directionsremain to be pursued.
Greater experience with existing therapies
Substantial advances have been made in developingand demonstrating the safety and efficacy of a numberof metabolic agents for the treatment of ischemic heartdisease. Indeed a number of these agents, e.g., rano-lazine and trimetazidine, have been tested in clinicaltrials. The paucity of use of these agents partiallyreflects the requirement for further education of clinicalcolleagues. However, perhaps a more trenchant rea-son for the lack of extensive use of these agentsderives from a lack of clarity about the ideal contextfor their use. Existing agents such as β-blockers,nitrates, calcium channel antagonists and specificrate-limiting agents, such as ivabradine, all success-fully mitigate the consequences of ischemia in manypatients. One of the challenges for many practitionersis, to identify the population in which these productswill offer them the most adapted benefits, e.g., thosewith concomitant metabolic disease such as themetabolic syndrome/diabetes mellitus or those withventricular dysfunction, whose cardiac metabolicdysfunction may respond especially well to metabolicmodulation.
Novel metabolic therapies
While inhibition of FFA oxidation continues to representa credible strategy for the treatment of chronic ische-mia, there is substantial potential for identifying novelmetabolic nodes for treatment. Existing interesting
MAIN CLINICAL ARTICLE - HOUMAN ASHRAFIAN
Heart Metab. (2011) 53:9–13 11

metabolic therapies such as dichloroacetate which,through liberating pyruvate dehydrogenase from itskinase inhibitors, augments carbon flux into theKreb’s cycle have been disappointing by virtue oftheir pharmacokinetics and their potential side effects.However, there are a number of novel avenues to pur-sue. Firstly, it is increasingly recognized that as well astheir roles in energy generation, metabolites canmarshal a wider group of biological responses thatare amenable to therapeutic modulation. For example,the Kreb’s cycle intermediate, succinate, acting throughG protein-coupled receptor-91, can determine angio-genesis as a response to chronic ischemic stress.This observation supports the contention that a broadervision of metabolic manipulation is likely to elevate met-abolic therapy beyond energy modulation [19]. Pursu-ing this theme further, established metabolic therapiessuch as GIK and more specifically agents such as glu-cosamine post-translationally modify serine and threo-nine residues of proteins by the O-linked attachmentof the monosaccharide β-N-acetyl-glucosamine (i.e.,O-GlcNAcation) [20]. As well as subserving metabolicbenefits, metabolic therapies also have profound influ-ences on other aspects of cardiac biology as diverse ascontractility and clock function [21].
Conclusion
Accordingly, the future of metabolic therapies likelylies in a redoubling of clinical efforts to apply existingtherapies to patients likely to benefit most from them,and to recognize the potentially beneficial conse-quences of metabolic therapies on exciting novel bio-logical pathways, a better understanding and applica-tion of which holds promise for conferring additionalbenefits to patients with acute or chronic cardiacischemia. ●
Acknowledgements This research is supported by theOxford British Heart Foundation Centre of Research Excel-lence Award.
References
1. Kim AS, Johnston SC (2011) Global variation in the relativeburden of stroke and ischemic heart disease. Circulation124:314–323
2. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F,McQueen M, Budaj A, Pais P, Varigos J, Lisheng L (2004)Effect of potentially modifiable risk factors associated with
myocardial infarction in 52 countries (the INTERHEART study):case-control study. Lancet 364:937–952
3. Ashrafian H, Frenneaux MP, Opie LH (2007) Metabolicmechanisms in heart failure. Circulation 116:434–448
4. Riquelme CA, Magida JA, Harrison BC, Wall CE, Marr TG,Secor SM, Leinwand LA (2011) Fatty acids identified in theBurmese python promote beneficial cardiac growth. Science334:528–531
5. Taegtmeyer H (2002) Switching metabolic genes to build abetter heart. Circulation 106:2043–2045
6. Dyck JR, Lopaschuk GD (2006) AMPK alterations in cardiacphysiology and pathology: enemy or ally? J Physiol 574:95–112
7. Endo J, Sano M, Katayama T, Hishiki T, Shinmura K, MorizaneS, Matsuhashi T, Katsumata Y, Zhang Y, Ito H, Nagahata Y,Marchitti S, Nishimaki K, Wolf AM, Nakanishi H, Hattori F,Vasiliou V, Adachi T, Ohsawa I, Taguchi R, Hirabayashi Y,Ohta S, Suematsu M, Ogawa S, Fukuda K (2009) Metabolicremodeling induced by mitochondrial aldehyde stress stimu-lates tolerance to oxidative stress in the heart. CirculationResearch 105:1118–1127
8. Howell NJ, Ashrafian H, Drury NE, Ranasinghe AM, ContractorH, Isackson H, Calvert M, Williams LK, Freemantle N, QuinnDW, Green D, Frenneaux M, Bonser RS, Mascaro JG, Gra-ham TR, Rooney SJ, Wilson IC, Pagano D (2011) Glucose-insulin-potassium reduces the incidence of low cardiac outputepisodes after aortic valve replacement for aortic stenosis inpatients with left ventricular hypertrophy / clinical perspective.Circulation 123:170–177
9. The CREATE-ECLA Trial Group (2005) Effect of glucose-insulin-potassium infusion on mortality in patients with acuteST-segment elevation myocardial infarction. JAMA 293:437–446
10. Apstein CS, Opie LH (2005) A challenge to the metabolicapproach to myocardial ischaemia. Eur Heart J 26:956–959
11. Zhao YT, Weng CL, Chen ML, Li KB, Ge YG, Lin XM, ZhaoWS, Chen J, Zhang L, Yin JX, Yang XC (2010) Comparison ofglucose-insulin-potassium and insulin-glucose as adjunctivetherapy in acute myocardial infarction: a contemporary meta-analysis of randomised controlled trials. Heart 96:1622–1626
12. Read PA, Hoole SP, White PA, Khan FZ, O’Sullivan M, WestNE, Dutka DP (2011) A pilot study to assess whetherglucagon-like peptide-1 protects the heart from ischemic dys-function and attenuates stunning after coronary balloon occlu-sion in humans. Circ Cardiovasc Interv 4:266–272
13. Bao W, Aravindhan K, Alsaid H, Chendrimada T, Szapacs M,Citerone DR, Harpel MR, Willette RN, Lepore JJ, Jucker BM(2011) Albiglutide, a long lasting glucagon-like peptide-1 ana-log, protects the rat heart against ischemia/reperfusion injury:evidence for improving cardiac metabolic efficiency. PLoS One6:e23570
14. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, StanleyWC (2010) Myocardial fatty acid metabolism in health and dis-ease. Physiol Rev 90:207–258
15. Noble D, Noble PJ (2006) Late sodium current in the patho-physiology of cardiovascular disease: consequences ofsodium-calcium overload. Heart 92 Suppl 4:iv1–iv5
16. Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Bohm M,O’Rourke B, Maack C (2010) Elevated cytosolic Na+increases mitochondrial formation of reactive oxygen speciesin failing cardiac myocytes. Circulation 121:1606–1613
17. McCormack JG, Barr RL, Wolff AA, Lopaschuk GD (1996)Ranolazine stimulates glucose oxidation in normoxic, ische-mic, and reperfused ischemic rat hearts. Circulation 93:135–142
18. Nash DT, Nash SD (2008) Ranolazine for chronic stableangina. Lancet 372:1335–1341
MAIN CLINICAL ARTICLE - HOUMAN ASHRAFIAN
12 Heart Metab. (2011) 53:9–13

19. Sapieha P, Sirinyan M, Hamel D, Zaniolo K, Joyal JS, Cho JH,Honore JC, Kermorvant-Duchemin E, Varma DR, Tremblay S,Leduc M, Rihakova L, Hardy P, Klein WH, Mu X, Mamer O,Lachapelle P, Di Polo A, Beausejour C, Andelfinger G, MitchellG, Sennlaub F, Chemtob S (2008) The succinate receptorGPR91 in neurons has a major role in retinal angiogenesis.Nat Med 14:1067–1076
20. Darley-Usmar VM, Ball LE, Chatham JC (2011) Protein O-linked beta-N-acetylglucosamine: A novel effector of cardio-
myocyte metabolism and function. J Mol Cell Cardiol doi:10.1016/j.yjmcc.2011.08.009
21. Durgan DJ, Pat BM, Laczy B, Bradley JA, Tsai JY, GrenettMH, Ratcliffe WF, Brewer RA, Nagendran J, Villegas-Montoya C, Zou C, Zou L, Johnson RL, Dyck JR, Bray MS,Gamble KL, Chatham JC, Young ME (2011) O-GlcNAcylation:a novel post-translational modification linking myocardialmetabolism and the cardiomyocyte circadian clock. J BiolChem doi: 10.1074/jbc.M111.278903
MAIN CLINICAL ARTICLE - HOUMAN ASHRAFIAN
Heart Metab. (2011) 53:9–13 13

Glucose metabolism as a markerof myocardial ischemia
Paolo G Camici, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy
Correspondence: Paolo G Camici, Via Olgettina 60, 20132 Milan, Italy.e-mail: [email protected]
AbstractAlthough the heart is omnivorous, glucose becomes the key substrate under conditions of stress.The utilization of exogenous glucose by the myocardium can be assessed non-invasively using positronemission tomography (PET) with the glucose analogue 18F-fluorodeoxyglucose (FDG). Several studieshave demonstrated that glucose utilization is increased at peak stress and during conditions of reducedoxygen supply. Moreover, glucose utilization remains elevated after an episode of transient ischemia,which constitutes a sort of “metabolic memory”. PET with FDG also permits the identification of“hibernating myocardium”. This allows a more accurate stratification of patients with post-ischemicleft ventricular dysfunction and identification of those that might benefit most from coronaryrevascularization.
Keywords: coronary artery disease; myocardial metabolism; myocardial ischemia; positron emissiontomography.
■ Heart Metab. (2011) 53:14–18
Introduction
The human heart in the fasting state extracts free fatty acid (FFA), glucose, lactate, pyruvate, andketone bodies from the systemic circulation. A small but consistent net uptake of circulating glu-cose by the heart is normally demonstrable in the fasting state with a reported arterial-venous(AV) difference ranging from 0.15 to 0.23 mmol/l, corresponding to a fractional uptake of only3% and to an average oxygen extraction ratio of ~27%. Measurements of the rate of glucoseoxidation by radiolabelled techniques in healthy volunteers have shown that, at the most, onlyabout 30% of the glucose uptake is rapidly oxidized, and about 15% is converted to lactate [1].
Cardiac glucose metabolism during fasted and fed states
There is a general consensus that FFA is the major fuel for cardiac muscle in the fasting, post-absorptive state. In various studies using the coronary sinus (CS) catheterization technique, netuptake of FFA from the arterial circulation has been found consistent. At arterial FFA levels inthe 0.5 to 0.9 mmol/l range, the reported AV differences is 0.14 to 0.20 μmol/ml, which cor-respond to oxygen extraction ratios of up to 40%. If a total coronary blood flow of ~250 ml/minis assumed, then the heart of fasting subjects at rest consumes up to about 50 μmol/min of FFA,or up to 10% of the whole body FFA turnover (8 μmol/min/kg), despite receiving only 5% ofcardiac output. In general, the fate of FFA is largely complete oxidation in the Krebs’ cyclewith a lesser component undergoing re-esterification to tissue triglycerides. The fact that the
Metab
olicI
magin
gMETABOLIC IMAGING - PAOLO G CAMICI
14 Heart Metab. (2011) 53:14–18

respiratory quotient of the heart in the fasting state ison average 0.74 indicates that the greater part of theextracted FFA is oxidized [1] (Fig. 1).
The oxidative use of lipid (FFA) and carbohydrate(glucose and lactate) fuel is reciprocally regulatedthrough the operation of Randle’s cycle [2]. Feeding,by increasing both insulin and glucose concentrationsshifts myocardial metabolism towards preferential car-bohydrate usage, both for oxidative energy generationand for glycogen synthesis (Fig. 1).
Cardiac glucose metabolism during conditionsof reduced oxygen supply
During conditions of reduced oxygen supply, the oxi-dation of all substrates is decreased while anaerobicmetabolism is activated (Fig. 2). In patients with coro-nary artery disease (CAD) and stable angina pectoris,
net lactate release in the CS can be demonstrated dur-ing pacing stress. However, this occurs in only 50% ofpatients, and no relationship can be demonstratedbetween lactate production and the severity of ische-mia [3]. In patients with chronic angina, a significantrelease of alanine in the CS and an increased myocar-dial uptake of glutamate could be demonstrated at restand following pacing [4–5). These two phenomenaresult from increased transamination of excess pyru-vate to alanine with glutamate serving as NH2 donor. Inaddition, release of citrate (a known inhibitor of glyco-lysis) in the CS can be demonstrated following pacingin patients with stable angina.
Positron emission tomography
The utilization of exogenous glucose by themyocardiumcan be assessed using positron emission tomography
GLYCOGEN
G – 6 – PF – 6 – P
F 1,6 bis P
TRIOSEGAPDH
PFK
PDH
LACTATE
Acetyl CoA
CITRATE
Fasted
excess
ATP
FFA
NADH2
O2
ADPPi
INHIBITION
G
P
–
–
–
+
GLYCOGEN
G – 6 – PF – 6 – P
F 1,6 bis P
TRIOSE
GAPDH
PFK
PDH
LACTATE
Acetyl CoA
CITRATE
Fed
normal
ATP
FFA
O2
ADPPi
NO INHIBITION
G
INS
P
� ��
�
Fig. 1 Left panel: Inhibited glycolysis in fasted state. Overall control of pathways of glycolysis, which is taken as the conversion of G-6-Pto P. The inhibition of glycolysis at the level of phosphofructokinase is the result of accumulation of excess citrate during oxidation of FFA.Pyruvate dehydrogenase is inhibited by accumulated NADH2 the result of β-oxidation of fatty acids. Right panel: Overall patterns of glyco-lysis in the fed state. Besides direct acceleration of glucose uptake as a result of high circulating levels of glucose and insulin, there is indi-rect acceleration of glycolysis as blood FFA levels decrease, and the inhibition of phosphofructokinase by citrate is removed. G-6-P glucose-6-phosphate, P pyruvate, G glucose, F-6-P fructose 1,6 phosphate, PFK phosphofructokinase, F 1,6 bis P fructose1,6 diphosphate,GAPDH glyceraldehyde phosphate dehydrogenase, NADH2 reduced form of nicotine edenine dinucleotide, PDH pyruvate dehydrogenase.
METABOLIC IMAGING - PAOLO G CAMICI
Heart Metab. (2011) 53:14–18 15

(PET) with the glucose analogue 18F-fluorodeoxyglu-cose (FDG). FDG is transported into the myocyteby the same trans-sarcolemmal carrier as glucoseand is then phosphorylated to FDG-6-phosphateby the enzyme hexokinase. This is essentially a unidi-rectional reaction and results in FDG-6-phosphateaccumulation within the myocardium, as no glucose-6-phosphatase (the enzyme that hydrolyses FDG-6-phosphate back to free FDG and free phosphate)has yet been identified in cardiac muscle. Thus, mea-surement of the myocardial uptake of FDG is propor-tional to the overall rate of trans-sacolemmal transportand hexokinase-phosphorylation of exogenous (circu-lating) glucose by heart muscle.
A number of kinetic modeling approaches havebeen used for the quantification of glucose utilizationrates using FDG. The major limitation of theseapproaches is that quantification of glucose meta-bolism requires the knowledge of the lumped con-stant, a factor that relates the kinetic behavior of FDG
to naturally occurring glucose in terms of the relativeaffinity of each molecule for the trans-sarcolemmaltransporter and for hexokinase. Unfortunately, thevalue of the lumped constant in humans under differ-ent physiological and pathophysiological conditions isnot known, thus making precise in vivo quantificationof myocardial metabolic rates of glucose practicallyimpossible. Still current measurements of the uptakeof FDG (particularly if obtained under standardizedconditions) allow comparison of absolute values fromdifferent individuals and may help to establish theabsolute rates of glucose utilization (in FDG units) innormal and pathologic myocardium.
FDG uptake in patients with CAD and stable angina
Different patterns of myocardial glucose utilizationhave been observed in patients with CAD studiedusing FDG. In patients with stable angina studied atrest, after overnight fast, regional myocardial glucoseutilization was homogeneously low and comparablewith that in normal subjects. In contrast, in patientswith unstable angina, myocardial glucose utilization atrest was increased even in the absence of symptomsand electrocardiographic signs of acute ischemia [6].In patients with stable angina, a prolonged increase inFDG uptake could be demonstrated in post-ischemicmyocardium in the absence of symptoms or perfusionabnormalities, which suggests a sort of post-ischemic“metabolic memory” [7]. Subsequent studies in ani-mals have indicated that this increased post-ischemicglucose utilization is mainly finalized to replenish myo-cardial glycogen stores which were depleted duringischemia [1].
PET with FDG for the identification of hibernatingmyocardium
In the current era of coronary revascularization andthrombolysis, it has become increasingly apparentthat restoration of blood flow to asynergic myocardialsegments may result in improved regional and globalLV function [8–10]. The greatest clinical benefit is seenin those patients with the most severe forms of dys-function. Initial studies indicated that myocardial ische-mia and infarction could be distinguished by analysis ofPET images of the perfusion tracer 13NH3 and the glu-cose analogue FDG acquired after an oral glucoseload. Regions which showed a concordant reductionin both myocardial blood flow and FDG uptake (“flow-
GLYCOGEN
G – 6 – P
F 1,6 bis P
TRIOSE
PFK
PDH
LACTATE
electron transport
Acetyl CoA
CITRATE
FFA
NADH2
NADH2
Wash out
NAD
O2
ADPPi
INHIBITION REMOVED
G
P
–
++
+
++
INS
HYPOXIA
B
ATP
Pi �
�
�
�
CP
�
�
�
�
�
�
ATP
�
Fig. 2 Mechanisms whereby mild ischemia increases glycolysis.Note especially a direct effect of hypoxia on glucose transport,and acceleration of phosphofructokinase (PFK) activity by decreas-ing adenosine triphosphate (ATP), creatin phosphate (CP)and an increase of inorganic phosphate (Pi) as well as a decreaseof citrate. Note inhibition of pyruvate dehydrogenase (PDH)by NADH2
METABOLIC IMAGING - PAOLO G CAMICI
16 Heart Metab. (2011) 53:14–18

metabolism match”) were labeled as predominantlyinfarcted, whereas regions in which FDG uptake wasrelatively preserved or increased despite having a per-fusion defect (“flow-metabolism mismatch”) were con-sidered to represent jeopardized viable myocardium[11]. The uptake of FDG by the myocardium, however,depends on many factors such as dietary state, car-diac workload, and response of the tissue to insulin,sympathetic drive and the presence and severity ofischemia. These factors contribute to variability inFDG imaging in the fasted or glucose-loaded state,confusing data interpretation.
With the recent suggestion that semi-quantitativeand quantitative analyses of FDG uptake may enhancedetection of viable myocardium, there was an urgentneed to rigorously standardize the study conditions.Furthermore, many patients with coronary artery dis-ease are insulin resistant, i.e., the amount of endoge-nous insulin released after feeding will not induce maxi-mal stimulation due to partial resistance to the actionof the hormone. This may result in poor FDG imagequality after an oral glucose load. To circumvent theproblem of insulin resistance, an alternative protocolhas been recently applied to PET viability studies.The protocol is based on the use of the hyperinsuline-
mic euglycemic clamp, essentially the simultaneousinfusion of insulin and glucose acting on the tissue asa metabolic challenge and stimulating maximal FDGuptake (see Fig. 3). This leads to optimization ofimage quality and enables PET studies to be per-formed under standardized metabolic conditions,which allows comparison of the absolute values ofthe metabolic rate of glucose (μmol/g/min) amongstdifferent subjects and centers [12].
Conclusion
Although the heart is omnivorous, glucose becomesthe key substrate under conditions of stress. Severalstudies have demonstrated that glucose utilization isincreased at peak stress and during conditions ofreduced oxygen supply. Moreover, glucose utiliza-tion remains elevated after an episode of transientischemia, which constitutes a sort of “metabolicmemory”. PET with FDG also permits the identifi-cation of “hibernating myocardium”. This allows amore accurate stratification of patients with post-ischemic left ventricular dysfunction and identifica-tion of those that might benefit most from coronaryrevascularization. ●
A B
SeptumLateral
Anterior0,14 µmol/g/min
Short Axis - Basal LV
SeptumLateral
InferoposteriorInferoposterior
Anterior
Short Axis - Mid LV
0,45 µmol/g/min
Fig. 3 Myocardial viability in two patients with coronary artery disease and severe chronic left ventricular dysfunction assessed by PET with18F-labelled fluorodeoxyglucose (FDG) during hyperinsulinemic euglycemic clamp. Both patients had previous myocardial infarctions. Thescan illustrated in panel A shows that FDG uptake in the previously infarcted antero-septal segment is 0.45 μmol/min/g, suggestingthe presence of viable myocardium. In the scan illustrated in panel B the uptake of FDG in the anterior wall and the interventricular septumis significantly reduced (0.14 μmol/min/g), suggesting absence of viability in this large area. A cut-off point of 0.25 μmol/min/g is routinelyused in our laboratory to differentiate between viable and non-viable myocardium. This cut-off value was derived from our databaseof patients with coronary artery disease and chronic left ventricular dysfunction who underwent FDG-PET and were subsequently revascu-larized. The proportion of dysfunctional segments that improved following revascularization increased linearly with FDG uptake. To deter-mine the value of FDG uptake above which the best prediction of improvement in functional class of at least one grade could be obtained,a receiver-operator characteristic curve (ROC) was constructed. According to this analysis the optimal operating point on the curve (pointof best compromise between sensitivity and specificity) was at the FDG uptake value of 0.25 μmol/min/g.
METABOLIC IMAGING - PAOLO G CAMICI
Heart Metab. (2011) 53:14–18 17

References
1. Camici PG, Ferrannini E, Opie LH (1989) Myocardial meta-bolism in ischemic heart disease: basic principles and applica-tion to imaging by positron emission tomography. Prog Cardi-ovasc Dis 32:217–238
2. Randle PJ, Hales CN, Garland PB et al (1963) The glucosefatty acid cycle. Its role in insulin sensitivity and the metabolicdisturbances of diabetes mellitus. Lancet 1:785–789
3. Markham RV, Winniford MD, Firth BG et al (1983) Symptom-atic electrocardiographic, metabolic, and hemodynamic altera-tions during pacing-induced myocardial ischemia. Am J Car-diol 51:1589–1594
4. Mudge GH, Mills RW, Taegtmeyer H et al (1976) Alter ations ofmyocardial amino acid metabolism in chronic ischemic heartdisease. J Clin Invest 58:1185–1192
5. Brodan V, Fabian J, Andel M et al (1978) Myocardial aminoacid metabolism in patients with chronic ischemic heart dis-ease. Basic Res Cardiol 73:160–170
6. Araujo LI, Camici PG, Spinks T, Jones T, Maseri A (1988)Abnormalities in myocardial metabolism in patients with unsta-
ble angina as assessed by positron emission tomography.Cardiovasc Drugs Ther 2:41–46
7. Camici PG, Araujo L, Spinks T et al (1986) Increased uptake ofF18-flurodeoxyglucose in postischemic myocardium of patientswith exercise-induced angina. Circulation 74:81–88
8. Marinho NVS, Keogh BE, Costa DC, Lammertsma AA, Ell PJ,Camici PG (1996) Pathophysiology of chronic left ventricular dys-function: New insights from the measurement of absolute myo-cardial blood flow and glucose utilization. Circulation 93:737–744
9. Camici PG, Wijns W, Borgers M, De Silva R, Ferrari R, KnuutiJ, Lammertsma AA, Liedtke AJ, PaternostroG, Vatner SF(1997) Pathophysiological mechanisms of chronic reversibleleft ventricular dysfunction due to coronary artery disease(hibernating myocardium). Circulation 96:3205–3214
10. Wijns W, Vatner SF, Camici PG (1998) Hibernating myo-cardium. N Engl J Med 339:173–181
11. Tillisch J, Brunken R, Marshall R, Schwaiger M, MandelkernM, Phelps M, Schelbert H (1986) Reversibility of cardiac wall-motion abnormalities predicted by positron tomography.N Engl J Med 314:884–888
12. Camici PG, Prasad S, Rimoldi O (2008) Stunning, hibernationand assessment of viability. Circulation 117:103–114
METABOLIC IMAGING - PAOLO G CAMICI
18 Heart Metab. (2011) 53:14–18

An expanded role for AMP kinase:self-renewal of the cardiomyocyte
Kedryn K. Baskin and Heinrich Taegtmeyer, Department of Internal Medicine, Division of Cardiology,University of Texas Medical School at Houston, University of Texas Health Science Center at Houston,
Houston, Texas, USA
Correspondence: Heinrich Taegtmeyer, MD, DPhil, Professor of Medicine,University of Texas Medical School at Houston, 6431 Fannin, MSB 1.246, Houston, TX 77030, USA.
Tel.: 713-500-6569, fax: 713-500-0637, e-mail: [email protected]
AbstractOur work on atrophic remodeling of the heart has caused us to appreciate a simple principle in biology:from the cell cycle to the Krebs cycle, there is no life without cycles. While the potential for cellularregeneration receives much attention, the dynamics of intracellular protein turnover have received onlyselective consideration. Although the concept of the “dynamic state of body constituents” has existedsince the 1940s, the idea that heart muscle cells renew themselves from within is relatively new. Therationale is as follows. For the last 30 years, we (and many others) have elucidated the interaction ofmetabolic pathways for energy provision and contraction of the heart. Work in the field has uncoverednovel metabolic regulators of enzyme action, yet much less attention has been given to the impact ofmyocardial energy metabolism on myocardial protein turnover. We therefore began to consider meta-bolic signals as putative regulators of myocardial protein synthesis and degradation. In a broad sense,we sought to establish mechanisms underlying the self-renewal of intact cardiomyocytes, because wehave observed that atrophic remodeling of the heart simultaneously activates pathways of intracellularprotein synthesis and degradation. We determined how metabolic signals regulate protein degradation,and tested the hypothesis that there is a direct link between intermediary metabolism and protein deg-radation and that the specific molecular mechanisms involve 5′ AMP-activated protein kinase (AMPK)regulation of ubiquitin ligases. We review our first results on metabolic signals as regulators of myocar-dial protein turnover that seek to broaden the role energy substrate metabolism from a provider of ATPto a regulator of self-renewal of the cardiomyocyte.
Keywords: AMPK; protein degradation; cardiac metabolism
■ Heart Metab. (2011) 53:19–24
Introduction
Our work on atrophic remodeling of the heart has led us to appreciate a simple principle in bio-logy: from the cell cycle to the Krebs cycle, there is no life without cycles. While cellular rege-neration of the heart receives much attention [1], the dynamics of intracellular protein turnoverhave received only selective consideration [2]. Undoubtedly stem (or precursor) cells contributeto the replacement of cardiomyocytes after injury, but they contribute little to cardiomyocyterenewal during normal aging [3]. Although Schoenheimer’s concept of the “dynamic state ofbody constituents” has existed for some time [4], the idea that heart muscle cells renew them-selves from within is relatively new. We will begin to address this concept with a review on the
NewTh
erape
uticA
pproa
ches
NEW THERAPEUTIC APPROACHES - KEDRYN K. BASKIN
Heart Metab. (2011) 53:19–24 19

transcriptional role for 5′ AMP-activated proteinkinase (AMPK) on protein degradation pathways.
For the last 50 years many laboratories have eluci-dated the interaction of metabolic pathways for energyprovision and contraction in the heart. Work in the fieldhas uncovered novel metabolic regulators of enzymeaction, yet the impact of myocardial energy metabo-lism on myocardial protein turnover has not been con-sidered. After we discovered that cardiac atropohy isnot a simple mirror image of hypertrophy [5], we arenow proposing that metabolic signals are putativeregulators of myocardial protein synthesis and degra-dation. In a broad sense we seek to establish mechan-isms underlying the self-renewal of the intact cardio-myocyte. The rationale arises from our observationthat atrophic remodeling of the heart simultaneouslyactivates pathways of intracellular protein synthesisand degradation [5] and the following considerations.
First, a large number of models already exist thatidentify molecular targets of myocardial hypertrophyand atrophy [6,7]. Secondly, metabolism is the firstresponder to any form of stress [8]. We have evidencesuggesting that the process of metabolic remodelingprecedes, triggers and sustains both structural andfunctional remodeling of the heart [9]. We proposethat modulation of metabolic stresses provides ameans to remove damaged or redundant proteinsand replace them with new, functional proteins (Fig. 1).Furthermore, the identification of metabolic signalswhich govern cardiac remodeling will set us also onthe path to develop novel strategies aiming at specificmetabolic intermediaries as modulators of cardiomyo-cyte size.
Intracellular protein turnover in perspective
The depressing statistics on heart failure are widelyknown [10]. Yet in spite of broad and formidableefforts, there is no cure in sight because the cellularand molecular mechanisms are still not completelyunderstood. Accepted features in the developmentof heart failure are cardiac hypertrophy and impairedATP production, which develop in response to bothendogenous (genetic) and exogenous (environmental)changes. We propose that metabolic remodeling(which is potentially reversible) precedes, triggers andsustains structural and functional remodeling [11]. Inorder for the heart to adapt to various types of stress,individual heart muscle cells change or “remodel” both
metabolically and structurally. Excessive remodelingresults in the enlargement of cardiomyocytes, whichtranslates into an overall increase in heart size. Thetransition from hypertrophy to heart failure, or the tran-sition from adaptation to maladaptation of the heart,remains elusive. Consequently it seems to us criticalto know more about the mechanisms that control therebuilding of the cardiomyocyte.
Our most recent ideas advance a new understand-ing of cardiac metabolism as an integral part of theself-renewing myocyte as highlighted below. Theseideas result from our investigations on switching ofmetabolic genes and atrophic remodeling of the cardio-myocytes in response to mechanical unloading.
We are operating under the premise that duringsteady state conditions rates of myocardial proteinsynthesis (PS) and protein degradation (PD) are equal[12,13]. The intrinsic mechanism of self-renewal of thecardiomyocyte requires the regulated degradation ofdamaged, misfolded, or useless proteins and theirreplacement by new and functional proteins (Fig. 1).Protein turnover therefore constitutes a major line ofdefense for protein quality control of the cardiomyo-cytes [14]. The rate of myocardial protein turnover ismuch faster than it is generally assumed, with thehalf-life of individual myocardial proteins ranging fromseveral hours to several days [12]. The term “self-
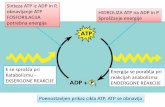
MetabolismATP/AMP, Metabolites
Protein Degradation
Protein Synthesis
(Proteins)
Ser
TrpArg
Val ProLys
Ile
AlaHisMet
Cys
Gin *PheAsnLeuHis
Gly
Tyr
AspThr Phe
(Amino Acids)
PD PS
Fig. 1 The balance of protein synthesis (PS) and degradation (PD)determines size and function of cardiomyocytes. Damaged,misfolded, or useless proteins are degraded to amino acids thatare used for the synthesis of new, functional proteins.
NEW THERAPEUTIC APPROACHES - KEDRYN K. BASKIN
20 Heart Metab. (2011) 53:19–24

renewal of the cardiomyocyte” gives exciting newmeaning to the concept of “cardiac plasticity” [6].
We do not know at present to what extent biochem-ical signals regulate protein degradation and proteinsynthesis. We have preliminary evidence which sug-gests that metabolic signals, i.e., changes in intracellu-lar metabolite levels in response to stress, may activatepathways of protein degradation and protein synthesis[5,15].
Lastly, and perhaps most importantly, for nearly acentury the study of cardiac metabolism has con-cerned itself with energy substrate metabolism andcontraction of the heart [16,17]. This focus has culmi-nated in a recent review proclaiming that the failingheart is an “engine out of fuel” [18]. We have ques-tioned this concept because the non-ischemic, failingheart is always well supplied with nutrients, and theheart is actually drowning in fuel [19]. Not surprisingly,attempts to restore normal contractile function in thefailing heart by metabolic interventions have not beenconsistently successful [20,21]. It is much more likelythat intermediary metabolism, rather than impaired fuelsupply, is the culprit. We consider altered fuel meta-bolism (leading to either a decrease or an increase ofcertain metabolic signals) as a root cause for alteredrates of intracellular protein turnover and, hence, self-renewal of the cardiomyocyte.
Taken together, we propose that the “metabolic”approach to myocardial protein synthesis and degra-dation provides a new framework that will expose newregulators driving self-renewal of cardiomyocytes fromwithin. We focus on the ubiquitin proteasome system(UPS).
The ubiquitin proteasome system and AMPK
Intracellular protein degradation is a complex andhighly controlled process that is integrated with theenvironment of the cell. We have recently identified ametabolic signal that regulates protein degradation inthe heart and the corresponding mechanisms bywhich it does so, specifically pertaining to the ubiquitinproteasome system (UPS). Our studies suggest thatthe adenine nucleotides ATP and AMP are metabolicsignals that regulate protein degradation. AMP-activated protein kinase (AMPK) supports energy pro-vision in the cell by sensing changes in the ratio [ATP]:[AMP]. Therefore, our working hypothesis was thatmetabolic signals (decrease in [ATP]:[AMP]) and the
subsequent activation of AMPK, regulate protein deg-radation. We have tested the hypothesis by modulat-ing AMPK in vitro and in vivo to define the mechanismsby which AMPK is involved in protein degradation [22].
Intracellular protein degradation in cardiomyocytesis controlled by independent but interrelated pro-cesses: UPS-mediated proteolysis and autophagy.While autophagy can degrade whole organelles, indi-vidual proteins are degraded through the UPS [13].Ubiquitin ligases confer specificity to the system bythe selective ubiquitination of target proteins whichare then degraded by the proteasome [2]. Twomuscle-specific ubiquitin ligases, muscle atrophyF-box (MAFbx/atrogin-1) and muscle ring finger-1(MuRF1), are critical regulators of cardiac protein deg-radation and myocardial mass. Studies in vivo havedemonstrated that overexpressing atrogin-1 in theheart attenuates the development of hypertrophy[23], while the deletion of MuRF1 results in increasedhypertrophy [24]. These experiments highlight theimportance of atrogin-1 and MuRF1 in regulatingheart size. However, the mechanisms by which theligases themselves are regulated are not completelyunderstood.
Early studies in the heart in vivo demonstrated thatnutrient deprivation decreases protein synthesis andincreases fractional rates of protein degradation [25].Starvation decreases the intracellular concentration ofATP and, consequently, AMPK is activated in order toprovide energy to maintain normal cellular function. It iswell established that AMPK regulates energy substratemetabolism, inhibits protein synthesis [26], and regu-lates transcription of metabolic genes [27]. Although ithas recently been reported that starvation inducesautophagy in cardiomyocytes through AMPK [28], arole of AMPK in the cardiac UPS had never been con-sidered before.
In order to investigate the role of AMPK in the UPS,we first verified that substrate deprivation in cardio-myocytes (CM) enhances protein degradation (PD),as has been shown already in vivo [25]. Protein degra-dation was enhanced in CM during starvation, butdecreased with bortezomib, a proteasome inhibitor,or with 3-methyladenine (3-MA), an inhibitor of auto-phagy. These results suggest that, like autophagy [28],proteasome-mediated degradation is important duringnutrient starvation in CM. Given the importance ofatrogin-1 and MuRF1 in regulating protein degradation
NEW THERAPEUTIC APPROACHES - KEDRYN K. BASKIN
Heart Metab. (2011) 53:19–24 21

and cardiac size [23,24], we quantified their expres-sion in parallel experiments. Atrogin-1 and MuRF1levels were significantly increased with starvation,which also correlated with enhanced AMPK activity(Fig. 2). We also found that direct AMPK activation,independent of nutrient starvation, increased bothatrogin-1 and MuRF1 expression, which was signifi-cantly impaired with AMPK inhibition. Consequently,protein degradation in the heart is increased with
AMPK activation, but proteasome-mediated proteindegradation downstream of AMPK requires MuRF1[22]. The conclusions are shown in the schematic(Fig. 3).
Perspective
We have shown that AMPK regulates ubiquitin ligasesin the rodent heart. The present work extends the longestablished concept of the “dynamic state of bodyconstituents” [4] to a specific situation when the heartadapts to changes in its metabolic environment. Pro-tein turnover constitutes a major line of defense forprotein quality control of the cardiomyocytes [14] andis a major mechanism of adaptation in the heart.Therefore it is of interest to understand how proteindegradation is regulated under various circumstancesin the heart. Markers of the UPS are upregulated in theheart in several settings of cardiac remodeling [13], butit is not clear exactly how the markers themselves areregulated. AMPK regulates cellular homeostasis in partby inhibiting the mTOR pathway [26] and thus bydecreasing protein synthesis, while at the same timeAMPK activates autophagy [28].
It is well known that AMPK is a central regulator offuel homeostasis, but studies have until now predomi-nantly focused on the effects of AMPK activation onenergy substrate metabolism [29]. The active subunitof AMPK is highly expressed in the heart, and is pref-erentially localized to the nucleus [30]. It is therefore notsurprising that AMPK also transcriptionally regulatesmetabolic gene expression. Earlier reports in livershow that AMPK activation represses transcription,but little is known about AMPK-regulated transcriptionin the heart. AMPK activates transcription [27], and theactivation of PGC1α by AMPK leads to increasedmito-chondrial gene expression [31]. Still, the importance ofAMPK in transcription is only now coming into focus.AMPK regulates entire transcriptional programs, andnot only transcription of individual genes, by regulatinghistone 2B [32]. We have now expanded the role ofAMPK in both cellular homeostasis and transcriptionalregulation in the heart [22].
The AMPK activator and anti-diabetic drug metfor-min has proven to have beneficial outcomes in heartfailure patients with diabetes [21]. The role of proteinturnover in hearts of these patients could not beinvestigated. However, based on our experimentalfindings, AMPK-regulated protein degradation may be
A
B
C
Atrogin-1
MuRF1
Nutrient Deprivation (hrs)
6
4
2
0
3
4
2
0
1
0 2 4 8 24
Rel
ativ
e m
RN
A le
vels
(Fo
ld c
hang
e)
Fold
Cha
nge
(no
rmal
ized
to
GA
PD
H)
Pro
tein
Deg
rad
atio
n (%
to
tal p
rote
in)
pAMPK/AMPKAtrogin-1MuRF1
Untreated
Control Nutrient Deprivation
Bortezomib3-MA
Control
GAPDH
MuRF1
Atrogin-1
AMPK
pAMPK
Nutrient Deprivation
Control Nutrient Deprivation
6
8
10
4
0
2
Fig. 2 Nutrient deprivation increases expression of ubiquitin ligasesand enhances protein degradation. (A) Atrogin-1 and MuRF1 mRNAexpression in nutrient deprived NRVM. (B) Relative protein levelsand quantification in NRVM after 24 hours of nutrient deprivation.(C) Protein degradation in NRVM after 24 hours of nutrient depriva-tion with 1μmol/L Bortezomib or 10μmol/L 3-methyladenine treat-ment. Data are mean ± SEM of 3 independent experiments per-formed in triplicate. *P<0.01 vs control or untreated, †P<0.01 vscontrol or complete nutrients (reprinted with permission from [22]©2011 Wolters Kluwer Health).
NEW THERAPEUTIC APPROACHES - KEDRYN K. BASKIN
22 Heart Metab. (2011) 53:19–24

protective because of enhanced protein quality control[14]. Activation of AMPK results in increased rates ofprotein degradation, and consequently leads to remo-deling of the heart. The immediate cardiometabolicenvironment may determine whether the remodelingis beneficial or detrimental. We speculate that the acti-vation of AMPK results in enhanced availability of intra-cellular amino acids for either ATP production or thesynthesis of new proteins as the heart adapts to anew physiologic state. This self-renewal of the cardio-myocytes would mean an expanded role for 5′ AMP-activated protein kinase in the heart. ●
Acknowledgements The authors’ lab is supported, in part,by grants from the US Public Health Service (2R01 HL-61483-10) and the American Heart Association (PredoctoralFellowship 11PRE5200006). We thank Mrs. Roxy A. Tate forexpert editorial assistance.
References
1. Chien KR (2008) Regenerative medicine and human models ofhuman disease. Nature 453:302–305
2. Willis MS, Townley-Tilson WH, Kang EY, Homeister JW, Patter-son C (2010) Sent to destroy: The ubiquitin proteasome systemregulates cell signaling and protein quality control in cardiovas-cular development and disease. Circ Res 106:463–78
3. Hsieh PC, Segers VF, Davis ME et al (2007) Evidence from agenetic fate-mapping study that stem cells refresh adult mam-malian cardiomyocytes after injury. Nat Med 13:970–974
4. Schoenheimer R (1942) The dynamic state of body constitu-ents. Harvard University Press, Cambridge, MA, 42 pp
5. Razeghi P, Sharma S, Ying J, Li YP, Stepkowski S, Reid MB,Taegtmeyer H (2003) Atrophic remodeling of the heart in vivosimultaneously activates pathways of protein synthesis anddegradation. Circulation 108:2536–2541
6. Hill JA, Olson EN (2008) Cardiac plasticity. N Engl J Med358:1370–1380
7. Baskin K, Taegtmeyer H (2011) Taking pressure off the heart:The ins and outs of atrophic remodeling. Cardiovasc Res doi:10.1093/cvr/cvr060
8. Goodwin GW, Taylor CS, Taegtmeyer H (1998) Regulation ofenergy metabolism of the heart during acute increase in heartwork. J Biol Chem 273:29530–29539
9. Taegtmeyer H, Golfman L, Sharma S, Razeghi P, van ArsdallM (2004) Linking gene expression to function: Metabolic flexi-bility in the normal and diseased heart. Ann N Y Acad Sci1015:202–213
10. Roger VL, Go AS, Lloyd-Jones DM et al (2011) Heart diseaseand stroke statistics—2011 update: A report from the Ameri-can Heart Association. Circulation 123:e18–e209
11. Young ME, Yan Z, Razeghi P et al (2007) Proposed regulationof gene expression by glucose in rodent heart. Gene RegSystems Biol 1:251–262
12. Morgan HE, Rannels D, McKee E (1979). Protein metabolismof the heart. Handbook of physiology: The cardiovascular sys-tem. R Berne. Bethesda, MD, American Physiological Society.Vol. 1: The Heart: pp. 845–871
13. Razeghi P, Baskin KK, Sharma S, Young ME, Stepkowski S,Essop MF, Taegtmeyer H (2006) Atrophy, hypertrophy, andhypoxemia induce transcriptional regulators of the ubiquitinproteasome system in the rat heart. Biochem Biophys ResCommun 342:361–364
14. Wang X, Robbins J (2006) Heart failure and protein qualitycontrol. Circ Res 99:1315–1328
15. Depre C, Shipley GL, Chen W et al (1998) Unloaded heart invivo replicates fetal gene expression of cardiac hypertrophy.Nat Med 4:1269–1275
16. Taegtmeyer H (1994) Energy metabolism of the heart: Frombasic concepts to clinical applications. Curr Prob Cardiol19:57–116
17. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, StanleyWC (2010) Myocardial fatty acid metabolism in health anddisease. Physiol Rev 90:207–258
18. Neubauer S (2007) The failing heart—an engine out of fuel.N Engl J Med 356:1140–1151
19. Taegtmeyer H (2007) The failing heart. N Engl J Med356:2545–2456; author reply 2546
Metabolic Stress(Nutrient Deprivation)
ATP
AMP
MEF2
AMPK
MuRF1
Protein Degradation CardiomyocyteRemodeling
Fig. 3 AMPK regulates MuRF1 transcription in a MEF2-dependent manner. This leads to increased protein degradation in the cardiomyo-cyte and increased remodeling (reprinted with permission from [22] ©2011 Wolters Kluwer Health).
NEW THERAPEUTIC APPROACHES - KEDRYN K. BASKIN
Heart Metab. (2011) 53:19–24 23

20. Singh S, Loke YK, Furberg CD (2007) Long-term risk of car-diovascular events with rosiglitazone: A meta-analysis. JAMA298:1189–1195
21. Aguilar D, Chan W, Bozkurt B, Ramasubbu K, Deswal A(2011) Metformin use and mortality in ambulatory patientswith diabetes and heart failure. Circ Heart Fail 4:53–58
22. Baskin KK, Taegtmeyer H (2011) AMP-activated proteinkinase regulates E3 ligases in rodent heart. Circ Resdoi:10.1161/CIRCRESAHA.111.252742
23. Li HH, Kedar V, Zhang C, McDonough H, Arya R, Wang DZ,Patterson C (2004) Atrogin-1/muscle atrophy f-box inhibitscalcineurin-dependent cardiac hypertrophy by participating inan scf ubiquitin ligase complex. J Clin Invest 114:1058–1071
24. Willis MS, Ike C, Li L, Wang DZ, Glass DJ, Patterson C (2007)Muscle ring finger 1, but not muscle ring finger 2, regulatescardiac hypertrophy in vivo. Circ Res 100:456–459
25. Samarel AM, Parmacek MS, Magid NM, Decker RS, Lesch M(1987) Protein synthesis and degradation during starvation-induced cardiac atrophy in rabbits. Circ Res 60:933–941
26. Bolster DR, Crozier SJ, Kimball SR, Jefferson LS (2002) AMP-activated protein kinase suppresses protein synthesis in ratskeletal muscle through down-regulated mammalian target ofrapamycin (mTOR) signaling. J Biol Chem 277:23977–23980
27. Stoppani J, Hildebrandt AL, Sakamoto K, Cameron-Smith D,Goodyear LJ, Neufer PD (2002) AMP-activated protein kinaseactivates transcription of the UCP3 and hkii genes in rat skel-etal muscle. Am J Physiol Endocrinol Metab 283:E1239–1248
28. Matsui Y, Takagi H, Qu X et al (2007) Distinct roles of autop-hagy in the heart during ischemia and reperfusion: Roles ofAMP-activated protein kinase and beclin 1 in mediating autop-hagy. Circ Res 100:914–922
29. Hardie DG (2008) Role of AMP-activated protein kinase inthe metabolic syndrome and in heart disease. FEBS Lett582:81–89
30. Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D,Hardie DG (1998) AMP-activated protein kinase: Greater AMPdependence, and preferential nuclear localization, of complexescontaining the alpha2 isoform. Biochem J 334 (Pt 1):177–187
31. Holmes BF, Kurth-Kraczek EJ, Winder WW (1999) Chronicactivation of 5′-AMP-activated protein kinase increases glut-4, hexokinase, and glycogen in muscle. J Appl Physiol87:1990–1995
32. Bungard D, Fuerth BJ, Zeng PY et al (2010) Signaling kinaseAMPK activates stress-promoted transcription via histone h2bphosphorylation. Science 329:1201–1205
NEW THERAPEUTIC APPROACHES - KEDRYN K. BASKIN
24 Heart Metab. (2011) 53:19–24

Beneficial effects of trimetazidine(Vastarel®MR) in patientswith chronic heart failure
Gabriele Fragasso, Luca Alberti and Ludovica Lauretta, Division of Metabolic and Cardiovascular Sciences,Istituto Scientifico H San Raffaele, Milano, Italy
Correspondence: Gabriele Fragasso MD, Heart Failure Unit, Division of Metabolic and Cardiovascular Sciences,Istituto Scientifico San Raffaele, Via Olgettina 60, 20132 Milano, Italy.
Tel.: +39 02 26437366, fax: +39 02 26437395, e-mail: [email protected]
AbstractThe possibility of modifying cardiac metabolism by switching the fuel used by the myocardium couldbecome increasingly important, especially in clinical conditions characterized by reduced energy avail-ability, such as heart failure. Trimetazidine (Vastarel®MR), an inhibitor of free fatty acid (FFA) oxidation,holds the characteristics to play a fundamental role in the therapeutic strategy of patients with heart fail-ure. More specifically, shifting the energy substrate preference away from FFA metabolism and towardglucose metabolism has been shown to be an effective adjunctive treatment in terms of myocardialmetabolism and left ventricular function improvement. These effects seem operative in heart failuresyndromes regardless of their etiopathogenetic cause and are not confined to those of ischemic origin.In this paper, the recent literature on the beneficial therapeutic effects of trimetazidine on left ventriculardysfunction and heart failure is reviewed and discussed.
Keywords: systolic-dysfunction heart failure; left ventricular function; metabolic therapy; energyexpenditure
■ Heart Metab. (2011) 53:25–28
Introduction
Trimetazidine (TMZ) (1-[2,3,4-trimethoxybenzyl] piperazine dihydrochloride) (Vastarel®MR) hasbeen reported to exert anti-ischemic properties without affecting myocardial oxygen consump-tion and blood supply [1]. The beneficial effect of this agent has been attributed to preservationof phosphocreatine and ATP intracellular levels [2] and reduction of cell acidosis [3–4], calciumoverload [4] and free-radical-induced injury caused by ischemia [5]. More importantly, TMZ MRaffects myocardial substrate utilization by inhibiting oxidative phosphorylation and by shiftingenergy production from free fatty acids (FFA) to glucose oxidation [6–7]. This effect appearsto be predominantly caused by a selective block of long chain 3-ketoacyl CoA thiolase activity,the last enzyme involved in β-oxidation [8].
Effects of TMZ in patients with chronic heart failure
In chronic heart failure therapeutic strategies have traditionally focused on the modification ofhemodynamic alterations that occur in the failing heart. However, in addition to hemodynamic
Focu
sonVa
starel
®MR
FOCUS ON VASTAREL®MR - GABRIELE FRAGASSO
Heart Metab. (2011) 53:25–28 25

alterations, heart failure causes deep changes both insystemic and in cardiac metabolic milieu. In this con-text, recent studies performed in small groups ofpatients with post ischemic left ventricular dysfunc-tion, have shown that TMZ may be beneficial in termsof left ventricular function preservation and control ofsymptoms [9–15]. On this basis, it has been shownthat this pharmacological approach could also beuseful in the treatment of patients with heart failure ofvarious etiologies [16–19].
The beneficial effect of TMZ on left ventricular func-tion has been attributed to preservation of intracellularphosphocreatine (PCr) and adenosintriphosphate(ATP) [2]. Previous clinical studies using phosphorus-31 magnetic resonance spectroscopy to measurePCr/ATP ratios in human myocardium have shownthat this ratio is reduced in failing human myocardium[20]. The PCr/ATP ratio is a measure of myocardialenergetics, and its reduction may depend on imbal-ance of myocardial oxygen supply and demand [21]and reduction of the total creatine pool, a phenome-non known to occur in heart failure [22]. In a recentstudy performed in patients with heart failure of differ-ent etiologies who were receiving full standard medicaltherapy, TMZ-induced improvement of functionalclass and left ventricular function was associated witha 33% improvement of the PCr/ATP ratio, supportingthe hypothesis that TMZ probably preserves myo-cardial high-energy phosphate intracellular levels [23].These results appear particularly interesting in view ofprevious evidence indicating that the PCr/ATP ratio is asignificant predictor of mortality [24].
Effects of TMZ on whole body energy metabolismof patients with heart failure
A higher resting metabolic rate has been observedin patients with heart failure [25–27], and this factorprobably contributes to progressive worsening of thedisease. Rate of energy expenditure is related toincreased serum FFA oxidation and both energyexpenditure and serum FFA oxidation are inverselycorrelated with left ventricular ejection fraction andpositively correlated with growth hormone concentra-tions, epinephrine and norepinephrine [28]. Norepi-nephrine increases whole body oxygen consumption,circulating FFA concentrations, and FFA oxidation [29].These changes have been attributed to stimulation of
hormone-sensitive lipase in adipose tissue, and tostimulation of oxygen consumption independent oflipolysis by norepinephrine [30]. This data, togetherwith close correlations between plasma norepineph-rine concentrations, energy expenditure at rest andFFA oxidation, make increased sympathetic activitythe most likely explanation for alterations in fuelhomeostasis in patients with HF [30]. Therefore, inter-vention strategies aimed at optimizing global and car-diac metabolism, could be useful for interrupting thevicious circle of reduced function at greater metabolicexpenses in different cardiac conditions [31]. In a veryrecent study, it has been shown that 3 months of treat-ment with TMZ added to usual treatment consistentlyreduces whole body resting energy expenditure alongwith improved functional class, quality of life and leftventricular function in patients with systolic heart fail-ure, regardless of its etiology and diabetic status [32](Fig. 1). The observation that the beneficial effect ofTMZ on left ventricular function is also paralleled by areduction of whole body rate of energy expenditurewhen compared to patients on conventional treatmentunderlies the possibility that the effect of TMZ may bemediated through a reduction of metabolic demand atthe level of the peripheral tissues and, in turn, in somesort of central (cardiac) relief. Therefore, reduction ofwhole body energy demand could be one of the prin-cipal mechanisms by which TMZ could improve symp-toms and left ventricular function in patients with heartfailure.
p: 0.03
Kcal / die
ConventionalTherapy
Conventional Therapy+ TMZ
1679± 304
1690± 337 1677
± 264
1580± 263
Baseline
3 months follow-up
1700
1650
1600
1550
1500
1450
1400
1350
1300
Fig. 1 Rate of energy expenditure (Kcal/die) measured by indirectcalorimetry at baseline and 3 months follow-up in patients with heartfailure receiving conventional therapy alone (left histograms) or con-ventional therapy plus trimetazidine (right histograms) (adapted withpermission from reference [32]).
FOCUS ON VASTAREL®MR - GABRIELE FRAGASSO
26 Heart Metab. (2011) 53:25–28

Additional potential beneficial pharmacologicaleffects of TMZ in heart failure
It has been observed that TMZ could reduce endothe-lin release in cardiac patients [12, 33–34]. Growthfactors, vasoactive substances and mechanical stressare involved in the endothelin-1 (ET-1) increase in heartfailure patients. Despite the known adaptive aspect ofsupporting contractility of the failing heart, persistentincreases in cardiac ET-1 expression in the failingheart have a pathophysiological maladaptive aspectand are associated with the severity of myocardial dys-function [35].
TMZ-induced reduction of intracellular acidosis inischemic myocardium could not only influencemyocardial but also endothelial membranes [5]. Bydecreasing endothelial damage, TMZ could inhibitET-1 release that, in turn, will finally decrease myo-cardial damage. A second hypothesis is that, by justdecreasing the effects of chronic myocardial ischemia,TMZ could inhibit ET-1 release. Therefore, theobserved decrease in ET-1 release with TMZ, couldlikely be linked to TMZ-induced reduction of myocar-dial ischemia. Finally, keeping in mind the close relationbetween endothelium and insulin sensitivity, theobserved effects of TMZ on endothelial functioncould also explain the beneficial action of TMZ on glu-cose metabolism. In fact, apart from improving leftventricular function in cardiac patients, it has beenrecently shown that TMZ could also improve overallglucose metabolism in the same patients, indicatingan attractive ancillary pharmacological property ofthis class of drugs [12, 33]. In fact, the known insulinresistant state in most cardiac patients is certainlyaggravated in those patients with overt diabetes. Thisis particularly relevant in patients with both diabetesand left ventricular dysfunction. In this context, theavailability of glucose and the ability of cardiomyocytesand skeletal muscles to metabolize glucose are grosslyreduced. Indeed, since a major factor in the develop-ment and progression of heart failure is already areduced availability of ATP, glucosemetabolism altera-tions could further impair the efficiency of cardio-myocytes to produce energy. By inhibiting fatty acidoxidation, TMZ stimulates total glucose utilization,including both glycolysis and glucose oxidation. Theeffects of TMZ on glucose metabolism could thereforebe dependent by a) improved cardiac efficiency;b) improved peripheral glucose extraction and utiliza-
tion. Both mechanisms could definitely be beneficial inheart failure patients.
Systematic literature search on the beneficialeffect of TMZ in heart failure
A systematic search of the literature was recently con-ducted by Gao et al. to identify randomized controlledtrials of TMZ for heart failure [36]. They consideredreports of trials comparing TMZ with placebo controlfor chronic heart failure in adults, with outcomesincluding all-cause mortality, hospitalization, cardio-vascular events, changes in cardiac function para-meters and exercise capacity. The results of thesearch identified 17 trials with data for 955 patients.TMZ therapy was associated with a significantimprovement in left ventricular ejection fraction inpatients with both ischemic (7.37%; 95% CI 6.05to 8.70; p<0.01) and non-ischemic heart failure(8.72%; 95% CI 5.51 to 11.92; p<0.01). With TMZtherapy, New York Heart Association classificationwas also improved (p<0.01), as was exercise duration(p<0.01). More importantly, TMZ had a significant pro-tective effect for all-cause mortality (RR 0.29; 95% CI0.17 to 0.49; p<0.00001) and cardiovascular eventsand hospitalization (RR 0.42; 95% CI 0.30 to 0.58;p<0.00001). These data confirm that TMZ might bean effective strategy for treating heart failure and thata large multicenter randomized controlled trial shouldbe performed, in order to clarify its therapeutic role inthis setting.
Conclusion
TMZ could have an important role in the therapeuticstrategy of patients with heart failure. More specifically,shifting the energy substrate preference away fromfatty acid metabolism and toward glucose metabolismappears as an effective adjunctive treatment inpatients with heart failure, in terms of left ventricularmetabolism and function improvement. These effectsare operative in heart failure syndromes regardless oftheir etiopathogenetic cause and are not confined tothose of ischemic origin.
However, despite a very recent meta-analysis hasevidenced that these benefits also translate intoimproved survival, a randomized placebo controlledmulticenter trial is definitely warranted in order toobjectively investigate the role of TMZ in the therapeu-tic armamentarium of heart failure. ●
FOCUS ON VASTAREL®MR - GABRIELE FRAGASSO
Heart Metab. (2011) 53:25–28 27

References
1. Chierchia S, Fragasso G (1993) Metabolic management ofischaemic heart disease. Eur Heart J 14 Suppl G:2–5
2. Lavanchy N, Martin J, Rossi A (1987) Anti-ischemia effects oftrimetazidine: 31P-NMR spectroscopy in the isolated rat heart.Arch Int Pharmacodyn Ther 286:97–110
3. Lagadic-Gossmann D, Le Prigent K, Feuvray D (1996) Effectsof trimetazidine on pHi in the rat isolated ventricular myocyte.Br J Pharmacol 117:831–838
4. Renaud JF (1988) Internal pH, Na+, and Ca2+ regulation bytrimetazidine during cardiac cell acidosis. Cardiovasc DrugsTher 1:677–686
5. Maridonneau-Parini I, Harpey C (1985) Effects of trimetazidineon membrane damage induced by oxygen free radicals inhuman red cells. Br J Clin Pharmacol 20:148–151
6. Kay L, Finelli C, Aussedat J, Guarnieri C, Rossi A (1995)Improvement of long-term preservation of the isolated arrestedrat heart by trimetazidine: Effects on the energy state andmitochondrial function. Am J Cardiol 76:45B–49B
7. Fantini E, Demaison L, Sentex E, Grynberg A, Athias P (1994)Some biochemical aspects of the protective effect of trimeta-zidine on rat cardiomyocytes during hypoxia and reoxygena-tion. J Mol Cell Cardiol 26:949–958
8. Kantor PF, Lucien A, Kozak R, Lopaschuk GD (2000) The anti-anginal drug trimetazidine shifts cardiac energy metabolismfrom fatty acid oxidation to glucose oxidation by inhibitingmitochondrial long-chain 3-ketoacyl coenzyme A thiolase.Circ Res 86: 580–588
9. Brottier L, Barat JL, Combe C, Boussens B, Bonnet J,Bricaud H (1990) Therapeutic value of a cardioprotectiveagent in patients with severe ischemic cardiomyopathy.Eur Heart J 11:207–212
10. Lu C, Dabrowski P, Fragasso G, Chierchia SL (1998) Effects oftrimetazidine on ischemic left ventricular dysfunction in patientswith coronary artery disease: a double-blind, placebo-controlled, crossover study. Am J Cardiol 82:898–901
11. Belardinelli R, Purcaro A (2001) Effects of trimetazidine on thecontractile response of chronically dysfunctional myocardiumto low-dose dobutamine in ischemic cardiomyopathy. EurHeart J 22:2164–2170
12. Fragasso G, Piatti Md PM, Monti L et al (2003) Short and longterm beneficial effects of partial free fatty acid inhibition in dia-betic patients with ischemic dilated cardiomyopathy. Am HeartJ 146:E1–8
13. Vitale C, Wajngaten M, Sposato B et al (2004) Trimetazi-dine improves left ventricular function and quality of life inelderly patients with coronary artery disease. Eur Heart J25:1814–1821
14. Di Napoli P, Taccardi AA, Barsotti A (2005) Long term cardio-protective action of trimetazidine and potential effect on theinflammatory process in patients with ischaemic dilated car-diomyopathy. Heart 91:161–165
15. Sisakian H, Torgomyan A, Barkhudaryan A (2007) The effectof trimetazidine on left ventricular systolic function and physicaltolerance in patients with ischaemic cardiomyopathy. ActaCardiol 62:493–499
16. Essop MF, Opie LH (2004) Metabolic therapy for heart failure.Eur Heart J 25:1765–1768
17. Thrainsdottir I, von Bibra H, Malmberg K, Ryden L (2004)Effects of trimetazidine on left ventricular function in patientswith type 2 diabetes and heart failure. J Cardiovasc Pharmacol44:101–108
18. Fragasso G, Palloshi A, Puccetti P et al (2006) A randomizedclinical trial of trimetazidine, a partial free fatty acid oxidation
inhibitor, in patients with systolic dysfunction heart failure.J Am Coll Cardiol 48:992–998
19. Tuunanen H, Engblom E, Naum A et al (2008) Trimetazidine, ametabolic modulator, has cardiac and extracardiac benefits inidiopathic dilated cardiomyopathy. Circulation 118:1250–1258
20. Conway MA, Allis J, Ouwerkerk R et al (1991) Detection of lowPCr to ATP ratio in failing hypertrophied myocardium by 31Pmagnetic resonance spectroscopy. Lancet 338:973–976
21. Yabe T, Mitsunami K, Inubushi T, Kinoshita M (1995) Quanti-tative measurements of cardiac phosphorus metabolites incoronary artery disease by 31P magnetic resonance spectros-copy. Circulation 92:15–23
22. Nascimben L, Ingwall JS, Pauletto P et al (1996) The creatinekinase system in failing and nonfailing human myocardium.Circulation 94:1894–1901
23. Fragasso G, De Cobelli F, Perseghin G, et al: Effects of meta-bolic modulation by trimetazidine on left ventricular functionand phosphocreatine/adenosine triphosphate ratio in patientswith heart failure. Eur Heart J 2006, 27:942–948.
24. Neubauer S, Horn M, Cramer M et al (1997) Myocardialphosphocreatine-to-ATP ratio is a predictor of mortality inpatients with dilated cardiomyopathy. Circulation 96:2190–2196
25. Peabody FW, Meyer AL, Du Bois EF (1916) The basal metab-olism of patients with cardiac and renal disease. Arch Int Med17:980–1009
26. Riley M, Elborn JS, McKane WR et al (1991) Resting energyexpenditure in chronic cardiac failure. Clin Sci 80:633–639
27. Poehlman ET, Scheffers J, Gottlieb SS et al (1994) Increasedresting metabolic rate in patients with congestive heart failure.Ann Intern Med121:860–862
28. Lommi J, Kupari M, Yki-Järvinen H (1998) Free fatty acidkinetics and oxidation in congestive heart failure. Am J Cardiol81:45–50
29. Steinberg D, Nestel PJ, Buskirk ER et al (1964) Calorigeniceffect of norepinephrine correlated with plasma free fatty acidturnover and oxidation. J Clin Invest 43167–176
30. Landsberg L, Saville ME, Young JB (1984) Sympathoadrenalsystem and regulation of thermogenesis. Am J Physiol 247:E181–E189
31. Beadle RM, Frenneaux M (2010) Modification of myocardialsubstrate utilisation: a new therapeutic paradigm in cardiovas-cular disease. Heart 96:824–830
32. Fragasso G, Salerno A, Lattuada G et al (2011) Effect of partialinhibition of fatty acid oxidation by trimetazidine on whole bodyenergy metabolism in patients with chronic heart failure. Heart97(18):1495–1500
33. Monti LD, Setola E, Fragasso G et al (2006) Metabolic andEndothelial Effects of Trimetazidine on Forearm Muscle inPatients with Type 2 Diabetes and Ischemic Cardiomyopathy.Am J Physiol Endocrinol Metab 290:E54–E59
34. Fragasso G, Piatti P, Monti L et al (2002) Acute effects ofheparin administration on the ischemic threshold of patientswith coronary artery disease: evaluation of the protective roleof the metabolic modulator trimetazidine. J Am Coll Cardiol39:413–419
35. Yamauchi-Kohno R, Miyauchi T, Hoshino T et al (1999) Role ofendothelin in deterioration of heart failure due to cardiomyopa-thy in hamsters: increase in endothelin production in the heartand beneficial effect of endothelin A antagonist on survival andcardiac function. Circulation 99:2171–2176
36. Gao D, Ning N, Niu X, Hao G, Meng Z (2011) Trimetazidine: ameta-analysis of randomised controlled trials in heart failure.Heart 97:278–286
FOCUS ON VASTAREL®MR - GABRIELE FRAGASSO
28 Heart Metab. (2011) 53:25–28

Myocardial power delivery is impairedin progressive left ventricular pump failure:a case report
Frank Lloyd Dini, Cardiac, Thoracic and Vascular Department, University of Pisa, Pisa, Italy.
Correspondence: Frank Lloyd Dini, Cardiovascular Diseases Unit 1, Cardiac, Thoracic and Vascular Department,Azienda Universitaria Ospedaliera Pisana, Via Paradisa 2, 56100 Pisa, Italy
Tel.: +39 (050) 995307; fax: +39 (050) 995308, e-mail: [email protected]
AbstractThe driving force for the flow of blood is the total energy imparted to it that corresponds to the mechan-ical, external work performed by the ventricle. In the cardiovascular system, the pumping power of theheart can be determined by cardiac power output, which is the product of cardiac output and meanblood pressure. Similarly to power-to-weight ratio, that is a measurement of the actual performanceof any engine, maximal left ventricular power output-to-left ventricular mass is an index of cardiacperformance potentially useful in patients with heart failure. In this case report, an example of theusefulness of this parameter is described in a patients with advanced heart failure secondary to dilatedcardiomyopathy.
Keywords: heart failure; dilated cardiomyopathy; echo stress
■ Heart Metab. (2011) 53:29–32
Introduction
In attempting to characterize a system composed of an energy source and pipes conductingthis energy, the usual parameters used for this purpose are the power of the energy generatorand the resistance of the conducting pipes [1]. In the cardiovascular system, the pumpingpower of the heart can be determined by cardiac power output, which is the product of car-diac output (CO) and mean blood pressure (BP). By incorporating both flow and pressure in asingle entity, cardiac power output represents the amount of energy imparted by the left ventri-cle to the volume of blood ejected per second [2]. Cardiac power output can be invasively andnoninvasively assessed during maximal exercise or pharmacological stress test [3–7].
Power-to-weight ratio is a measure that is widely applied to mechanical engines to comparethe performance of vehicles, aircrafts, and other mobile power sources. Similarly to power-to-weight ratio, peak power output-to-left ventricular (LV) mass (peak power-to-mass) is anindex of LV performance potentially useful in patients with cardiac diseases. This parameterallows us to assess the relationship between cardiac power measurements and most ofthe recruitable myocardial reserve available at maximum workload. As a result, peakpower-to-mass may be interpreted as a measure of myocardial efficiency, that is a ratio thatincorporates the degree of the external work per unit of time and the maximal work possible.
Case
Repo
rtCASE REPORT - FRANK LLOYD DINI
Heart Metab. (2011) 53:29–32 29

Although the denominator of this equation cannot bemeasured, it can be argued that in normal ventriclesthe amount of LV mass is comparative to myocardialpower delivery, whereas a disproportion between LVperformance and mass is suggestive of the maladap-tive features of LV remodeling [1]. To date, peakpower-to-mass can be easily assess during exerciseor dobutamine stress echocardiography and resultedto be valuable to measure cardiac pumping capacityespecially in patients with cardiomyopathies. In thefollowing case report, the clinical significance of peakpower-to-mass is described.
Case report
The clinical, biochemical and echocardiographic dataof a 64-year-old man with idiopathic dilated cardio-myopathy hospitalized because of symptoms ofcongestive heart failure (HF) are reported. After stabiliza-tion, cardiac right-sided catheterization was carried outusing a 7F MPA1 catheter (Cordis, Miami, FL). Meanpulmonary capillary wedge pressure was determinedautomatically by the monitoring system (Horizon 9000WS, Mennen Medical Ltd, Israel). LV end-diastolicpressure was recorded using a 6F 145° pigtail catheter(Cordis, Miami, FL). Hemodynamic measurementswere acquired before any injection of the contrastmedium. LV end-systolic and end-diastolic meridionalwall stresses were estimated using invasive measure-ments of LV pressures. The patient was submitted toa comprehensive transthoracic echocardiographyusing commercially available Acuson Sequoia C256ultrasound instrument (Mountain View, CA) with2nd-harmonic imaging and a 3.5-MHz transducer.Two-dimensional and color-flow Doppler imageswere obtained in standard parasternal and apicalviews. The LV mass was determined by using theM-mode method according to the recommendationsof the European Society of Echocardiography [2]. Asymptom-limited graded bicycle semi-supine exercisewas performed at an initial workload of 20 watts lastingfor one minute; thereafter the workload was increasedstepwise by 10 watts every minute. A 12-lead electro-cardiogram and blood pressure determination wereperformed at baseline and every minute thereafter. Atbaseline and at peak exercise, Doppler-derived CO atLV outflow tract, heart rate (HR) and arterial systolicblood pressure (BP) and diastolic BP (by cuff sphygmo-manometer) were measured. Mean BP was estimated
as follows: diastolic BP + 1/3 (systolic BP – diastolicBP). Stroke volume (SV) was calculated as strokedistance × LV outflow tract area and CO as SV × HRas previously described [3]. LV power output wasmeasured as the product of CO and mean BP.In meter-kilogram-second units, the conversion is106 ml/m3 for SV, and 133 pa (pascal)/mmHg for pres-sure. Power-to-mass was calculated as LV power out-put per 100 gram of LV mass: 100 x LV power outputdivided by LV mass (watt/100 g).
The characteristics of the patient during the hospi-talization are shown in Table 1 and Table 2. He was inNYHA class III and his LV ejection fraction (EF) was21%. The electrocardiogram showed a sinus rhythmand a complete left branch bundle block. B-type natri-uretic peptide (BNP) level was 498 pg/ml. The work-load reached at the end of the stress test performedthe day before discharge was 70 watts. At maximumexercise, mean BP was 107mmHg, SV was 80 ml andHR was 145 beats per minute; entering these values inthe above formulas, we get: BP = 133 × 103 = 13,699pa, SV = 35 × 10-6 m3 and then we can calculatepower as (13,699 × 35 × 145 × 10-6)/60 = 1.15 watt.LV mass was 349 g. A simplified formula to calculatepower output-to-mass is: 0.222 × CO (l/min) × meanBP (mmHg)/LV mass (g) = (0.222 × 5 × 103)/349 =0,33 watt/100 g. The patient underwent cardiac resyn-chronization therapy and was implanted with an auto-matic cardioveter defibrillator. Then, he was dis-charged with a therapy that included furosemide,angiotensin converting inhibitors, aldosterone antago-nists, beta-blockers and digoxin. Six months later, he
Variable
Age, years 64Body surface area 26Right atrial pressure (mmHg) 10Pulmonary artery systolic pressure (mmHg) 58Pulmonary artery diastolic pressure (mmHg) 30Pulmonary artery mean pressure (mmHg) 30Pulmonary capillary wedge pressure (mmHg) 33Left ventricular end-diastolic pressure (mmHg) 25Cardiac output (l/min) 3.4Cardiac index (l/min/m2) 1.7Left ventricular end-systolic stress (kdyne/cm2) 53Left ventricular end-diastolic stress (kdyne/cm2) 183
Table 1 Clinical and hemodynamic characteristics of the patientdescribed in the case report.
CASE REPORT - FRANK LLOYD DINI
30 Heart Metab. (2011) 53:29–32

was re-hospitalized for worsening HF. LV EF was notdifferent with respect to the previous hospitalizationand no relevant changes were apparent for most ofthe other echocardiographic and Doppler parameters.Pre-discharge BNP was 1072 pg/ml. The exercisestress test was repeated before discharge. Peakpower output-to-mass at exercise stress test was0.16 watt/100 g. Four months later the patient dieddue to refractory progressive pump failure.
Discussion
This case report describes a case of dilated cardio-myopathy accompanied by a severe deterioration ofcardiac pumping capacity. The patient developed aprogressive refractory heart failure, which was clearlyevident at the time of the first exercise stress test,where peak cardiac power output showed only ablunted increase during the exercise. Neither opti-mized medical treatment nor cardiac resynchroniza-
tion therapy were able to retard the progression ofthe disease.
This report shows the importance of measuring car-diac pumping capacity during exercise stress echo-cardiography for risk stratification of patients withadvanced HF. It is interesting to note that resting LVEF did not change between the first and the secondhospitalization. LV EF is the most frequently used indexof LV performance, but it may not accurately reflectmyocardial contractility and provides little prognosticinformation in patients with advanced HF. The inter-pretation of an EF less than 30% in a NYHA class Ipatient with mild LV dilation may be quite differentfrom that an individual with class III symptoms asso-ciated with a severely dilated left ventricle. Both EFdeclines reflect chamber remodeling despite relativepreserved stroke volume. However, in one instance,LV remodeling dominates this decline, with a near nor-mal residual myocardium capable of providing ade-quate cardiac reserve. In the other, the entire LV myo-cardium is depressed with little reserve pumpingcapacity. These two situations may look similar whenassessed by conventional measures, such as EF orwall motion score index, yet be very different byalternative methods, such as cardiac power outputperformed under stress.
Although cardiac power output is a well-establishedparameter of ventricular function that can be noninva-sively acquired during exercise testing, it does not con-sider alterations in cardiac size and structure that mayhave an impact on the outcome of patients with HF.The novelty of peak power-to-mass (and peak mass-to-power) is that it encompasses LV mass, that is amajor feature of the alterations in ventricular structurethat occurs as a part of normal growth or due to apathologic process, and similarly to EF, that is theratio between the stroke volume and LV end-diastolicvolume, provides integrate information on cardiacfunction and ventricular remodeling. The amount ofLV mass may be equated to the energy stored in themyocardium according to the principle of equivalenceof mass and energy as affirmed by Einstein’s theory ofrelativity. An example of this is physiological hypertro-phy that is induced by exercise training, whereas thephenotypes that appear in chronically overloadedventricles are pathological because they are accompa-nied by maladaptive changes [4,5]. The discrepancybetween a severely depressed cardiac power output
Before After
Resting variablesHeart rate (bpm) 81 65Mean blood pressure (mmHg) 89 60End-diastolic volume (ml) 205 206End-systolic volume (ml) 162 161Stroke volume (ml) 43 45Stroke volume index (ml/m2) 23 24Ejection fraction (%) 21 22Cardiac output (l/min) 3.5 2.9Cardiac index (l/min/m2) 1.8 1.5Power output (watt) 1.15 0.39Power output-to-mass (watt/100 g) 0.20 0.11
ExerciseWorkload (watt) 70 60Heart rate (bpm) 145 85Mean blood pressure (mmHg) 103 73End-diastolic volume (ml) 165 181End-systolic volume (ml) 130 141Stroke volume (ml) 35 40Stroke volume index (ml/m2) 18 21Ejection fraction (%) 21 22Cardiac output (l/min) 5.0 3.4Cardiac index (l/min/m2) 2.6 1.8Power output (watt) 0.69 0.55Power output-to-mass (watt/100 g) 0.33 0.16
Table 2 Clinical and echo-Doppler-derived hemodynamiccharacteristics of the patient described in the case report.
CASE REPORT - FRANK LLOYD DINI
Heart Metab. (2011) 53:29–32 31

albeit an increased LV mass is likely to reveal the pres-ence of maladaptive LV remodeling and this mayreflect the inefficiency of the system to comply withthe body’s metabolic needs. Adverse or maladaptiveLV remodeling is a major factor that affects the out-come of patients with advanced HF due to LV systolicdysfunction [6].
The effects of intervening LV hypertrophy on recrui-table cardiac power output are important to establishthe significance of LV remodeling. When the recruita-ble power output per unit of LV mass decreases due toprogressively decreasing ability of the myocardium togenerate force to overcome the load, LV functionrapidly deteriorates. Furthermore, the high LV end-diastolic volume and pressure promote subendo-cardial ischemia that aggravates LV dysfunction andneurohormonal activation, decreases exercise capac-ity and increases the risk of ventricular arrhythmias.Another factor that may contribute to maladaptive LVremodeling is the inadequate growth of myocardialmicrovasculature accompanying myocardial hyper-trophy [7].
Peak power-to-mass provided incremental prog-nostic information over resting LV EF as well as otherLV parameters recorded under stress. In our experi-ence, the cutoff value for peak cardiac power-to-mass that accurately predicts all-cause mortalityor HF hospitalization is 0.58 watt/100 g, but its impacton prognosis is clearer if the patient achieves a peakcardiac power-to-mass less than 1.0 watt/100 g afteroptimal tailored therapy or myocardial revasculari-zation with interventional cardiac procedures or resyn-chronization therapy [6].
By coupling peak exercise LV power output and LVmass, peak power-to-mass is useful to identifypatients with adverse LV remodeling during stressechocardiography and may provide additional prog-nostic information either in association with restingechocardiographic studies or cardiopulmonary exer-cise testing. LV hypertrophy is almost always presentin patients with chronic systolic HF accompanied by
LV dilatation and low EF, is typically eccentric, and isfrequently associated with a normal or lower than nor-mal LV wall thickness [8]. Despite that changes in LVgeometry and wall thickness may be temporarily usefulin maintaining myocardial pump function, they occursat significant high cost and are commonly followed bythe unfavorable consequences of dilation. LV enlarge-ment increases the force that must be generated toexceed end-diastolic wall stress and to achieve agiven level of cavity pressure. ●
References
1. Dini FL (2011) Assessment of cardiac dynamics during stressechocardiography by the peak power output-to-left ventricularmass ratio. Future Cardiol 7:347–356
2. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E,Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J,Solomon S, Spencer KT, St John Sutton M, Stewart W (2006)Recommendations for chamber quantification: a report from theAmerican Society of Echocardiography’s Guidelines and Stan-dards Committee and the Chamber Quantification WritingGroup, developed in conjunction with the European Associationof Echocardiography, a branch of the European Society of Car-diology. Eur J Echocardiography 7:79–108
3. Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA(2002) Doppler Quantification Task Force of the Nomenclatureand Standards Committee of the American Society of Echocar-diography. Recommendations for quantification of Dopplerechocardiography: a report from the Doppler QuantificationTask Force of the Nomenclature and Standards Committee ofthe American Society of Echocardiography. J Am Soc Echocar-diogr 15:167–184
4. Ingwall JS, Weiss RG (2004) Is the failing heart energy starved?On using chemical energy to support cardiac function. Circ Res95:135–145
5. Neubauer S (2007) The failing heart-an engine out of fuel.N Engl J Med 356:1140–1451
6. Dini FL, Mele D, Conti U, Ballo PC, Citro R, Menichetti F, MarzilliM (2010) Peak power output-to left ventricular mass: An indexto predict ventricular pumping performance and morbidity inadvanced heart failure. J Am Soc Echocardiogr 23:1259–1265
7. Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, JafriS, Hawkins ET, Goldstein S (1991) A canine model of chronicheart failure produced by multiple sequential coronary microem-bolizations. Am J Physiol 260:H1379–H1384
8. Dini FL, Capozza P, Donati F, Simioniuc A, Corciu AI, FontaniveP, Pieroni A, Di Bello V, Marzilli M (2011) Patterns of left ventric-ular remodeling in chronic heart failure: Prevalence and prog-nostic implications. Am Heart J 161:1088–1095
CASE REPORT - FRANK LLOYD DINI
32 Heart Metab. (2011) 53:29–32

Impact of fatty acid oxidationon cardiac efficiencyNatasha Fillmore and Gary D. Lopaschuk, Cardiovascular Research Centre, Mazankowski Alberta Heart Institute,
University of Alberta, Edmonton, Canada
Correspondence: Dr. Gary Lopaschuk, 423 Heritage Medical Research Center, University of Alberta,Edmonton, Canada T6G 2S2.
Tel.: (780) 492-2170; fax: (780) 492-9753, e-mail: [email protected]
AbstractAlterations in energy substrate preference by the heart can lead to significant changes in cardiacefficiency. Cardiac efficiency, which is the amount of work produced by the heart per energy (O2) con-sumed, is dependent not only on the efficiency of producing energy (ATP), but also on the efficiency ofusing energy to produce contractile work. Using fatty acids as a source of fuel has the potential to alterboth of these pathways. The mitochondrial oxidation of fatty acids utilizes more O2 per molecule of ATPproduced than most other sources of fuel. High rates of fatty acid oxidation also inhibit glucose oxida-tion in the heart, which can result in alterations in ionic homeostasis, such that more of the ATPproduced in the heart is used for non-contractile purposes. Combined, the excessive use of fatty acidsby the heart can result in a significant decrease in cardiac efficiency. In certain heart pathologies, suchas during and following ischemia or in the failing heart, cardiac efficiency is also decreased. Alterationsin the balance between fatty acid and carbohydrate use contribute to these alterations in cardiacefficiency. In this review we will focus on how alterations in cardiac energy metabolism alter cardiac effi-ciency, as well as on how alterations in energy metabolism that occur in heart failure and ischemia resultin decreased cardiac efficiency.
Keywords: fatty acid β-oxidation; uncoupling proteins; mitochondrial thioesterase; glucose oxidation;glycolysis
■ Heart Metab. (2011) 53:33–37
Introduction
The heart has a very high energy demand, while essentially having no energy reserves. Forexample, consumption of the main energy currency of the heart, adenosine triphosphate(ATP), is so high that in the contracting heart the entire pool of ATP turns over approximately6 to 8 times a minute [1]. In order to produce this large amount of ATP, the heart consumes anumber of different energy substrates, including fatty acids (FAs), glucose, lactate, ketones,pyruvate, and amino acids. Most of the ATP produced requires the consumption of O2 formitochondrial oxidative metabolism (Fig. 1) [1]. However, the efficiency of producing ATP canvary dramatically depending on the type of energy substrate used. An example of this is theutilization of FAs, which while being a plentiful source of energy, is also a particularly inefficientsource of energy [1]. Different cardiac pathologies can also alter cardiac efficiency, both as aresult of a decreased efficiency of producing ATP or alterations in the efficiency of using ATPto produce contractile work [1].Re
fresher
Corner
REFRESHER CORNER - NATASHA FILLMORE
Heart Metab. (2011) 53:33–37 33

Cardiac efficiency is often expressed as a measureof the amount of cardiac work produced per amount ofenergy (O2) consumed by the heart (cardiac work/MVO2 ratio) [2]. It is not surprising that alterations inenergy metabolism can alter cardiac efficiency, sincecardiac work requires ATP and production of ATP viamitochondrial oxidative metabolism requires O2. Thetype of substrate utilized in the production of ATP viaoxidative metabolism affects cardiac efficiency. Inaddition, metabolic by-products produced duringATP production also have the potential to alter cardiacefficiency.
Energy metabolism and cardiac efficiency
Efficiency of ATP production
A major determinant of cardiac efficiency is the type offuel being utilized for ATPproduction. The efficiencywithwhich FAs and glucose are utilized to produce ATP dif-fers [1]. The production of 31 ATP by one glucose mol-ecule going through glycolysis and glucose oxidation(GO) requires 6 O2. For the production of 105 ATP bypalmitate oxidation, 23O2 are required. Therefore,moreoxygen is used per ATP produced during fatty acid oxi-dation (FAO) compared to coupled GO, making FAs a
A BFatty acid
Fatty acid
Fatty acyl CoA
Fatty Acyl CoA
Glucose
Glucose
Pyruvate
Pyruvate
Lactate
Glycolysis
2ADP + 2H+2ATP
H+
ATP
Acetyl CoA
TCA CycleNADH, FADH2
MitochondrialMatrix
Cytosol
Cytosol
Sarcolemma
Extracellular
ETC
PDH
FACS
GLUT4CD36/FATH+
H+H+
H+
H+
H+
H+H+
H+H+
Na+
Na+
Na+
Ca2+
Ca2+
H+
H+
H+
H+
H+ H+
Na+ / H+
Transporter
Na+ / Ca2+
Exchanger
Na+ / Ca2+
Exchanger
Fig. 1 Cardiac efficiency is decreased by elevated glycolysis and fatty acid oxidation. A. Elevated fatty acid oxidation can reduce cardiacefficiency through a number of mechanisms including inhibition of glucose oxidation and increasing glycolysis. Fatty acyl CoA inhibits PDH,thereby inhibiting glucose oxidation. Under certain conditions glycolysis can become elevated to make up for reduced oxidative metabo-lism. Protons accumulate because the co-transport of protons with pyruvate into the mitochondria is decreased and cause acidosis. Acido-sis can decrease contractile force by decreasing the responsiveness of myofilaments to calcium. The use of ATP for ionic homeostasis canreduce cardiac efficiency. B. Return of intracellular pH (low pH caused by uncoupled elevated glycolysis) to normal upon reperfusiondecreases cardiac efficiency by affecting ionic homeostasis. A large trans-sarcolemmal proton gradient forms that increases the exchangeof the Na+/H+ transporter resulting in further elevation of intracellular Na+. In response, the Na+/Ca2+ exchanger works in reverse mode,moving calcium into the cell. This results in an overload of intracellular calcium. More energy is expended maintaining calcium homeostasis.PDH pyruvate dehydrogenase
REFRESHER CORNER - NATASHA FILLMORE
34 Heart Metab. (2011) 53:33–37

less efficient substrate than glucose for energy produc-tion. In addition, FAO inhibits GO [1]. When the FA sup-ply to the heart rises, assumingO2 availability, the rate ofFAO increases and GO decreases. This explains whyunder conditions in which circulating free FAs are ele-vated (such as heart failure [HF], ischemia, and type IIdiabetes) cardiac efficiency is decreased.
Futile cycling
In addition to being less efficient at ATP production perO2 consumed, FAs can also decrease cardiac effi-ciency through a number of other mechanisms. Thisis evident from the fact that there is a large discrepancybetween the degree of inefficiency observed in ATPproduction/O2 consumed, and actual measurementsof cardiac efficiency in the heart (cardiac work/MVO2)[1]. At most, elevated FAO should decrease efficiencyby 10 to 12% [1]. In reality, cardiac efficiency has beenshown to be decreased by as much as 30% [3]. Onepossible mechanism to explain this is long-chain FAactivation of Ca2+ channels in the sarcolemma [4]. Arise in cytosolic Ca2+ results in more energy beingexpended to keep cytosolic Ca2+ levels normal.Another mechanism that has been proposed involvesFA inhibition of ATP removal from the mitochondriaby inhibition of adenine nucleotide transferase [1]. Yetanother potential mechanism involved in FA-inducedinefficiency is the presence of futile cycles.
One such futile cycle involves the uncoupling pro-teins. The uncoupling proteins UCP2 and UCP3 arepresent in ventricular muscle [2]. These proteins areclassically believed to work by dissipating the intermem-brane proton gradient (Fig. 2). FAs are believed to workthrough UCP2 and UCP3 to mediate the uncoupling ofoxidative phosphorylation [2,5]. Further, UCP2 andUCP3 expression correlate positively with circulatingFA levels in the failing human heart [6]. UCP3 may alsocontribute to cardiac efficiency by transporting FAanions out of the mitochondrial matrix [2,7].
FAs in the cytoplasm can also cycle between theiracyl-coenzyme A (CoA) moieties and intracellular tria-cylglycerol pools [1]. Two high energy phosphates arerequired to esterify FA to CoA, which can then eitherbe directed to mitochondrial FA β-oxidation or com-plex lipid synthesis in the heart (such as triacylglycer-ols). FAs liberated from the triacylglycerol pool prior tosubsequent β-oxidation create a futile cycle, potentiallycontributing to a decreased cardiac efficiency.
Fatty acid inhibition of glucose oxidation
Another pathway by which FAs may decrease cardiacefficiency is secondary to GO inhibition [1]. FAO pro-ducts can inhibit GO (i.e., the Randle Cycle) (Fig. 1).This FA inhibition of GO is more dramatic than theeffects of FAs on glycolysis [1]. This can result in a sce-nario where glycolysis is uncoupled from GO, which
Fatty acyl CoA
Fatty acyl CoA
Fatty acid anion
Electrontransportchain
Fatty acid anion
Mitochondrialmatrix
Intermembrane
H+
H+
H+
H+
H+H+
H+
H+
ADPUCP
UCP
ATP
CoA
I
II
III
IV
H+
H+
F0/F1ATPase
Mitochondrialthioesterase
Fig. 2 Mechanisms of uncoupling protein reduction of cardiac inefficiency. Uncoupling proteins dissipate the high proton concentrationin the mitochondrial intermembrane space by transferring the hydrogen back into the mitochondrial matrix. UCP3 may also reduce cardiacefficiency by transferring fatty acid anions out of the mitochondrial matrix. CoA coenzyme A, UCP uncoupling protein
REFRESHER CORNER - NATASHA FILLMORE
Heart Metab. (2011) 53:33–37 35

can result in elevated lactate and proton levels causingintracellular acidosis [2,8]. The co-transport of protonswith pyruvate into the mitochondria is decreasedbecause pyruvate is not taken into the mitochondriawhen glycolysis is uncoupled fromGO. Although glycol-ysis produces ATP without consuming O2, an increasein glycolysis in the presence of low GO rates can resultin the accumulation of metabolic byproducts in theheart, that include both lactate and protons [1] (Fig. 1).The clearance of these protons can result in Na+ andCa2+ accumulation in the heart (Fig. 1), requiring ATP toremove these ions [1]. This can lead to a decrease incardiac efficiency, as ATP is redirected away from con-tractile function and towards ionic homeostasis [1].
Altered energy metabolism in ischemic heart disease
Prominent alterations in energy metabolism occur inthe setting of myocardial ischemia and reperfusionthat result in reduced cardiac efficiency (Fig. 3a) [1].In the ischemic heart, energy metabolic rates aredependent upon the degree of ischemia. Because ofthe reduced oxygen availability both GO and FAO arereduced in the ischemic heart [2,9,10]. Interestingly,during mild ischemia FAO predominates as the source
of oxidative metabolism in the heart [2,11,12]. This islikely explained by the exposure of ischemic hearts tohigh plasma FAs and to direct changes in the intracel-lular control of FAO, which combine to inhibit GO[1,11]. It is important to note that unlike the normalheart, exposure of the ischemic heart to FAs doesnot inhibit glycolysis, and that glycolytic rates areincreased in the ischemic heart [11].
Increased glycolysis and impaired GO in the ischemicheart result in lactate and proton accumulation in theischemic myocardium. Indeed, uncoupling of glycolysisfromGO can largely explain the acidosis observed in theseverely ischemic heart. As described above, accumu-lation of protons can lead to Na+ and Ca2+ overload inthe heart, resulting in decreased cardiac efficiency asATP is used to attempt to restore ionic homeostasis [1].
Lack of recovery of cardiac function and efficiencyupon reperfusion is also explained by the alterations inmetabolism during ischemia and reperfusion [2,13,14].Return of intracellular pH to normal upon reperfusioncan be deleterious. This is because, by modulating theNa+/H+ transporter and the Na+/Ca2+ exchanger,changes in intracellular ion concentrations occur thatcontribute to impaired cardiac function and efficiency
A Ischemia B Heart Failure
Fatty acids Mitochondrial oxidation
Glucose oxidation
Glycolysis
Protons
Cardiacefficiency
Intracellular calcium and sodium
ATP utilization for noncontractilepurposes
Oxygen consumption/ATPproduced
Fatty acid oxidation Glycolysis
Protons
Cardiac efficiency
Intracellular calcium and sodium
ATP utilization for noncontractile purposes
Fig. 3 Cardiac inefficiency in the ischemic heart and the failing heart. A. During ischemia and reperfusion fatty acid levels rise resultingin elevated fatty acid oxidation. This results in decreased glucose oxidation and elevated glycolysis. As a result of glycolysis and glucoseoxidation being uncoupled, proton levels rise causing an overload of sodium and calcium. More energy is expended to maintain ionichomeostasis resulting in a decrease in cardiac efficiency. Through other mechanisms, fatty acids also reduce cardiac efficiency. B. In heartfailure, a reduction in overall oxidative metabolism results in elevated uncoupled glycolysis resulting in decreased cardiac efficiency throughsimilar mechanisms as described for ischemia
REFRESHER CORNER - NATASHA FILLMORE
36 Heart Metab. (2011) 53:33–37

[2]. The large trans-sarcolemmal proton gradient thatforms increases the exchange of the Na+/H+ trans-porter resulting in further elevation of intracellular Na+.In response, the Na+/Ca2+ exchanger reverse mode isactivated. The movement of calcium into the cell viathe Na+/Ca2+ exchanger results in an overload of intra-cellular calcium and thus more energy being expendedto maintain calcium homeostasis.
As would be expected, cardiac efficiency and func-tion is reduced in mildly ischemic hearts exposed tohigh levels of FAs [11]. If a heart is subjected to globalischemia, FA β oxidation decreases secondary to alack of O2 availability for mitochondrial oxidative phos-phorylation [15]. During reperfusion FAO recovers,resulting in GO remaining low [2,8,13,14]. Therefore,while glycolysis can remain high during reperfusion, itcan still be uncoupled from GO resulting in elevatedlactate and proton production [2,13]. Changes inFA supply, the type of oxidative metabolism, andincreased glycolysis are responsible for the cardiacinefficiency observed during ischemia and reperfusion.
Energy metabolism and cardiac efficiencyin heart failure
Alterations in energy substrate metabolism accompa-nying HF are extremely complex, in part due to the het-erogeneous nature of HF [1]. In general, however, as HFprogresses, what is observed is a decrease in overallmitochondrial oxidative capacity and an increase in glu-cose uptake and glycolysis [1]. FAO rates have beenshown to be elevated, unchanged or decreased in HF(see [1] for review). The increase in glycolysis in HF is anadaptive response to compensate for decreased mito-chondrial oxidative capacity. This increase in glycolysiswith a lowmitochondrial capacity to oxidize glucose canexacerbate lactate and proton production, in a mannersimilar to that seen in the ischemic heart (Fig. 3b). SinceFAOcompeteswithGO, FAs can further exacerbate thisuncoupling. Support for this concept comes from anumber of clinical studies in which FAO inhibition in HFimproved both cardiac efficiency and function.
Conclusions
Alterations in cardiac energymetabolismcan profoundlyaffect cardiac efficiency. Excessive use of FAs has beenshown to be especially important, either by decreasingthe efficiency of producing ATP, or by decreasing ATPavailability for contractile function. Strategies aimed at
optimizing cardiac energy metabolism have the abilityto improve cardiac efficiency and function. For example,the Randle cycle is being targeted in order to increaseGO and decrease FAO [1]. Therefore, understandinghow energy metabolism affects cardiac efficiency isimportant for improving the treatment of heart disease. ●
Acknowledgements Gary D. Lopaschuk is a Scientist of theAlberta Heritage Foundation for Medical Research.
References
1. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, StanleyWC (2010) Myocardial fatty acid metabolism in health anddisease. Physiol Rev 90:207–258
2. Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD(2011) Targeting fatty acid and carbohydrate oxidation - Anovel therapeutic intervention in the ischemic and failingheart. Biochim Biophys Acta 1813:1333–1350
3. How OJ, Aasum E, et al (2005) Influence of substrate supplyon cardiac efficiency, as measured by pressure-volume analy-sis in ex vivo mouse hearts. Am J Physiol Heart Circ Physiol288:H2979–2985
4. Huang JM, Xian H, Bacaner M (1992) Long-chain fatty acidsactivate calcium channels in ventricular myocytes. Proc NatlAcad Sci U S A 89:6452–6456
5. Boehm EA, et al (2001) Increased uncoupling proteins anddecreased efficiency in palmitate-perfused hyperthyroid ratheart. Am J Physiol Heart Circ Physiol 280: H977–983
6. Murray AJ, Anderson RE, et al (2004) Uncoupling proteins inhuman heart. Lancet 364:1786–1788
7. Seifert EL, Bezaire V, Estey C, Harper ME (2008) Essential rolefor uncoupling protein-3 in mitochondrial adaptation to fastingbut not in fatty acid oxidation or fatty acid anion export. J BiolChem 283:25124–25131
8. Dennis SC, Gevers W, Opie LH (1991) Protons in ischemia:where do they come from; where do they go to? J Mol CellCardiol 23:1077–1086
9. Lloyd SG, et al (2004) Impact of low-flow ischemia on sub-strate oxidation and glycolysis in the isolated perfused ratheart. Am J Physiol Heart Circ Physiol 287:H351–62
10. Whitmer JT, Idell-Wenger JA, Rovetto MJ, Neely JR (1978)Control of fatty acid metabolism in ischemic and hypoxichearts. J Biol Chem 253:4305–4309
11. Folmes CD, et al (2009) High rates of residual fatty acid oxida-tion during mild ischemia decrease cardiac work and effi-ciency. J Mol Cell Cardiol 47:142–148
12. Neely JR, Liedtke AJ, Whitmer JT, Rovetto MJ (1975) Rela-tionship between coronary flow and adenosine triphosphateproduction from glycolysis and oxidative metabolism. RecentAdv Stud Cardiac Struct Metab 8:301–321
13. Liu Q, Docherty JC, et al (2002) High levels of fatty acids delaythe recovery of intracellular pH and cardiac efficiency inpost-ischemic hearts by inhibiting glucose oxidation. J Am CollCardiol 39:718–725
14. McVeigh JJ, Lopaschuk GD (1990) Dichloroacetate stimula-tion of glucose oxidation improves recovery of ischemic rathearts. Am J Physiol 259: H1079–1085
15. Neely JR, Morgan HE (1974) Relationship between carbohy-drate and lipid metabolism and the energy balance of heartmuscle. Annu Rev Physiol 36:413–459
REFRESHER CORNER - NATASHA FILLMORE
Heart Metab. (2011) 53:33–37 37

Statin therapy: reduction in cardiovascularevents still pays in new-onset diabetes
Alda Huqi, Cardiovascular Medicine Division, Cardio Thoracic Department, University of Pisa, Pisa, Italy,and University of Alberta, Edmonton, Alberta, Canada
Correspondence: Dr. Alda Huqi, Cardiovascular Medicine Division, Cardio Thoracic Department, University of Pisa,Via Paradisa, 2, 56100 Pisa, Italy. And: 423 Heritage Medical Research Center, University of Alberta,
Edmonton, Alberta T6G 2S2, Canada.Tel: +39 32972 56426 e-mail: [email protected]
With a few exceptions [1], in the past two decades the benefit of statin therapy has beenreproducible irrespective of the individual drug, population subset or prevention strategy
(i.e., primary or secondary) used [2]. In addition to the established benefits, the decision to usestatin therapy has recently been reinforced by the introduction of generic formulation drugs(i.e., simvastatin, lovastatin and pravastatin) in the market. Accordingly, the need for expandingthe indications was met in the 2011 European Society of Cardiology (ESC) guidelines for themanagement of dyslipidemia [3]. These guidelines confirm that patients with a risk score of≥10%, those with established cardiovascular disease (CVD), type II or I diabetes or chronic kid-ney disease have a class I indication, level of evidence (LoE) A to receive aggressive statintreatment in order to achieve low density lipoprotein cholesterol (LDL-C) levels of less than70 mg/dl. In these guidelines, it is also recommended that a drug therapy with statins be con-sidered in patients with a risk score of <1% who have LDL-C levels of ≥190 mg/dl (Class IIa,LoE A), and in those patients with a risk score from 1 to 5% who have LDL-C levels of100–190 mg/dl (Class IIa recommendation, LoE A), or ≥190 mg/dl (Class I recommendationand LoE A).
However, recent data suggest that statin therapy is associated with an increased incidenceof new-onset diabetes. The incident finding in large clinical trials [4–6] of increased new-onsetdiabetes was in fact confirmed recently in two well-conducted metanalysis [7,8]. The first one[7], including 91,140 patients, showed that, as compared to patients receiving placebo,patients receiving statin therapy had a 9% increase in relative risk for developing diabetes(odds ratio [OR] 1.09, 95% confidence interval [CI] 1.02–1.17). More specifically, 1 out of255 patients would develop diabetes during a 4-year period of statin therapy. The secondmetanalysis [8] assessed whether, among those patients receiving statins, an intensive-dosetreatment regimen would further increase the incidence of new-onset diabetes. Data from fiveclinical trials including 32,752 patients showed that, as compared to moderate-dose therapy,an intensive treatment strategy was associated with a further 12% increase in relative risk fordeveloping new-onset diabetes (OR 1.12, 95% CI 1.04-1.22). Therefore, during a year of statintreatment, one out of 498 patients would develop new-onset diabetes if in the intensive treat-ment arm. However, on the other hand, 1 out of 155 of the same patient population wouldexperience less cardiovascular events because of the intensive treatment. Although no plausi-ble biological effects can yet be identified, the dose-dependence relationship observed in the
HotT
opics
HOT TOPICS - ALDA HUQI
38 Heart Metab. (2011) 53:38–39

latter study further confirmed that statin therapy isassociated with an increased risk for new-onsetdiabetes.
The status quo of statin therapy can therefore besummarized as follows: on one hand, as supportedalso by the availability of generic formulations andestablishment of new guidelines, there is a strongwillingness to extend treatment indications; however,on the other hand, there is also an increasing concernregarding the incidence of new-onset diabetes, partic-ularly for the low risk patient population.
Given such statements, how is the clinician sup-posed to weight the benefits and risks of statin treat-ment in the individual patient?
Following the recent meta-analysis, another, veryelegant study, that might actually help answer thisquestion was published [9]. This study used an estab-lished computer simulation model to project cost-effectiveness of statin therapy in an era where a moreaggressive statin treatment is being sought, low costgeneric formulations are available and side effectssuch as new-onset diabetes are better defined. Theauthors found that lowering LDL-C thresholds to<130 mg/dl for patients with no risk factors and to<100 mg/dl for patients with one risk factor and treat-ing all moderate and moderately high risk patientsregardless of LDL-C levels would provide additionalhealth benefits. Most importantly, these benefits werenot negatively affected by the inclusion in the analysisof statin-associated diabetes or other severe hypo-thetical side effects.
In conclusion, as outlined by the recently publishedESC guidelines [3], broadening of the indications tostatin therapy appears reasonable. With regards tothe individual patient, one should keep in mind that
the odds of developing diabetes from statin therapyare lower than those of reducing cardiovascularevents. Therefore, if the former occurs, statin therapyis theoretically supposed to provide a payback byreducing the risk for cardiovascular events, one ofthe major complications of diabetes. ●
References
1. Ray KK, Seshasai SR, Erqou S et al (2010) Statins and all-cause mortality in high-risk primary prevention: a meta-analysisof 11 randomized controlled trials involving 65,229 participants.Arch Intern Med 170(12):1024–1031
2. Mills EJ, Wu P, Chong G et al (2010) Efficacy and safety ofstatin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trials. QJM104(2):109–124
3. Reiner Z, Catapano AL, De Backer G et al (2010) ESC/EASGuidelines for the management of dyslipidaemias: The TaskForce for the management of dyslipidaemias of the EuropeanSociety of Cardiology (ESC) and the European AtherosclerosisSociety (EAS). Eur Heart J 32(14):1769–1818
4. Freeman DJ, Norrie J, Sattar N et al (2001) Pravastatin and thedevelopment of diabetes mellitus: evidence for a protectivetreatment effect in the West of Scotland Coronary PreventionStudy. Circulation 103(3):357–362
5. Sever PS, Dahlof B, Poulter NR et al (2003) Prevention of coro-nary and stroke events with atorvastatin in hypertensive patientswho have average or lower-than-average cholesterol concen-trations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomisedcontrolled trial. Lancet 361(9364):1149–1158
6. Ridker PM, Danielson E, Fonseca FA et al (2008) Rosuvastatinto prevent vascular events in men and women with elevatedC-reactive protein. N Engl J Med 359(21):2195–2207.
7. Sattar N, Preiss D, Murray HM et al (2010) Statins and risk ofincident diabetes: a collaborative meta-analysis of randomisedstatin trials. Lancet 375(9716):735–742
8. Preiss D, Seshasai SR, Welsh P et al (2011) Risk of incidentdiabetes with intensive-dose compared with moderate-dosestatin therapy: a meta-analysis. JAMA. Jun 305(24):2556–2564
9. Lazar LD, Pletcher MJ, Coxson PG, Bibbins-Domingo K, Gold-man L (2011) Cost-effectiveness of statin therapy for primaryprevention in a low-cost statin era. Circulation 124(2):146–153
HOT TOPICS - ALDA HUQI
Heart Metab. (2011) 53:38–39 39

AMP-activated protein kinase (AMPK)AMPK is a key kinase that controls many cellular pro-cesses, particularly pathways involved in cellularenergy status. AMPK is activated during metabolicstress, where it then can either activate energy-producing pathways or inhibit energy-consumingpathways. For these reasons, it has been termed a“fuel gauge” of the cell.
mTOR pathwayMammalian target of rapamycin (mTOR) is a kinasethat is a member of the phosphatidylinositol kinasefamily. The mTOR pathway functions as a key signal-ing pathway involved in the control of cell growth andproliferation. mTOR is activated in response to a num-ber of upstream signaling molecules, including insulinand growth factors such as IGF-1 and IGF-2. The termmTOR arose since rapamycin was originally shown toinhibit the mTOR pathway.
PGC-1αPeroxisome proliferator-activated receptor-γ coacti-vator-1α (PGC-1α) is a transcription co-activator thatplays a key role in the regulation of cellular energymetabolism. Activation of PGC-1α increases mito-chondrial biogenesis. In muscle PGC-1α activationresults in a muscle that is more oxidative and lessglycolytic.
PPARαPeroxisome proliferator-activated receptor α (PPARα)is a nuclear receptor involved in the transcriptionalregulation of proteins. This includes the transcription
of key proteins involved in the control of fatty acidoxidation.
ProteosomesProteosomes are large protein complexes inside cellsthat function to degrade damaged proteins. Proteasesin the proteasome degrade these proteins into shortamino acid peptides.
SERCA2Sarcoplasmic/endoplasmic reticulum calcium ATPase2 (SERCA2) is the enzyme primarily responsible for thetransport of calcium into intracellular sarcoplasmicreticulum (SR) and endoplasmic reticulum (ER). SR isan intracellular organelle in heart and skeletal musclethat stores calcium. During excitation-contraction cou-pling, release of calcium for the SR is the major sourceof calcium that initiates contraction. SERCA2 initiatesrelaxation of muscle by re-sequestering the calciumback up into the SR.
UbiquitinationUbiquitination is a process in cells in which proteins are“tagged” with a small protein called ubiquitin. This canlead to further ubiquitination of the protein, which thentargets the protein for degradation by proteasomes.
Ubiquitin ligasesUbiquitin ligases are enzymes in cells that catalyze theubiquitination reaction. These ubiquitinated proteinsare then targeted for degradation by proteasomes.As a result, ubiquitine ligases are key enzymes involvedin cellular protein degradation. ●
GLOSSARY - GARY D. LOPASCHUK
40 Heart Metab. (2011) 53:40