Confocal laser microscopy: Scanning new structural … · Confocal laser microscopy: Scanning new...
Transcript of Confocal laser microscopy: Scanning new structural … · Confocal laser microscopy: Scanning new...

Confocal laser microscopy: Scanning new structural and functional insights of host-microbe interactions
Mohd Sajjad Ahmad Khan*1, Mohd Musheer Altaf2, Abdullah Safar Althubiani3 and Iqbal Ahmad#2
1Department of Biology, College of Medicine, Imam Abdulrahman Al Faisal University, Dammam 31451 KSA 2Department of Agricultural Microbiology, Aligarh Muslim University, Aligarh 202002 India 3Department of Biology, Faculty of Applied Sciences, Umm Al Qura University, Makkah KSA *Corresponding author’s email id: * [email protected]; # [email protected]
Microorganisms under natural conditions do not exist in isolation but form complex ecological interaction webs. They undergo various kinds of interactions with their ecological hosts viz. plants and humans. These interactions may be either beneficial or deleterious to each other. Such behaviors are of fundamental importance to natural cycles and ecosystem functions and are of agricultural, environmental and biomedical interests for humans. In some cases, these are caused by planktonic cells, whereas in most cases they are associated with the structured aggregates of cells termed biofilms. The ability of detecting and investigating these various types of host-microbe interactions are studied through a number of varied methods varying from classical microscopy to advanced microscopy and molecular techniques. Various molecular methods are being used for examining the microbial diversity and activity of environmental samples. However, spatial information at micro-scale, which is fundamental to understand the dynamics of host-microbe interactions, is usually lost during the sample processing. Therefore, it is useful to complement the indirect molecular techniques with direct visualization methods. Confocal laser scanning microscopy (CLSM) allows the exploration of microbial habitats at a spatial resolution level unattainable with molecular methods. In this chapter, the application of CLSM for the study of host-microbe interactions, including both planktonic and biofilm ways of life, are addressed in the lights of newly developed molecular methods including fluorescent protein tags and advanced microscopic approaches. The application of CLSM including its operating modes, sample preparation methods and results obtained on ligand–receptor interactions, and cytoplasmic illustrations in various hosts with particular emphasis on plants and humans are discussed. Finally, we discuss recent progress in the understanding of the role of bacterial interactions in medicine within the framework of this techniques and concepts.
Keywords: Confocal laser scanning microscopy, host-microbe interactions, microbial niche, pathogens.
1. Introduction
Microorganisms play a central role in many processes that are of fundamental importance to natural cycles and ecosystem function by interacting with human activities. Various host-microbe interactions, namely, infection, commensalism, colonization, persistence, and diseases are perceived [1]. Host provides space for the microbe and transports nutrients to encourage its growth, metabolism and multiplication inside the host cell. These activities are responsible for running biogeochemical cycles, promotion of plant health and soil fertility, and other clinical relevance to humans. Man has exploited these activities for industrial, agricultural and medicinal benefits. Microbial interactions with either plants or humans are sometimes beneficial such as plant growth promoting activities and human micro-biota, and may also be damaging by causing diseases. In some cases, these diseases are caused by planktonic cells, whereas others are associated with the organized aggregates of cells, biofilms. During the progression of infection, many pathogens avoid the host immune defenses and bind to host cell surfaces and undermine fundamental cellular processes [2,3]. Because of their importance, microbes and microbial functions have been studied extensively in the modern scientific era. One of the most successful methods to study these host-microbe interactions is using microscopic approaches. A new era of biological imaging has been come up with recent developments of fluorescent probes and new high-resolution microscopes, and is presently having an intense impact on the way research is being lead in the life sciences [4]. Now, biologists are becoming more and more dependent on imaging technologies to visualize subcellular components and processes in vivo, both structurally and functionally. Observations can be made in two, three and even in four dimensions, at different wavelengths, feasibly with time-lapse imaging to inspect cellular dynamics [3,5]. In most natural and engineered systems microorganisms grow as spatially organised, matrix-enclosed multi-species communities in aggregates termed biofilms. The advent of the fluorescent in situ hybridisation (FISH) using rRNA targeted gene probes in association with confocal laser scanning microscopy (CLSM) allows to directly analyze the microbial community structure of such systems and to acquire evidences on the localization of definite bacterial groups [6]. FISH efficiently outspreads epifluorescence microscopy and has allowed fast detection and enumeration of explicit microorganisms [7]. All host-microbe interactions are commencing at the level of the cell and have long been recognized as multifaceted and critical for both susceptibility and resistance to microbial growth. Subsequent to its introduction, the electron microscope has become the instrument of choice to examine subcellular interactions between plants and microbes. Also, its usefulness has been boosted by the development of a variety of immunological, cytochemical, and analytical procedures to identify and localize specific enzymatic activities, chemical structures, or
Microscopy and imaging science: practical approaches to applied research and education (A. Méndez-Vilas, Ed.)
62
___________________________________________________________________________________________

atomic level interaction inside the cell [8]. In spite of this electron microscopy has not yet become routine in the study of plant-microbe interactions because of technical difficulties in its application or the requisite for specialized equipment and artifacts induced by the fixation procedures necessary to prepare tissue for electron microscopy [9]. Although the light microscope does not attain the resolution provided by the electron microscope, it has recently gain popularity in plant research. The light microscope is now frequently used for the detection of gene expression at the cellular level by in situ hybridization or the use of visually detectable reporter genes such as GUS (β-glucuronidase) [8,10,11]. More importantly, the usefulness of the light microscope in plant research has been further enhanced by recent parallel developments of confocal laser scanning and video microscopy, computerized image processing, and an ever-increasing array of fluorescent probes that can be applied to living cells [5]. CLSM not only remains as a widely applicable methodology for studying plant-microbe interactions, but also can be extended and integrated by other microscopic techniques, and therefore serves as a central technology in medicinal studies. Some of newer technologies, such as molecular imaging, may improve existing imaging modalities in amalgamation with nanotechnology, biotechnology, bioinformatics, leading to innovative approaches to clinical imaging such as in gastrointestinal human–microbe interface [12,13,14]. Confocal scanning microscopy has been extensively used to study bacterial adhesion and biofilms on tissue surfaces including oral biofilms [15,16]. Fluorescent antisera and fluorescent in situ hybridization probes may empower us to identify specific organisms within a mixed biofilm community. Interestingly, green fluorescent protein (GFP), a constitutively produced, plasmid-mediated molecule, can allow biofilms to be examined noninvasively, without fixation or staining [17]. Therefore, methods based on the GFP are widely being used to detect specific bacterial cell types within biofilms [18]. Collectively, these techniques and strategies have opened up new and exciting opportunities for directly imaging cellular components and activities during the course of host-microbe interactions. It is on these techniques that this chapter focuses and provides the most comprehensive information on exploitation of CLSM in studying host-microbe interactions at the cell biological level especially with reference to plants and humans. Moreover, this chapter is expected to be an inspiration for integrating microscopy with molecular methods in research on host-microbe interaction. In the following paragraphs we explain basic principles of the technique and subsequently discuss applications of confocal microscopy in microbiology and medicine, including analyses of host-colonization and interactions with pathogens, critical methodological aspects, and integration with complementary methods.
2. Basic principles of confocal laser scanning microscopy
Confocal laser scanning microscopy (CLSM) has been used in the biological sciences since 1961, when the concept of “optical sectioning” of a biological specimen was introduced. CLSM produces optical sections of thick samples in which out-of-focus effects are removed. CLSM is a variant of white light microscopy in which specimens are imagined by exciting light emission by a low power laser beam after application of fluorescent contrast agents. CLSM offers the aptitude for direct, non-invasive, successive optical sectioning of intact, thick, live specimens with a least amount of sample preparation. And, it also displays a fringing enhancement in lateral resolution [19]. Confocal laser scanning microscopes are basically high-tech machines based on the procurement of fluorescent light and composed of hundreds of mechanical, optical and electronic parts [5]. Most commercially available systems being used in biological researches use small air-cooled lasers with visible wavelengths along with UV lasers [8]. CLSM allows the detection of three types of objects; (1) molecules, cells and tissues stained with one or more fluorochromes (2) genetically modified organisms (GMO) that express fluorescent proteins (3) autofluorescent cells, tissues and substrates [5]. Since, autofluorescence of biological and synthetic substrates is typically considered as a negative aspect of CLSM images, efforts often aim toward avoiding autofluorescent signals [20]. The “heart” of a confocal microscope is “the pinhole”. The photons of light emanated by the specimen are filtered here, and only those coming from the focal plane can touch the detector or photomultiplier that converts them into an electron flow, which is finally digitalized by the software. The outcome of this process is an exceptionally sharp image, representing an “optical slice” of the sample [21]. The thickness of this optical slice depends on both the magnification and the pinhole diameter. The resulting image is relatively free of the hazy light produced by the fluorescence of out-of-focus regions of the specimen, which can reduce the resolution of the image in normal fluorescence microscopes. Continuous optical slices are serially acquired along the Z-axis to form a ‘confocal stack’, which can then be used to create bi-dimensional projections, or visualized in the three-dimensional space [5,21]. Exclusive softwares, such as Imaris (Bitplane, Zuerich, Switzerland) and AMIRA (TGS Inc., US) and several other freeware tools such as DAIME (www.microbial-ecology.net/daime/), Image Surfer (http://imagesurfer.cs.unc.edu/) and ImageJ are available for qualitative and quantitative analysis of CLSM stacks. DAIME (Digital Image Analysis in Microbial Ecology) [22], COMSTANT [23] and IMARIS are particularly helpful in analyzing 3D images and quantifying Z-stacks [24]. ImageJ was initially established for analysis of medical images but nowadays many plugins (http://rsbweb.nih.gov/ij/plugins/) have been developed and applied for CLSM analyses of microbial communities. In plants, these softwares have helped to analyze rhizosphere and phyllosphere communities [25,26,27]. Image surfer was developed with the specific purpose of imaging confocal stacks and it is not open to plugin implementation [28]. Yet, it includes sophisticated visualization tools which allow the analysis of complex systems such as host-microbes
Microscopy and imaging science: practical approaches to applied research and education (A. Méndez-Vilas, Ed.)
63
___________________________________________________________________________________________

interactions in the rhizosphere [29]. DAIME is a tool for quantitative analysis of complex microbial communities, such as biofilms, and also includes procedures for evaluation of fluorescence in situ hybridization probes [22]. Dedicated software tools can create isosurfaces and spheres, three-dimensional objects that replace the original fluorescent signals. Such ‘three-dimensional models’ ease the exploration of microbe-microbe and host-microbe interactions by vividly increasing the resolution level of the images [21]. The discovery that truly brought fluorescence microscopy to the lead came in 1994, when Chalfie et al. [30] succeeded in expressing a naturally fluorescent protein, the now-famous green fluorescent protein (GFP), in living organisms. The GFP is obtained from the jellyfish Aequorea victoria that delivers a label for living bacteria and can be used as a biological probe and reporter molecule for the examination of gene expression or protein localization [31]. This advent of GFP became a landmark progress in the biological field that opened a whole new class of tagging methods. Since the first experiments with GFP, many variants have been engineered and discovered. From the naturally occurring GFP, called wild-type GFP, and from similar fluorescent proteins occurring in other marine organisms, newer and more influential mutants tags have been derived. Their properties vary from different excitation and emission spectra to stronger fluorescence and higher resistance to photobleaching. The currently available fluorescent protein tags offer a wide choice of wavelengths within the visible spectrum. Two such widespread examples are cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP), named for their distinctive emission spectra [5,32]. The availability of this fluorescent protein tagging techniques has led to a fundamental change in the way biological research is conducted and to an explosion of experimental possibilities.
2.1. Combination of CLSM with other techniques: current state and perspectives
CLSM not only remains as a commonly applicable methodology for studying host-microbe interactions, but also can be extended and integrated by other microscopic techniques, and therefore serves as a central technology in such studies. The combination of CLSM with other microscopic methods could offer supplementary benefits. The optical resolution of the confocal microscopes is constrained by the physical properties of the light. Therefore, fluorescent microscopy was used in a correlative approach in combination with electron microscopy, in order to identify target objects first, and then imaging them at nanometric scale [33]. A further correlative approach is already being employed in medical sciences but not yet in plant microbiology, is the combination of CLSM with atomic force microscopy (AFM). It could potentially deliver structural information, such as interaction forces between beneficial microorganisms, pathogens and hosts [34]. Correlative FISH-CLSM with nano-SIMS (secondary ion mass spectrometry) could be of special interest, as this will offer information on functional contributions of specific taxonomical groups of bacteria. However, nano SIMS has been used in plant-microbe interactions only to visualize differential partitioning of NH+4 between roots and soil microbial communities at nanometric scale [35]. It has been shown that FISH, a technique that employs fluorescence labelled species-specific DNA probes, is a valuable method for the revealing of bacteria without disruption of their natural environment or biofilms ([36,37]. Several authors have used this combination to obtain images of three-dimensional reconstructions of the natural microbiological environments of in vivo dental plaque-biofilm [38,39]. Many authors have employed a variety of fluorochromes, including acridine orange, fluorescein, DAPI, fluorescein diacetate plus ethidium bromide, and SYTO 9 plus propidium iodide (PI). Both fluorescein plus ethidium bromide and SYTO 9 plus PI offer the possibility of selectively staining live and dead bacteria. Therefore, these fluorochromes have been widely used to analyse bacterial viability in the majority of studies on undisturbed in vivo dental plaque biofilm [16]. We summarize here few examples of using several microbiological and microscopic methods applied in combination with CLSM with reference to oral biofilms. Some molecular biology approaches are being used in combination to CLSM. Like Palmer et al. [40] while studying clinical isolates of Veillonella spp. obtained from undisturbed in vivo dental plaque-biofilm, not only used fluorescence-labelled antibodies/CLSM but also simultaneously applied molecular techniques including phylogenetic characterization of clinical isolates by 16S rRNA gene sequencing. Those authors then analyzed the relationship between the isolates at strain level using enterobacterial repetitive intergenic consensus (ERIC) PCR fingerprinting. Chalmers [41] used fluorescence-labelled polyclonal antibodies in combination with CLSM to look at the initial stages of biofilm development (at 4, 6, and 8 hours), and at the specific interactions between early colonizers of the tooth surface (such as Streptococcus spp., Actinomyces spp., and Veillonella spp.). Dige et al. [37] have suggested that the combined use of FISH/CLSM and stereological methods is an attractive tool for quantification of bacterial populations in undisturbed in vivo biofilm. This technique provides an unbiased and reliable determination of the numerical contribution of specific species in mixed bacterial communities. Moreover, Schaudinn et al. [42] have also proposed a combined method of using more than one microscopic techniques to provide exclusive information about a structure that is difficult or even impossible to obtain with a single imaging procedure. Von Ohle et al. [43] validated the utility of microelectrodes to provide a non-destructive, real-time measurement of the effects of sucrose and CHX on the physiology of dental plaque-biofilm. The microelectrode data were correlated with microscopic examination by CLSM of the biofilm structure, thus allowing a relationship to be made between biofilm physiology and structure. CLSM is also being used in combination with other methods of microscopy, such as scanning electron microscopy (SEM), transmission electron microscopy (TEM), or epifluorescence microscopy [16]. Furthermore, technological
Microscopy and imaging science: practical approaches to applied research and education (A. Méndez-Vilas, Ed.)
64
___________________________________________________________________________________________

advances in miniaturization has prompted that CLSM can be integrated into a conventional flexible endoscope, or into transendoscopic probes, a technique known as confocal endomicroscopy (CEM) or confocal laser endomicroscopy (CLE). This newly developed technology has enabled endoscopists to collect real-time, in vivo histological images or “virtual biopsies” of the gastrointestinal tract. It’s clinical applications has shown usefulness in not only studying of precancerous and cancerous lesions but also the identification of Helicobacter pylori infection and diagnosis of disorders such as coeliac disease and ulcerative colitis [44,45]. A most recent combination of spinning disc confocal coupled with two-photon microscopy has upgraded our ability to study visualize host-microbe interactions in real time (living cell-cell interactions) in three dimensions. It affords dynamic live-cell imaging to explore our understanding of the relative contributions of neutrophils and macrophages in the phagocytosis of pathogens in vitro and may provide some clues with regard to the role phagocyte subsets in murine models and human disease [46]. Additionally, modern commercial instruments and high-performance camera systems have imparted it high-acquisition speeds with acceptable contrast and minimal photobleaching at the low-light levels. This unique feature has enabled this technique to rapidly acquire images in four dimensions.
3. Application of CLSM to host-microbe interactions
In nature, the majority of microorganisms are adherent to the surfaces on which they grow, and form biofilms. Biofilms forming ability of rhizospheric microorganisms extensively enhances the plant growth and soil fertility [35]. The interactions of microorganisms with plants and humans are of great interest for the researchers working in the area of agricultural and medical sciences [33]. The gastro-intestinal and oral biofilm specifically, dental plaque, is considered to be an example of a specialized microbial biofilm [16]. Biofilm formation is controlled by quorum sensing genes. CLSM are useful to observe morphological changes in biofilms formed by pathogens and have been applied to study their formation with mutations in quorum sensing (QS) genes [47,48,49]. QS processes are directly involved in inhibition or development of biofilm growth. Therefore, better understanding of these mechanisms would contribute to control the negative concerns of biofilm growth. Usually molecular methods are employed to characterize the whole microbiome but spatial information at micro-scale, which is fundamental to understand the dynamics of host-microbe interactions, is usually lost during the sample processing. Therefore, it is useful to complement the indirect molecular techniques with direct visualization methods. Especially, CLSM allows the examination of microbial habitats at a spatial resolution level unachievable with molecular methods [21]. Moreover, FISH has been successfully used to characterize microorganisms within biofilms and to detect pathogens in drinking water samples [50,51].
3.1. CLSM in Biofilm characterization
The biochemical composition of biofilms, particularly the exopolysaccharide substances (EPS) matrix components like carbohydrates, proteins, lipids and nucleic acids are usually quantified via chemical assay techniques. But microscopy based approaches are also gaining importance to assess chemical constituents of matrix. In order to use chemical assays it is necessary to first isolate the EPS from the cellular fraction of the biofilm and confirm the isolate is free from intracellular contaminants due to cell lysis. Moreover, these techniques also require the sample to be physically disturbed. This makes difficult to evaluate different biochemical components within the biofilm [47]. On the other hand microscopy techniques are non-invasive and offer a way to overcome some of the limitations of extraction techniques. And provides the possibility of monitoring, quantifying and visualizing cells and other biofilm components in situ, without tormenting their structure [38,46] Therefore, CLSM in combination with different fluorescent dyes has become a common and useful approach for biofilm-related research. A wide range of fluorescent dyes are being exploited to detect and quantify different biofilm components. Some of these most commonly used ones are DAPI for cells, Syto-60 and Syto-84 for nucleic acids, FITC and Sypro red for proteins, Nile red for lipids, and concanavalin A (ConA) labelled with Alexa fluor 488 for carbohydrates [52,53]. Fluorescent staining and CLSM have been reported to be successfully used in assessing the EPS carbohydrates and proteins of flocs [53], granules [55] and single-species cultured biofilms [56,57].
3.1.1. CLSM in determining strength of biofilms
Bacterial biofilms have been reported to comprehend discrete regions of viable and nonviable bacteria in the three dimensional networks of biofilms stacks. CLSM, in combination with vital stains and image analysis techniques, can expose viability patterns in biofilms. It can also be used to measure the dimensions of these structures. Biofilm thickness is especially important for calculation of heat exchange, diffusion rates of antimicrobials and nutrients through a biofilm, also for evaluation of the mechanical properties of a biofilm [58,59]. Biofilm "stacks" possess an outer layer of viable bacteria surrounding an internal core of nonviable bacteria. Using image analysis tools, we can measure the thickness of this outer viable region, in the x-y plane, from single confocal optical sections, and determined the mean angle (theta) of these portions of the biofilm stack [60]. Mohle et al. [61] determined the structure and shear strength of microbial biofilms by CLSM and fluid dynamic gauging (FDG). In their study, the thickness of
Microscopy and imaging science: practical approaches to applied research and education (A. Méndez-Vilas, Ed.)
65
___________________________________________________________________________________________

heterotrophic mixed culture biofilms was found to depend on substrate concentration and shear force at the biofilm surface during the cultivation. By using CLSM technique, a stable base biofilm with a high amount of stained EPS glycol-conjugates could be visualized after gauging.
3.2. Studying plants-microbe interactions
Plants form complex mosaics of different microhabitats colonized and adapted by microorganisms ranging from beneficials to pathogens. Pathogens exemplify a special case of plant-microbe interactions, where, pathogen virulence depends on a combination of genetic and ecological traits. Infections usually target specific plant tissues, and occur only when the responsible microbial genes are expressed. CLSM are being used as a tool to probe such coordinated mechanisms and assisting in understanding the dynamics of the infection process [34]. In addition, transgenic plants and cells can be generated in which specific components are fluorescently labeled without any invasive experimental manipulation. The application of such techniques to plant microbe interactions has revealed microbe-induced changes in cytosolic calcium levels, cytoskeleton rearrangements, the visualization of reactive oxygen species generation, DNA cleavage, and the detailed resolution of intercellular and intracellular trafficking of viral components [8].
3.2.1. Selected examples of application of CLSM to the cell biology of plant-microbe interactions
The combined use of fluorescent protein fusions with laser-based confocal imaging has yielded novel insights into the spatial and temporal processes associated with the invasion of plant tissues in numerous pathosystems. Fluorescent-tagged proteins can be used in several ways to study plant–microbe interactions. Tagged proteins can be used as general markers to visualize the spatial and temporal dynamics of the plant cell, the pathogen, or both [62]. Different patterns of ectophytic vs. endophytic colonization in plant-microbe associations can be discriminated using CLSM. The extremely accurate localization of microorganisms within the host, along with targeted staining of specific structures or taxa, CLSM has become a technique of choice to explore insights into their ecology and interaction with the host [63]. Here we summarize some of the uses of CLSM in plant microbiology research. Newman et al. [64] investigated the mechanism responsible for the Xylella fastidiosa-induced degenerative disease of Vitis vinifera. Using CLSM, they identified that the vessel to vessel movement of the bacterial cells is responsible for this. Li et al. [65] applied confocal microscopy to study the modulation of vir gene expression in Agrobacterium tumefaciens during the infection process after downstream insertion of gfp genes. Invasion of transport vessels at root level has been demonstrated by CLSM for several bacteria, such as Enterobacter gergoviae [66] and Bacillus subtilis [67]. The inference of such observations are significant in the case of human pathogens like Enterobacteriaceae, which are very commonly persist in soil and first colonize crop roots and then move to the edible parts of the plant [68]. Information onto the mechanisms underlying bacterial invasion and dynamics of their internal translocation can improve food safety by development of targeted measures for outbreak prevention. CLSM has been used to show that Escherichia coli colonizes Lactuca sativa (green lettuce) leaves preferentially beneath the epithelium; similarly, Salmonella enterica encroaches the lettuce leaves through the open stomata and resides in the endosphere [69]. Bragina et al. [70] showed by FISH-CLSM that the hyalocytes (dead cells which contain water) of Sphagnum mosses are the ideal colonization sites of various bacteria. Complementary molecular studies demonstrated their potential involvement in nitrogen fixation and methane degradation. CLSM has been used to analyze comparative analysis of environmental samples for the presence certain groups within the plant-associated microbiome. As Koberl et al. [71] showed for Bacillus and Streptomyces in an Egyptian desert farm which appeared to be recruited by plants as effective biocontrol agents. Thomas and Reddy [72] performed microscopic revelation of endophytic bacteria colonizing the cell wall plasma membrane perispace in the shoot tip tissue of banana. Their study was intended to generate microscopic evidences of intra-tissue colonization in banana leaf-sheath. Confocal and epifluorescence microscopy enabled them to obtain clear microscopic evidences of the extensive endophytic bacterial inhabitation in the perispace in shoot tissue of banana. These findings suggested that there is a possible involvement of endophytes in the biology of the host besides recognizing cell wall plasma membrane perispace as a major niche for plant-associated bacteria. Using CLSM, Grube et al. [73] studied Lichens which are considered as the typical example of mutualistic symbiosis between fungi and algae/cyanobacteria and observed unexpected microbial hot spots. CLSM observations revealed a clear succession of bacterial communities from young to older thallus regions suggesting that the lichens harbor a stable, active and functionally adapted microbiome, which may contribute to the growth and survival under extreme conditions. CLSM has also helped in elucidating the mechanisms of action of beneficial microbes in rhizosphere. Plant growth promoting rhizobacteria (PGPR) and biocontrol agents caught the attention of the scientific community due to their promising biotechnological potential for sustainable agriculture. They are regarded as environmental friendly measures to replace chemical fertilizers and pesticides [21]. Interestingly, in several case studies, CLSM showed that neither endophytism nor direct contact with the pathogen was the discriminative feature of efficient biocontrol strains [74.75]. Notably, it was shown by Kamilova et al. [76] that an in vitro inefficient Collimonas fungivorans strain effectively reduced disease occurrence in vivo.
Microscopy and imaging science: practical approaches to applied research and education (A. Méndez-Vilas, Ed.)
66
___________________________________________________________________________________________

Plant cell death plays key roles during plant-pathogen interactions. To study pathogen-induced cell death, there is a need for cytological tools that allow determining not only host cell viability, but also cellular events leading to cell death with visualization of pathogen development. Regarding this Kiersun [77] have described a live cell imaging method to provide insights into the dynamics of cell death in rice (Oryza sativa). This method uses live-cell confocal microscopy of rice sheath cells mechanically damaged or invaded by fluorescently-tagged Magnaporthe oryzae together with fluorescent dyes fluorescein diacetate (FDA) and propidium iodide (PI). FDA stains the cytoplasm of live cells exclusively, thus also visualizing the vacuole, whereas PI stains nuclei of dead cells. Recently, our group [78] has exploited confocal microscopy to highlight importance of biofilm forming ability of PGPR to enhance their colonization, survival and sustenance. Bacillus subtilis BCH4 showed strong biofilm formation on glass coverslip and root surfaces of Triticum aestivum (Figure 1). In the recent past, the significance of biofilm forming beneficial rhizobacteria in the survival of bioinoculant on plant root surfaces and plant-bacterial communication have gained increased attention. Plant root associated biofilms assist in the preservation of plant nutrients available in the form of rhizodeposits or root exudates and make them available to beneficial bacteria and, therefore, depriving the phytopathogens of these nutrients.
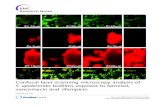
(a) (b)
Figure 1 Confocal laser scanning micrographs of biofilm formed by Bacillus subtilis BCH4 (a) on glass coverslip; magnification is 100x, scale bars = 5 µm. (b) on the Triticum aestivum root hairs and root surface; magnification is 20x, scale bars = 50 µm.
3.3. Studying humans-microbe interactions
The human microbiome is evolving as a key player governing human health and disease. However, a detailed understanding of the fundamental molecular mechanisms underlying host–microbe interactions and their potential impact on drug metabolism, nutrition, immune regulation, and infection remain largely intangible [79,80]. Lively imaging of host-pathogen interactions has recently revealed novel detail and unsuspected mechanistic insights, facilitating the dissection of the phagocytic process into its component parts [46]. Scientist have achieved advanced knowledge about host-pathogen interactions, including the specific effects of microbial cell viability, cell wall composition and morphogenesis on the phagocytic process and try to define the relative contributions of neutrophils and macrophages to the clearance of microbial pathogens in vitro and the infected host.
3.3.1. Selected examples of application of CLSM to the human-microbe interactions
The possible earliest application of CLSM has been reported in in vivo clinical dental research in the early 1990s [81,82]. They were the first investigators to use this non-invasive method to analyse the thickness and bacterial viability of undisturbed human dental plaque biofilm. Later, some authors designed special removable oral appliances that held a number of discs on which growth of the dental plaque-biofilm took place and this undisturbed biofilm was then analysed using CLSM [38,83,84]. At present, the scientific community considers that this methodological design is the most suitable approach for studying the in vivo architecture and physiology of dental plaque-biofilm formation on dental materials, as well as the antibacterial effect of antimicrobials on this microbial structure. Recent high resolution molecular analyses along with confocal microscopic studies have linked microbial community disequilibria (dysbiosis), primarily in the gastrointestinal tract (GIT), to several idiopathic diseases, including obesity, diabetes, inflammatory bowel disease, cancer and neurodegenerative diseases [14]. We have summarized some of the latest experimental utilization of CLSM in studying human microbiome research.
Microscopy and imaging science: practical approaches to applied research and education (A. Méndez-Vilas, Ed.)
67
___________________________________________________________________________________________

Mascari and Ross [85] quantified staphylococcal-collagen binding interactions in whole blood by use of a Confocal Microscopy Shear-Adhesion assay. S. aureus–collagen binding interactions, mediated by both S. aureus collagen adhesin (CNA) and protein A–von Willebrand factor (vWf), were evaluated using mutant strains and antibody-blocking techniques. Confocal laser microscopy assisted in determining the position of adherent bacteria (at the collagen surface or above the surface in the platelet aggregate). Their study demonstrated significant CNA-collagen interactions and protein A–vWf–collagen binding interactions under physiological shear conditions. They concluded that collagen binding interactions are important in the expansion of infected thrombi. Jefferson et al. [86] used confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. CLSM also helped Cerca et al. [87] to elucidate the effect of farnesol, vancomycin and rifampicin against S. epidermidis biofilms after staining with a Live/Dead stain, a known indicator of cell viability, related with cell membrane integrity. They observed that rifampicin killed a significant proportion of biofilm cells. While farnesol is not very efficient at killing biofilm bacteria, rather it damages cell membrane, as determined by the live/dead staining, in a similar way as vancomycin. Confocal microscopy techniques has enabled the microstructural analysis of the in vivo cornea by allowing fresh insight into corneal microstructure in health, and in inherited and acquired corneal disease. It has been utilised clinically to aid in the diagnosis of infectious keratitis, in particular Acanthamoeba and fungal keratitis, and has also established a role in the diagnosis and phenotyping of corneal dystrophies [88]. Ordinola-Zapata [89] exploited confocal microscopy to investigate the pattern of dentin infection provided by different infection models used in endodontic research that includes laboratory and in situ infected dentin. Confocal pictures of in vivo infected dentin from necrotic root canals are also presented. The ability to confocal techniques to allow 3D observation of bacterial colonization has the potential to significantly contribute to in situ morphological studies of endodontic infections as well as the ex vivo antibacterial effects of endodontic substances and procedures. Jackson et al. [90] exploited confocal three-dimensional fluorescence imaging technology to visualize and quantitate abundant macroautophagy in Varicella-zoster virus infected cells. A study employing spinning disc confocal microscopy assessed stage-specific C. albicans phagocytosis by human monocyte-derived macrophages and human PMNs in isolation with C. albicans and when both phagocyte subsets were present [91]. This study has surprisingly showed that when only one immune cell type was present, macrophages, not PMNs, had the greater capacity for C. albicans uptake despite engulfing fungal cells at a lower rate after establishing cell-cell contact. Marzorati et al. [92] developed the host-microbiota interaction (HMI) module and employed CLSM and FISH combined with molecular approaches to make evidence about the effect of a specific treatment at the level of the luminal microbial community and of the host surface colonization and signaling. The HMI module using fluorescent techniques has enabled them to co-culture gut representative microbial community with enterocyte-like cells up to 48 h. This facilitated in the mechanistic understanding of host-microbiome interactions. Kima [93] developed a human gut-on-a-chip microdevice to study the contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation. In this model, they coculture multiple commensal microbes in contact with living human intestinal epithelial cells for more than a week in vitro. Using confocal microscopy and immunolabelled techniques they analyzed how gut microbiome, inflammatory cells, and peristalsis-associated mechanical deformations independently contribute to intestinal bacterial overgrowth and inflammation. Guirado et al. [94] developed and characterized an in vitro granuloma model derived from human peripheral blood mononuclear cells (PBMCs) and autologous serum. They interrogated this model for its ability to discriminate between host and bacterial determinants in individuals with and without latent TB infection (LTBI). Using confocal and fluorescent labeled techniques in this model they provided first evidence that granuloma formation, bacterial survival, lymphocyte proliferation, pro- and anti-inflammatory cytokines, and lipid body accumulation are significantly altered in LTBI individuals. Moreover, they showed a specific transcriptional signature of Mycobacterium tuberculosis associated with survival within human granuloma structures depends on the host immune status. This was fundamentally new information on how the human host immune status and bacterial transcriptional signature may dictate early granuloma formation and outcome. Wako et al. [95] evaluated temporal dynamics of bacterial microbiota in the human oral cavity using confocal microscopy combined with electron microscopy. Using an in situ model of dental biofilms, they showed comprehensive composition of the bacterial microbiota involved in the health of the human oral cavity. Shah et al. [14] presented a microfluidics-based model (HuMiX, human–microbial crosstalk), which allows co-culture of human and microbial cells under conditions representative of the gastrointestinal human–microbe interface. HuMiX facilitates investigations of host–microbe molecular interactions and confocal fluorescence microscopy provided insights into a range of fundamental research questions linking the gastrointestinal microbiome to human health and disease. Recently, Wang et al. [96] exploited confocal laser endomicroscopy (CLE) to unravel poorly understood relationship of microbiota interacting with the human host at the colorectal mucosa by providing in vivo physiological information of the mucosa. The mucosal microbiota was quantified using 16 s rDNA pyro-sequencing. They found that human mucosal microbiota is agglomerated to three major clusters dominated by Prevotella, Bacteroides and Lactococcus.
Microscopy and imaging science: practical approaches to applied research and education (A. Méndez-Vilas, Ed.)
68
___________________________________________________________________________________________

Furthermore, these microbiota clusters did not significantly correlate with the disease status or biopsy sites but closely correlated with the mucosal niche physiology, which was non-invasively revealed by CLE. They suggested that a close correlation exists between the mucosal microbiota and the colorectal mucosal physiology, and CLE is a clinically available tool that can be used to facilitate the study of the in vivo correlation between colorectal mucosal physiology and the mucosal microbiota. In overall, CLSM studies of cellular responses to pathogen infection may lead to new knowledge about fundamental processes in animal cells, such as mechanisms underlying subcellular trafficking and targeting of proteins and other molecules.
4. Conclusion
Substantial advances in imaging technologies including laser-based confocal microscopy and the discovery and widespread use of fluorescent proteins as tags have provided the first glimpses of the dynamic nature of the processes of defense and pathogenicity. Prior to the development of these techniques, high resolution imaging by electron microscopy gave only a static picture of these dynamic events and live cell imaging was significantly limited in resolution as well as the availability of relevant stains and markers. CLSM is the method of choice for the monitoring of structure formation of live biofilms in laboratory flow-cell reactors. As a result of its non-invasiveness and non-destructive character, CLSM enables the in vivo reconstitution of the 3D structure of microbial biofilms in their naturally hydrated form. Moreover, biofilm formation can be observed in detail by scanning the same site repeatedly. Interactions between plant cells and microbial pathogens involve highly dynamic processes of cellular trafficking and reorganization that can be mapped using confocal and fluorescent labeled techniques. Moreover, the human microbiota establishment and its visualization in real time using confocal techniques in association with epifluorescence and molecular tools have enabled to genetically manipulate both human host and microbial associates. In overall, this technique of confocal microscopy in its variant forms has opened the door to fascinating new questions about the host cellular response either beneficial or harmful to the microbial interactions. These techniques, integrated with electron microscopy, molecular genetics, and other types of investigations, are likely to play an increasingly important role in future studies of host responses to microbial pathogens or mutualists.
Acknowledgment We acknowledge research support received from department of Scientific Research, Imam Abdulrahman Al Faisal University for completion of this work.
References [1] Casadevall A, Pirofski L. Host-pathogen interactions: Basic concepts of microbial commensalism, colonization, infection, and
disease. Infection and Immunity. 2000; 68:6511-18. [2] Ray K, Marteyn B. Sansonetti PJ, Tang CM. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nature Review
Microbiology. 2009; 7:333-40. [3] Haghighat A, Seveau S. Quantification of host–microbe interactions by automated fluorescence microscopy. Journal of
Immunological Methods. 2010; 352:186-91. [4] Larrainzar E, O’Gara F, Morrissey JP. Applications of autofluorescent proteins for in situ studies in microbial ecology. Annual
Review Microbiology. 2005; 59:257-77. [5] Vonesch C, Aguet F, Vonesch JL, Unser M. The colored revolution of bioimaging. IEEE Signal Processing Magazine. 2006;
23:20-31. [6] Lee N, Nielsen PH, Andreasen KH, Juretschko S, Nielsen JL, Schleifer KH, Wagner M. Combination of fluorescent in situ
hybridization and microautoradiography- A new tool for structure-function analyses in microbial ecology. Applied and Environmental Microbiology. 1999; 65:1289-97.
[7] Wagner M, Amann R, Lemmer H, Schleifer KH. Probing activated sludge with oligonucleotides specific for proteobacteria: inadequacy of culture-dependent methods for describing microbial community structure. Applied and Environmental Microbiology. 1993; 59:1520-25.
[8] Heath MC. Advances in imaging the cell biology of plant-microbe interactions. Annual Review of Phytopathology. 2000; 38:443-59.
[9] Kuo J. Processing plant tissues for ultrastructural study. Methods in Molecular Biology. 2014; 1117:39-55. [10] Escalante R, Sastre L. Investigating gene expression: in situ hybridization and reporter genes. Methods in Molecular
Biology. 2006; 346:247-60. [11] Koo J, Kim Y, Kim J, Yeom M, Lee IC, Nam HG. A GUS/Luciferase fusion reporter for plant gene trapping and for assay of
promoter activity with luciferin-dependent control of the reporter protein stability. Plant Cell Physiology. 2007; 48:1121-31. [12] Wolbarst AB, Hendee WR. Evolving and experimental technologies in medical imaging. Radiology. 2006; 238:16-39. [13] Moussata D, Goetz M, Gloeckner A, Kerner M, Campbell B, Hoffman A, Biesterfeld S, Flourie B, Saurin JC, Galle PR, Neurath
MF, Watson AJM, Kiesslich R. Confocal laser endomicroscopy is a new imaging modality for recognition of intramucosal bacteria in inflammatory bowel disease in vivo. Gut. 2011; 60:26-33.
[14] Shah P, Fritz JV, Glaab E, Desai MS, Greenhalgh K, Frachet A, Niegowska M, Estes M, Jager C, Seguin-Devaux C, Zenhausern F, Wilmes P. A microfluidics-based in vitro model of the gastrointestinal human–microbe interface. Nature Communications. 2016, 7:11535.
Microscopy and imaging science: practical approaches to applied research and education (A. Méndez-Vilas, Ed.)
69
___________________________________________________________________________________________

[15] Garrett TR, Bhakoo M, Zhang Z. Bacterial adhesion and biofilms on surfaces. Progress in Natural Science. 2008; 18:1049-56. [16] Tomas I, Henderson B, Diz P, Donos N. In vivo oral biofilm analysis by confocal laser scanning microscopy: methodological
approaches. In. Mendez-Vilas A, Diaz J, editors. Microscopy: Science, technology, applications and education. Spain: Formatex; 2010, p. 597-606.
[17] Bloemberg GV, O'Toole GA, Lugtenberg BJJ, Kolter R. Green fluorescent protein as a marker for Pseudomonas spp. Applied and Environmental Microbiology. 1997; 63:4543-51.
[18] Wouters K, Maes E, Spitz JA, Roeffaers MBJ, Wattiau P, Hofkens J, Springael D. A non-invasive fluorescent staining procedure allows confocal laser scanning microscopy based imaging of Mycobacterium in multispecies biofilms colonizing and degrading polycyclic aromatic hydrocarbons. Journal of Microbiological Methods. 2010; 83:317-25.
[19] Wright SJ, Wright DJ. Introduction to confocal microscopy. Methods in Cell Biology. 2002; 70:1-85. [20] LoPiccolo S, FerraroV, Alfonzo A, Settanni L, Ercolini D, Burruano S, Settanni L, Ercolini D, Burruano S, Moschetti G.
Presence of endophytic bacteria in Vitisvinifera leaves as detected by fluorescence in situ hybridization. Annals of Microbiology. 2010; 60:161-67.
[21] Cardinale M, Berg G. Visualization of plant-microbe interactions. In. Lugtenberg B, editor. Principles of plant-microbe interactions. Switzerland: Springer; 2015. P. 299-306.
[22] Daims H, Lucker S, Wagner M. DAIME, a novel image analysis program for microbial ecology and biofilm research. Environmental Microbiology. 2006; 8:200-13.
[23] Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000; 146:2395-2407.
[24] Hall-Stoodley L, Nistico L, Sambanthamoorthy K, Dice B, Nguyen D, Mershon W, Johnson C, Ze Hu F, Stoodley P, Ehrlich G, Post JC. Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiology. 2008; 8:173.
[25] Iverson SL, Maier RM. Effects of compost on colonization of roots of plants grown in metalliferous mine tailings, as examined by fluorescence in situ hybridization. Applied and Environmental Microbiology. 2000; 75:842-47.
[26] Downie H, Holden N, OttenW, Spiers AJ, Valentine TA, Dupuy LX. Transparent soil for imaging the rhizosphere. PLoS ONE 2012; 7:e44276.
[27] Lee J, Teitzel GM, Munkvold K, del Pozo O, Martin GB, Michelmore RW, Greenberg JT. TypeIII secretion and effectors shape the survival and growth pattern of Pseudomonas syringae on leaf surfaces. Plant Physiology. 2012; 158:1803-18.
[28] Feng D, Marshburn D, Jen D, Weinberg RJ, Taylor RMII, Burette A. Stepping in to the third dimension. The Journal of Neuroscience. 2007; 27:12757-760.
[29] Zachow C, Fatehi J, Cardinale M, Tilcher R, Berg G. Strain-specific colonisation pattern of Rhizoctonia antagonists in the root system of sugarbeet. FEMS Microbiology Ecology. 2010. 74:124-35.
[30] Chalfie M, Tu Y, Euskirchen G, Ward W, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994; 263:802-05.
[31] Rodenacker K, Bruhl A, Hausner M, Kuhn M, Liebscher V, Wagner M, Winkler G, Wuertz S. Quantification of biofilms in multi-spectral digital volumes from confocal laser scanning microscopes. Image Analysis and Steriology. 2000; 19:151-56.
[32] Zhang J, Campbell RE, Ting AY, Tsien RY. Creating new fluorescent probes for cell biology. Nature Reviews Molecular Cell Biology. 2002; 3:906-18.
[33] Jahn KA, Bartonb DA, Kobayashia K, Ratinac KR, Overall RL, Braet F. Correlative microscopy: providing new understanding in the biomedical and plant sciences. Micron. 2012; 43:565-82.
[34] Haupt BJ, Pelling AE, Horton MA. Integrated confocal and scanning probe microscopy for biomedical research. The Scientific World Journal. 2006; 15:1609-18.
[35] Clode PL, Kilburn MR, Jones DL, Stockdale EA, Cliff JB, Herrmann AM, Murphy DV. In situ mapping of nutrient uptake in the rhizosphere using nanoscale secondary ion mass spectrometry. Plant Physiology. 2009; 151:1751-57.
[36] Gilbride KA, Lee DY, Beaudette LA. Molecular techniques in wastewater: understanding microbial communities, detecting pathogens, and real-time process control. J. Microb. Methods. 2006; 66:1-e20.
[37] Dige I, Nyengaard JR, Kilian M, Nyvad B. Application of stereological principles for quantification of bacteria in intact dental biofilms. Oral Microbiology and Immunology. 2009; 24:69-75.
[38] Dige I, Nilsson H, Kilian M, Nyvad B. In situ identification of streptococci and other bacteria in initial dental biofilm by confocal laser scanning microscopy and fluorescence in situ hybridization. European Journal of Oral Sciences. 2007; 115:459-67.
[39] Jung DJ, Al-Ahmad A, Follo M, Spitzmüller B, Hoth-Hannig W, Hannig M, Hannig C. Visualization of initial bacterial colonization on dentine and enamel in situ. Journal of Microbiological Methods. 2010; 81:166-74.
[40] Palmer RJ Jr, Diaz PI, Kolenbrander PE. Rapid succession within the Veillonella population of a developing human oral biofilm in situ. Journal of Bacteriology. 2006; 188:4117-24.
[41] Chalmers NI, Palmer RJ Jr, Du-Thumm L, Sullivan R, Shi W, Kolenbrander PE. Use of quantum dot luminescent probes to achieve single-cell resolution of human oral bacteria in biofilms. Applied and Environmental Microbiology. 2007; 73:630-36.
[42] Schaudinn C, Carr G, Gorur A, Jaramillo D, Costerton JW, Webster P. Imaging of endodontic biofilms by combined microscopy (FISH/CLSM-SEM). Journal of Microscopy. 2009; 235:124-27.
[43] von Ohle C, Gieseke A, Nistico L, Decker EM, DeBeer D, Stoodley P. Real-time microsensor measurement of local metabolic activities in ex vivo dental biofilms exposed to sucrose and treated with chlorhexidine. Applied and Environmental Microbiology. 2010; 76:2326-34.
[44] De Palma GD. Confocal laser endomicroscopy in the in vivo histological diagnosis of the gastrointestinal tract. World Journal of Gastroenterology. 2009; 15:5770-75.
[45] Goetz M, Kiesslich R. Advances of endomicroscopy for gastrointestinal physiology and diseases. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2010; 298:G797-806.
Microscopy and imaging science: practical approaches to applied research and education (A. Méndez-Vilas, Ed.)
70
___________________________________________________________________________________________

[46] Bain J, Gow NAR, Erwig LP. Novel insights into host-fungal pathogen interactions derived from live-cell imaging. Seminars Immunopathology. 2015; 37:131-39.
[47] Purevdorj B, Costerton JW, Stoodley P. Influence of hydrodynamics and cell signaling on the structure and behavior of Pseudomonas aeruginosa biofilms. Applied and Environmental Microbiology. 2002; 68:4457-64.
[48] Cole SP, Harwood J, Lee R, She R, Guiney DG. Characterization of monospecies biofilm formation by Helicobacter pylori. Journal of Bacteriology. 2004; 186:3124-32.
[49] Huang Z, Meric G, Liu Z, Ma R, Tang Z, Lejeune P. luxS-based quorum-sensing signaling affects biofilm formation in Streptococcus mutans. Journal of Molecular Microbiology and Biotechnology. 2009; 17:12-19.
[50] Batte M, Mathieu L, Laurent P, Prevost M. Influence of phosphate and disinfection on the composition of biofilms produced from drinking water, as measured by fluorescence in situ hybridization. Canadian Journal of Microbiology. 2003; 49:741-53.
[51] Wilhartitz I, Mach RL, Teira E, Reinthaler T, Herndl GJ, Farnleitner AH. Prokaryotic community analysis with CARD-FISH in comparison with FISH in ultra-oligotrophic ground- and drinking water. Journal of Applied Microbiology. 2007; 103:871-81.
[52] Johnsen AR, Hausner M, Schnell A, Wuertz S. Evaluation of fluorescently labeled lectins for noninvasive localization of extracellular polymeric substances in Sphingomonas biofilms. Applied and Environmental Microbiology. 2000; 66:3487-91.
[53] Yang Y, Sreenivasan PK, Subramanyam R, Cummins D. Multiparameter assessments to determine the effects of sugars and antimicrobials on a polymicrobial oral biofilm. Applied and Environmental Microbiology. 2006; 72:6734-42.
[54] Schmid M, Thill A, Purkhold U, Walcher M, Bottero JY, Ginestet P, Nielsen PH, Wuertz S, Wagner M. Characterization of activated sludge flocs by confocal laser scanning microscopy and image analysis. Water Research. 2003; 37:2043-52.
[55] McSwain B, Irvine R, Hausner M, Wilderer P. Composition and distribution of extracellular polymeric substances in aerobic flocs and granular sludge. Applied and Environmental Microbiology. 2005; 71:1051-57.
[56] Chen M, Lee D, Tay J, Show K. Staining of extracellular polymeric substances and cells in bioaggregates. Applied Microbiology Biotechnology. 2007; 75:467-74.
[57] Shumi W, Lim J, Nam S, Kim S, Cho K, Yoon J, Park S. Fluorescence imaging of the spatial distribution of ferric ions over biofilms formed by Streptococcus mutans under microfluidic conditions. Biochip Journal. 2000; 3:119-24.
[58] Korstgens V, Flemming HC, Wingender J, Borchard W. Uniaxial compression measurement device for investigation of the mechanical stability of biofilms. Journal of Microbiological Methods 2001; 46:9-17.
[59] Stewart PS. Diffusion in biofilms. Journal of Bacteriology. 2003; 185:1485-91. [60] Hope CK, Wilson M. Measuring the thickness of an outer layer of viable bacteria in an oral biofilm by viability mapping. J of
Microbiological Methods. 2003; 54:403-10. [61] Mohle RB, Langemann T, Haesner M, Augustin W, Scholl S, Neu TR, Hempel DC, Horn H. Structure and shear strength of
microbial biofilms as determined with confocal laser scanning microscopy and fluid dynamic gauging using a novel rotating disc biofilm reactor. Biotechnology and Bioengineering. 2007; 98:747-55.
[62] Underwood W, Koh S, Somerville SC. Visualizing cellular dynamics in plant–microbe interactions using fluorescent-tagged proteins. In: McDowell JM, editor. Plant immunity: Methods and protocols, Methods in Molecular Biology. USA: Springer; 2011. p. 283-91.
[63] Cardinale M. Scanning a microhabitat: plant-microbe interactions revealed by confocal laser microscopy. Frontiers in Microbiology. 2014; 5:94.
[64] Newman KL, Almeida RP, Purcell AH, Lindow SE. Use of a green fluorescent strain for analysis of Xylella fastidiosa colonization of Vitis vinifera. Applied and Environmental Microbiology. 2003; 69:7319-27.
[65] Li L, Li Y, Lim TM, Pan SQ. GFP-aided confocal laser scanning microscopy can monitor Agrobacterium tumefaciens cell morphology and gene expression associated with infection. FEMS Microbiology Letters. 1999; 179:141-46.
[66] An Q, DongY, Wang W, Li Y, Li J. Constitutive expression of the nifA gene activates associative nitrogen fixation of Enterobacter gergoviae 57-7 an opportunistic endophytic diazotroph. Journal of Applied Microbiology. 2006; 103:613-20.
[67] Ji X, Lu G, GaiY, Zheng C, Mu Z. Biological control against bacterial wilt and colonization of mulberry by an endophytic Bacillus subtilis strain. FEMS Microbiology Ecology. 2008; 65:565-73.
[68] Cooley M, Carychao D, Crawford-Miksza L, Jay MT, Myers C, Rose C, Keys C, Farrar J, Mandrell RE. Incidence and tracking of Escherichia coli O157:H7 in a major produce production region in California. PLoS One 2007; 2:e1159.
[69] KroupitskiY, Golberg D, Belausov E, Pinto R, Swartzberg D, Granot D, Sela S. Internalization of Salmonella enterica in leaves is induced by light and involves chemotaxis and penetration through open stomata. Applied and Environmental Microbiology. 2009; 75:6076-86.
[70] Bragina A, Berg C, Cardinale M, Shcherbakov A, Chebotar V, Berg G. Sphagnum mosses harbour highly specific bacterial diversity during their whole lifecycle. ISME Journal. 2012; 6:802-13.
[71] Koberl M, Ramadan EM, Adam M, Cardinale M, Hallmann J, Heuer H, Smalla K, Berg G. Bacillus and Streptomyces were selected as broad spectrum antagonists against soilborne pathogens from arid areas in Egypt. FEMS Microbiology Letters. 2013; 342:168-78.
[72] Thomas P, Reddy KM. Microscopic elucidation of abundant endophytic bacteria colonizing the cell wall-plasma membrane peri-space in the shoot-tip tissue of banana. AoB Plants. 2013; 5.
[73] Grube M, Cardinale M, De Castro JV, Muller H, Berg G. Species-specific structural and functional diversity of bacterial communities in lichen symbiosis. ISME Journal. 2009; 3:1105-15.
[74] Maldonado-Gonzalez MM, Prieto P, Ramos C, Mercado-Blanco J. From the root to the stem interaction between the biocontrol root endophyte Pseudomonas fluorescens PICF7 and the pathogen Pseudomonas savastanoi NCPPB 3335 in olive knots. Microbial Biotechnology. 2013; 6:275-87.
[75] Gasser F, Cardinale M, Schildberger B, Berg G. Biocontrol of Botrytis cinerea by successful introduction of Pantoea ananatis in the grapevine phyllosphere. International Journal of Wine Research. 2012; 4:53-63.
[76] Kamilova F, Leveau JHJ, Lugtenberg B. Collimonas fungivorans, an unpredicted in vitro but efficient in vivo biocontrol agent for the suppression of tomato foot and root rot. Environmental Microbiology. 2007; 9:1597-03.
Microscopy and imaging science: practical approaches to applied research and education (A. Méndez-Vilas, Ed.)
71
___________________________________________________________________________________________

[77] Jones K, Kim DW, Park JS, Khang CH. Live-cell fluorescence imaging to investigate the dynamics of plant cell death during infection by the rice blast fungus Magnaporthe oryzae. BMC Plant Biology. 2016; 16:69.
[78] Altaf MM, Ahmad I. Plant growth promoting activities, biofilm formation and root colonization by Bacillus sp. isolated from rhizospheric soils. Journal of Pure and Applied Microbiology. 2016; 10:109-20.
[79] Gerber GK. The dynamic microbiome. FEBS Letters. 2014; 588:4131-39. [80] David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach
MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014; 505:559-63.
[81] Watson TF, Petroll WM, Cavanagh HD, Jester JV. In vivo confocal microscopy in clinical dental research: An initial appraisal. Journal of Dentistry. 1992; 20:352-58.
[82] Netuschil L, Reich E, Unteregger G, Sculean A, Brecx M. A pilot study of confocal laser scanning microscopy for the assessment of undisturbed dental plaque vitality and topography. Archives of Oral Biology. 1998; 43:277-85.
[83] Arweiler NB, Hellwig E, Sculean A, Hein N, Auschill TM. Individual vitality pattern of in situ dental biofilms at different locations in the oral cavity. Caries Research. 2004; 38:442-47.
[84] Auschill TM, Hein N, Hellwig E, Follo M, Sculean A, Arweiler NB. Effect of two antimicrobial agents on early in situ biofilm formation. Journal of Clinical Periodontology. 2005; 32:147-52.
[85] Mascari LM, Ross JM. Quantification of staphylococcal-collagen binding interactions in whole blood by use of a confocal microscopy shear-adhesion assay. The Journal of Infectious Diseases. 2003; 188:98-107.
[86] Jefferson KK, Goldmann DA, Pier GB. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Antimicrobial Agents and Chemotherapy. 2005; 49:2467-73.
[87] Cerca N, Gomes F, Pereira S, Teixeira P, Oliveira R. Confocal laser scanning microscopy analysis of S. epidermidis biofilms exposed to farnesol, vancomycin and rifampicin BMC Research Notes. 2012, 5:244.
[88] Niederer RL, McGhee CNJ. Clinical in vivo confocal microscopy of the human cornea in health and disease. Progress in Retinal and Eye Research. 2010; 29:30-58.
[89] Ordinola-Zapata R, Bramante CM, de Moraes IG, Bernardineli N, Porto VC, Campanelli AP, Garcia RB, Duarte MH. The use of confocal laser scanning microscopy for the study of dentin Infection. In: Mendez-Vilas A, Diaz J, editors. Microscopy: Science, Technology, Applications and Education. Spain: Formatex; 2010. p. 583-589.
[90] Jackson W, Yamada M, Moninger T, Grose C. Visualization and quantitation of abundant macroautophagy in virus-infected cells by confocal three-dimensional fluorescence imaging. Journal of Virological Methods. 2013; 193:244-50.
[91] Rudkin FM, Bain JM, Walls C, Lewis LE, Gow NA, Erwig LP. Altered dynamics of Candida albicans phagocytosis by macrophages and PMNs when both phagocyte subsets are present. M Biol. 2013; 4:810-13.
[92] Marzorati M, Vanhoecke B, De Ryck T, Sadabad MS, Pinheiro I, Possemiers S, Abbeele PV, Derycke L, Bracke M, Pieters J, Hennebel T, Harmsen HJ, Verstraete W, Wiele TV. The HMI™ module: a new tool to study the Host-Microbiota Interaction in the human gastrointestinal tract in vitro. BMC Microbiology. 2014; 14:133.
[93] Kima HJ, Lia H, Collins JJ, Ingber DE. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. PNAS. 2015; E7-E15.
[94] Guirado E, Mbawuike U, Keiser TL, Arcos J, Azad AK, Wang SH, Schlesinger LS. Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model. mBio. 2015; 6:e02537-14.
[95] Wake N, Asahi Y, Noiri Y, Hayashi M, Motooka D, Nakamura S, Gotoh K, Miura J, Machi H, Iida T, Ebisu S. Temporal dynamics of bacterial microbiota in the human oral cavity determined using an in situ model of dental biofilms. npj Biofilms and Microbiomes. 2016; 2:16018.
[96] Wang AH, Li M, Li CQ, Kou GJ, Zuo XL, Li YQ. Human colorectal mucosal microbiota correlates with its host niche physiology revealed by endomicroscopy. Scientific Reports. 2016; 6:21952.
Microscopy and imaging science: practical approaches to applied research and education (A. Méndez-Vilas, Ed.)
72
___________________________________________________________________________________________