Chemical Strategies of the Beetle Metoecus Paradoxus, Social Parasite of … · 2016-02-10 ·...
Transcript of Chemical Strategies of the Beetle Metoecus Paradoxus, Social Parasite of … · 2016-02-10 ·...

Chemical Strategies of the Beetle Metoecus Paradoxus, SocialParasite of the Wasp Vespula Vulgaris
Annette Van Oystaeyen1& Jelle S. van Zweden1
& Hilde Huyghe1 & Falko Drijfhout2 &
Wim Bonckaert1 & Tom Wenseleers1
Received: 25 June 2015 /Revised: 11 September 2015 /Accepted: 17 November 2015 /Published online: 28 November 2015# Springer Science+Business Media New York 2015
Abstract The parasitoid beetle Metoecus paradoxus fre-quently parasitizes colonies of the common wasp, Vespulavulgaris. It penetrates a host colony as a larva that attachesitself onto a foraging wasp’s body and, once inside the nest, itfeeds on a wasp larva inside a brood cell and then pupates.Avoiding detection by the wasp host is crucial when the beetleemerges. Here, we tested whether adultM. paradoxus beetlesavoid detection by mimicking the cuticular hydrocarbon pro-file of their host. The beetles appear to be chemically adaptedto their main host species, the common wasp, because theyshare more hydrocarbon compounds with it than they do withthe related German wasp, V. germanica. In addition, aggres-sion tests showed that adult beetles were attacked less bycommon wasp workers than by German wasp workers. Ourresults further indicated that the host-specific compoundswere, at least partially, produced through recycling of theprey’s hydrocarbons, and were not acquired through contactwith the adult host. Moreover, the chemical profile of thebeetles shows overproduction of the wasp queen pheromone,
nonacosane (n-C29), suggesting that beetles might mimic thequeen’s pheromonal bouquet.
Keywords Chemical mimicry . Cuticular hydrocarbons .
Social parasitism . Vespidae . Vespula germanica .
Rhipiphoridae
Introduction
The chemical strategies that parasites employ to surpass therecognition system of their hosts offer a fascinating object ofstudy within the field of chemical ecology (Bagnères andLorenzi 2010; Dettner and Liepert 1994). These strategiesoften involve mimicking host cuticular hydrocarbon (CHC)profiles that play a key role in nestmate recognition (Akinoet al. 2004; Dani et al. 2001; Gamboa 2004; Singer 1998; vanZweden et al. 2010; van Zweden and d’Ettorre 2010). Indeed,in many social insect species, colony members can distinguishnestmates from non-nestmates through comparison with theirown colony odor template (known as their BGestalt odor^),which often consists of a complex mix of different CHCs(Lenoir et al. 1999; Lorenzi et al. 1996; Singer 1998). Thesehydrocarbons typically are stored in the postpharyngeal gland(PPG) and can be exchanged between colony membersthrough trophallaxis, grooming, and physical contact, bywhich the colony Gestalt odor is spread among the colonymembers (Soroker et al. 1995). This fine-tuned recognitionsystem enables social insects to keep most parasites out ofthe colony.
Nevertheless, as a counter strategy, many parasites haveevolved mechanisms that mimic a host’s colony odor, a strat-egy commonly referred to as chemical mimicry (Howard et al.1980, 1990; VanderMeer andWojcik 1982). Generally, chem-ical mimicry is subdivided into two major types: chemical
Annette Van Oystaeyen and Jelle S. van Zweden contributed equally tothis work.
Electronic supplementary material The online version of this article(doi:10.1007/s10886-015-0652-0) contains supplementary material,which is available to authorized users.
* Annette Van [email protected]
* Jelle S. van [email protected]
1 Laboratory of Socioecology & Social Evolution, Department ofBiology, University of Leuven, Leuven, Belgium
2 Chemical Ecology Group, Lennard-Jones Laboratories, KeeleUniversity, Staffordshire, UK
J Chem Ecol (2015) 41:1137–1147DOI 10.1007/s10886-015-0652-0

mimicry sensu stricto and chemical camouflage. The first isdefined as a strategy in which the parasite activelybiosynthesizes host-specific compounds, while the latter isdefined as a strategy in which the parasite acquires host cuespassively through contact with the nest material (Bagnères andLorenzi 2010; Howard and Blomquist 2005; Nash andBoomsma 2008). In reality, however, both mechanisms oftenoccur simultaneously, making it difficult to distinguish onefrom the other (Bagnères and Lorenzi 2010). For example,caterpillars of the butterfly Maculinea rebeli use both strate-gies when parasitizing Myrmica schencki ant colonies. Theybiosynthesize some host-specific brood pheromones and lateracquire additional host-specific hydrocarbons through contactwith adult workers (Akino et al. 1999). Additionally, parasitescan employ an alternative strategy of chemical transparency,whereby they avoid detection by not synthesizing thechemicals that are used in their host’s recognition system,thereby making them chemically invisible (Lenoir et al.2001). An example of such chemical transparency is foundin the wasp Polistes atrimandibularis, which parasitizesPolistes biglumis colonies. It is thought that this social parasitesucceeds in usurping P. biglumis host colonies by dilutingrecognition cues, as they have three to four times less CHCsthan their host (Lorenzi and Bagnères 2002).
The subject of this study, the parasitic beetle Metoecusparadoxus, occurs mainly in nests of the eusocial waspVespula vulgaris. This beetle belongs to the subfamilyRhipiphorinae, of which all species are obligatory parasitesof various Hymenopteran taxa. Species belonging to the genusMetoecus specifically parasitize nests of eusocial Vespidae,and M. paradoxus is found almost exclusively in nests of thecommon wasp, Vespula vulgaris, although other host specieshave been reported (Heitmans and Peeters 1996; Spradbery1973). In spring and summer, V. vulgaris workers collectdecaying wood to build their nest, and it is at this time thatthe beetles’ eggs, hidden in the crevices of the wood, hatchand the triungulinid larvae emerge. These larvae cling onto theforaging wasps’ bodies and are carried into the nest wherethey enter a brood cell containing a fully grown wasp larva.Subsequently, the parasitoid consumes the entire wasp larvafrom the inside out, and pupates inside the closed brood cell.After pupation, the beetle ecloses and chews through thebrood cell cap, before leaving the colony to mate (Edwards1980; Heitmans and Peeters 1996; Spradbery 1973). The ex-act period of time that the adult beetle remains inside the nestis as yet unknown, but adult fully pigmented beetles are foundfrequently inside wasp nests, suggesting that they remain thereat least for 1 day after eclosing from the pupa. During theperiod that the adult beetle remains in the wasp nest, a crucialstep in its survival is to avoid being attacked by its host.
Here, we hypothesized that M. paradoxus chemicallymimics its host, the common wasp, Vespula vulgaris, thusavoiding detection before leaving the colony to mate. We
combined behavioral experiments and chemical analyses todetermine the level of chemical host specificity toV. vulgaris as compared to its sister species V. germanica.Next, we conducted an isolation experiment to determine ifbeetles produce host-specific compounds themselves or ac-quire them through contact with the nest material after emerg-ing from the brood cell. In addition, we used the fact thatdifferent wasp castes produce different hydrocarbon profilesto determine if the beetles might be recycling host-specificcompounds from the prey item that they parasitized. Finally,we compared CHC profiles of beetles with those of adultworkers and queens to test the hypothesis that the beetlesmostly mimic queens and produce some key queen com-pounds, thus avoiding attacks by workers.
Methods and Materials
Colony Collection From July to October 2011, 83V. vulgarisand 26V. germanica nests were collected in the vicinity ofLeuven, Belgium. After collection, colonies were anaesthe-tized with carbon dioxide, and thoroughly checked for thepresence of adult M. paradoxus beetles (Fig. 1). Nests wereplaced in wooden boxes (15×17×40 cm) with a small hole(Ø=4 cm), so that workers could forage freely, and wereplaced outside in an apiary. Nests were checked every 3–4 days for the presence of adult beetles. These beetles, ran-domly picked from colonies, were used for aggression tests(see below). In addition, several combs were incubated andchecked twice a day for newly emerged beetles that were usedfor chemical analysis (see below).
Aggression Tests In order to determine if the host V. vulgarisis less aggressive to the parasitic beetles than is its sister spe-cies, V. germanica, behavioral experiments were carried out toassess the degree of aggression toward introduced beetles. Forthese tests, observation colonies of the two wasp species wereset up, consisting of one comb and 200–300 workers, in anobservation box (15.0×18.0×20.5 cm) with transparent wallsand a copper wire to hold the comb in the middle of the box. Amirror was placed underneath the box, so that the bottom ofthe comb could be observed. These observation colonies wereplaced under red light, so that it appeared dark for the wasps,but allowed us to observe their behavior. The experimentsstarted approximately 30 min after the introduction of thewasps into the box (allowing time for the wasps to wake upafter sedation and acclimatize to the new box). A total of eightbeetles were introduced into each of the three different typesof experimental colonies in a pseudo-random order: 1) theirown V. vulgaris host colony, 2) a random alien V. vulgariscolony, and 3) a random alien V. germanica colony. Anothertwo beetles were introduced into a random alien V. vulgariscolony and a random alien V. germanica colony, because their
1138 J Chem Ecol (2015) 41:1137–1147

host colonies were too weak to be used in experiments.Beetles were introduced on the comb through a tube in thetop of the observation box. For 15 min, starting immediatelyafter introduction, the aggressive interactions of workers tobeetles were observed and counted. Five different types ofbehavioral interactions were observed: (1) antennation, (2)grabbing, (3) biting, (4) stinging attempts, and (5) dragging(sometimes accompanied by biting and/or stinging). The datawere analyzed using a generalized linear mixed model(GLMM) with a binomial error structure with a logit linkfunction. The numbers of aggressive (categories 2–5) andnon-aggressive behaviors (category 1) were entered as depen-dent variables, while individual beetle, original beetle hostcolony, and observation colony were included as random fac-tors. Two models were run, one with species as a fixed factor(levels: V. vulgaris and V. germanica), in which only data ofintroductions into alien colonies were used, and one modelwith observation colony type (levels: own host colony, alienV. vulgaris colony, and alien V. germanica colony) as a fixedfactor, in which only data of the eight beetles that had beenintroduced into all three experimental colony types were used.Statistical analyses were carried out in R v3.1.0 (RDevelopment Core Team) using the R package lme4 (Bateset al. 2015).
Cuticular Hydrocarbons, Comb Incubation, and IsolationExperiment To assess the degree to which the CHC profilesof M. paradoxus individuals resembled those of the hostV. vulgaris and of its sister species V. germanica, individualsof each of the species were washed for chemical analysis (seebelow). First, we collected several beetles and wasps fromtheir natural colony (Ncollected =14M. paradoxus, 38
V. vulgaris, and 10V. germanica) and froze them at −20 °Cfor later analysis. This was done early in the season (July), atwhich point the wasp nests produce only workers, so we couldsafely assume that all collected beetles were derived fromworker brood cells. Second, to obtain beetles from male andqueen brood cells, we placed combs of V. vulgaris, togetherwith a dozen workers, in nest boxes in a climate room at 28 °Cand 60–70 % RH. Water and sugar water were provided adlibitum. As soon as a pupal cap turned dark brown, meaningthat the adult beetle was about to emerge, the cap was re-moved, the beetle taken out with clean forceps, and frozen at−20 °C for later analysis (Nincubated=14M. paradoxus). It wasnoted from which type of brood cell (male or queen) each ofthe beetles emerged in order to be able to compare their pro-files with newly emerged V. vulgaris workers, males, and vir-gin queens. We used newly emerged wasps to make the agesmore comparable. Newly emerged workers were collectedfrom natural colonies and identified by their light pigmenta-tion. Newly emerged males and queens were obtained throughthe same incubation procedure as the beetles (Nincubated=18workers, 6 queens, 13 males).
Finally, an isolation experiment was carried out to as-sess whether M. paradoxus beetles obtain their hydrocar-bon profiles through active or passive contact withV. vulgaris workers. When a beetle was about to emergefrom a worker cell (Nisolated=16), it was taken from thecell with forceps and placed by itself in a plastic box (20×9.5×6 cm) with a cardboard floor. Beetles were kept un-der the same conditions in the climate room until theywere frozen at −20 °C for later chemical analysis. Weaimed to keep these isolated beetles alive for at least5 days (mean±SD=7.81±2.14 days), which is a relatively
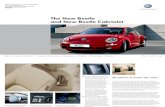
Fig. 1 Male Metoecus paradoxus (a) can be easily recognized by theirdarker orange color and their double-branched antennae compared tofemales (b) with single-branched antennae, lighter color, and anovipositor. Note the difference in size between beetles that developed inlarge queen cells (c) and beetles that developed in small worker brood
cells. The beetles received less aggression when introduced into coloniesof their most common host, Vespula vulgaris, compared to colonies of thesister species V. germanica. Alien colonies of V. vulgaris are ones fromwhich the beetles did not originate. Columns depict estimated means anderror bars depict 95 % confidence intervals; ** P=0.010
J Chem Ecol (2015) 41:1137–1147 1139

long isolation time, considering that the average life ex-pectancy of these beetles is only 7 and 8 days for femalesand males, respectively (Heitmans and Peeters 1996).
Chemical Analysis Cuticular hydrocarbons were extractedby immersing entire individuals in 600 μl of HPLC-gradepentane (Acros Organics, Belgium) for the workers, malesand beetles from small brood cells, while 1 ml of pentanewas used for queens and beetles from large (queen) broodcells (Fig. 1). For the first and last 15 s of the 10-minextraction, the samples were vortexed at 800 rpm.Thereafter, solvent was allowed to evaporate at room tem-perature in a laminar flow cabinet. Extracts subsequentlywere redissolved in 50 μl of pentane for workers, males,and beetles from small brood cells, and in 100 μl of pen-tane for queens and beetles from large brood cells. Twomicroliters of the extract were injected in an Agilent 6850gas chromatograph (GC; Agilent Technologies, US),equipped with an HP-1 capillary column (30 m×320 μm×25 μm), a splitless injector at 280 °C, a flameionization detector (FID), and helium as carrier gas at1.1 ml.min−1. The GC oven program was held at 70 °Cfor 1 min, after which the temperature was increased to150 °C at 20 °C.min−1 , and then to 320 °C at3.5 °C.min−1, and held for 5 min. Compounds were iden-tified using an Agilent 7890A GC equipped with a ZB-5HT capillary column (30 m×320 μm×25 μm) coupled toa 5975C Inert XL EI/CI mass spectrometric detector withelectron ionization (70 eV), using the same temperatureprogram as above. The transfer line was set to 280 °C andthe helium carrier gas flow was 1.5 ml.min−1.
The peak areas of identified compounds were quanti-fied using Agilent ChemStation (v. A.09.01, AgilentTechnologies) and normalized to relative concentrationsusing a log-ratio transformation (Aitchison 1986) scaledto unit variance. Retention indices of identified com-pounds were calculated by a cubic spline interpolationfunction (Halang et al. 1978) using an Excel macro (avail-able from the authors on request), as this provided esti-mates with better reproducibility across different instru-ments and temperature programs (Messadi et al. 1990).The data were analyzed by principal component analysis(PCA) and multiple analysis of variance (MANOVA).Because we noticed that the M. paradoxus individualshad much higher relative proportions of linear alkanesand some alkenes on their cuticle than did Vespulavulgaris individuals (Fig. 2; Table 2), the data were ana-lyzed for all compounds, together and separately, for thelinear alkanes, methylalkanes, and alkenes. For each ofthese compound groups, the raw data were again log-ratio transformed and scaled to ascertain independenceof the other compound groups. All statistical analyses
were carried out using R v3.1.0 (R Development CoreTeam 2011).
Results
Collection A total of 154M. paradoxus beetles (143 adultsand 11 pupae) were collected from the Vespula vulgaris nests.There were no beetles found in any of the 26V. germanicanests. The common wasp colonies contained between 0 and11 beetles, with an average of 1.73±2.02 (mean±SD) percolony, with 66.3 % of all colonies containing at least onebeetle. We found that the sex ratio of the adult beetles wasbiased toward females, as we collected approximately twice asmany females (N=95) as males (N=48).
Host Specificity: Aggression Tests Workers of V. vulgarisfrom a colony alien to the beetles were less aggressive towardthe beetles than were alien V. germanica workers (binomialGLMM with species as fixed factor, z=2.017, P=0.044).However, when observation colony type was used as a fixedexplanatory factor (levels: own V. vulgaris host colony, alienV. vulgaris colony, or alien V. germanica), there was no dif-ference in the level of aggression between V. vulgarisworkersof the beetles’ own host colony and V. vulgaris workers froman alien colony (binomial GLMM with observation colonytype as fixed factor, z=1.423, P=0.155) (Fig. 1). On the otherhand, V. germanica workers were more aggressive to the in-troduced beetles than were V. vulgaris of the beetles’ own hostcolony (Fig. 1; binomial GLMMwith observation colony typeas fixed factor, z=2.565, P=0.010).
Host Specificity: Resemblance in CHC Profiles In total, weidentified 67GC peaks in the beetles and the two wasp species(Fig. 2; Table 1). Of the 56 typicalM. paradoxus compounds,51 (91.1 %) were also found in the V. vulgaris samples. Thefive additional beetle-specific compounds, 4 alkenes and 1alkadiene, co-eluted with peaks 2, 9, 22, 28, and 37(Table 1). In V. vulgaris, all of these peaks contained only a3-methyl- or 4-methylalkane (Table 1). These peaks wereregarded as methylalkanes in further quantitative analyses,since the alkenes/alkadienes were low in relative concentra-tion and this made the comparison with wasps more appropri-ate since the two species shared the methylalkanes. By con-trast, only 34/56 (60.7 %) typical M. paradoxus compoundswere also found in the V. germanica worker samples, so thequalitative resemblance with V. vulgaris was greater.
From a quantitative point of view,M. paradoxus resembledV. vulgaris more than it did V. germanica (Fig. 3; Table 1),althoughmimicry of the host’s CHC profile was not perfect. Ina PCA, based on the 34 CHC variables shared by the threespecies and only individuals from natural colonies, the com-mon wasp workers (N=31) plotted closer on PC1 to the
1140 J Chem Ecol (2015) 41:1137–1147

beetles (N=14) than to the German wasps (N=10) (Fig. 3;M. paradoxus: −5.84±0.31 (estimate±S.E.), V. vulgaris:1.04±0.17, V. germanica: 4.96±0.35; V. vulgaris vs.
M. paradoxus, t=−22.08, P<0.001; V. vulgaris vs.V. germanica, t=11.17, P<0.001). As expected, based onthe chromatograms in Fig. 2, the compounds that were found
(ec
nad
nu
bA
pA
)
1 3
59 11 13
1516
17
19
20
21
22
23
24
25
26 2728
29
3031
33
34
36
37
38
39
40
4142
44 46
47
48
49
50
53
5758
59 60 61 62 63 64
Vespula germanica worker
20 25 30 35 40 45 50
Retention time (min)
1 2 3 4
5
67
8
9
10
111213
14
16
1718
1920
21
22
23
24
25
26
28
3132
3334
35
36
37
3839
40
414344
45
46 47
Vespula vulgaris worker
50
5152
53
5455
57 58 59
12 3 4
5
6
7
89101112
13
14
16
17
18
19
20
2122
23
2425
26
28
31
32
33
3435
3637
38
39
40
41
43
44
45
46
4752 53
504849
51
57
54
55 58 59
Metoecus paradoxus male
Metoecus paradoxus female
12 3 4
5
7
89101112
13
14
16
186
17
19
20
2122
23
2425
26
28
31
33
3435
3637
38
39
40
41
43
44
45
46
4752 53
504849
51
57
54
55 58 59
1 2 3 4
56 78
91011
1213
14
16
Vespula vulgaris queen
1718
19
20
21
22
23
24
252628
3132
33
34
35
36
37
38
3940
414344
46
4547
50
52
51 535455
575859
2.6·106
3.2·106
6.5·106
4.0·106
3.4·106
0
0
0
0
0
Fig. 2 Typical gas chromatograms of cuticular hydrocarbons of different types of individuals:Metoecus paradoxus females and males,Vespula vulgarisqueens and workers, and V. germanica workers. Labels refer to identification in Table 1
J Chem Ecol (2015) 41:1137–1147 1141

Tab
le1
Identifiedcuticular
hydrocarbons
forthespecies,Metoecusparadoxus,VespulavulgarisandV.germ
anica,
with
labelnumber(corresponding
tothatin
Fig.
2),retentio
nindex,
andaverage
percentage
inthedifferentspecies.*The
alk(adi)enes
inthesepeakswereonly
foundin
M.paradoxus
samples
Peak
Nr.
Identifiedcompound
Retentio
nIndex
M.paradoxus
female
M.paradoxus
female-isolated
M.paradoxus
male
M.paradoxus
male-isolated
V.vulgaris
queen
V.vulgaris
worker
V.germ
anica
worker
1n-C21
2100
0.71
0.48
1.04
0.93
0.09
0.35
0.13
23-MeC
21+C22:1*
2173
0.22
0.41
0.07
0.01
0.05
0.22
-
3n-C22
2200
0.66
1.21
0.29
0.21
0.11
0.27
0.13
4C23:1
2275
0.83
1.74
0.37
0.19
0.16
0.34
-
5n-C23
2300
8.79
13.79
8.65
10.15
0.76
3.05
0.79
67+9+11-M
eC23
2337
0.40
0.21
0.45
0.34
0.20
2.22
-
85-MeC
23
2351
0.09
0.02
0.08
0.05
0.09
0.88
-
93-MeC
23+C24:1*
2374
1.53
1.84
1.80
1.30
0.43
2.72
0.35
105,y-diMeC
232385
0.07
0.03
0.07
0.05
0.09
0.66
-
11n-C24
2400
1.54
3.07
0.88
1.35
0.99
1.86
0.46
123,y-diMeC
232409
0.19
0.12
0.14
0.10
0.21
1.43
-
1310
+12
+14-M
eC24
2434
0.32
0.14
0.24
0.17
0.28
1.98
0.33
146-MeC
24
2443
0.08
0.03
0.08
0.19
0.07
0.47
-
155-MeC
24
2451
--
--
--
0.03
164-MeC
24
2455
0.41
0.29
0.25
0.37
0.24
1.25
0.29
17C25:1
2465
3.94
5.38
31.33
29.59
0.53
1.58
0.37
184,y-diMeC
242489
0.16
0.10
0.14
0.17
0.16
1.00
-
19n-C25
2500
8.51
8.09
6.95
13.27
16.14
11.92
6.98
207+9+11
+13-M
eC25
2537
2.35
0.85
2.07
1.31
2.55
12.20
8.30
215-MeC
25
2551
0.48
0.15
0.35
0.19
0.54
2.46
0.38
223-MeC
25+C26:1*
2576
4.46
5.51
1.79
1.96
3.43
7.28
6.82
235,9-diMeC
25
2586
0.33
0.16
0.23
0.17
0.56
2.49
1.51
24n-C26
2600
0.95
1.19
0.48
0.46
4.43
1.82
1.37
253,11-diM
eC25
2610
0.48
0.29
0.31
0.23
0.96
4.21
0.69
268+10
+12-M
eC26
2634
0.74
0.42
0.36
0.31
0.59
2.16
3.08
276-MeC
26
2644
--
--
--
0.41
284-MeC
26+C27:2*
2657
0.39
0.37
0.25
0.35
0.52
0.89
0.08
2910,y-diM
eC26
2656
--
--
--
0.54
303-MeC
26
2667
--
--
--
1.90
31C27:1
2669
6.16
10.40
3.10
4.74
1.42
2.11
0.64
324,y-diMeC
262690
0.18
0.10
0.12
0.11
0.24
1.13
-
33n-C27
2700
10.19
6.88
6.88
5.87
28.48
5.47
8.35
347+9+11
+13-M
eC27
2733
1.47
0.56
0.93
0.53
2.47
6.22
22.40
355-MeC
27
2749
0.24
0.15
0.12
0.08
0.31
0.51
-
1142 J Chem Ecol (2015) 41:1137–1147

Tab
le1
(contin
ued)
Peak
Nr.
Identifiedcompound
Retentio
nIndex
M.paradoxus
female
M.paradoxus
female-isolated
M.paradoxus
male
M.paradoxus
male-isolated
V.vulgaris
queen
V.vulgaris
worker
V.germ
anica
worker
3611,15+9,13
+7,11-diM
eC27
2762
0.21
0.17
0.09
0.08
0.35
0.86
6.90
373-MeC
27+C28:1*
2774
1.59
2.08
0.38
0.39
7.40
2.97
8.22
385,15-diM
eC27
2783
0.14
0.07
0.08
0.05
0.35
1.03
1.15
39n-C28
2800
1.62
1.35
1.13
0.85
2.06
0.50
0.47
403,11-diM
eC27
2808
0.33
0.16
0.19
0.12
1.21
3.09
1.26
4110
+12
+14-M
eC28
2833
0.19
0.11
0.10
0.05
0.31
0.71
1.70
429,y+10,y-diM
eC28
2865
--
--
--
0.49
434-MeC
28
2860
0.300
0.25
0.13
0.08
0.57
0.26
-
44C29:1
2872
2.80
4.96
0.52
0.53
1.34
0.98
1.35
454,y-diMeC
282891
0.17
0.10
0.12
0.11
0.20
0.45
-
46n-C29
2900
24.65
16.64
20.15
15.80
6.35
1.18
1.04
477+9+11
+13-M
eC29
2930
1.07
0.79
0.55
0.32
1.48
1.51
3.21
485-MeC
29
2946
--
--
--
0.43
4913,17+11,15+9,13-diM
eC29
2964
--
--
--
1.93
503-MeC
29
2969
0.77
1.21
0.14
0.11
4.51
0.35
0.90
515,9-diMeC
29
2977
0.10
0.05
0.06
0.04
0.18
0.52
-
52n-C30
3000
0.74
0.58
0.53
0.39
0.59
0.71
-
5310
+11
+12
+13
+14-M
eC30
3046
1.01
1.13
0.52
1.83
1.35
2.05
2.03
54x,y-diMeC
30
3054
0.49
0.77
0.13
0.12
0.15
0.13
-
55C31:1
3071
1.11
1.88
0.28
0.19
0.36
0.31
-
564-MeC
30
3090
0.06
0.05
0.05
0.06
0.22
0.11
-
57n-C31
3100
4.88
3.02
4.55
3.73
0.44
0.21
0.33
5811
+13
+15-M
eC31
3134
0.49
0.26
0.30
0.20
0.69
0.41
0.56
5911,17+13,17+15,19-diMeC
31
3162
0.41
0.41
0.21
0.21
2.75
0.49
0.21
6011
+13
+15-M
eC33
3370
--
--
--
0.14
6111,21-diMeC
33
3403
--
--
--
0.19
6211
+13
+15
+17-M
eC35
3693
--
--
--
0.09
6311,21-diMeC
35
3766
--
--
--
0.27
647,11-diM
eC35
3801
--
--
--
0.27
6511
+12
+13
+17-M
eC37
4000
--
--
-0.19
6613,23-diMeC
37
4011
--
--
--
0.18
6711,21-diMeC
37
4040
--
--
--
0.14
J Chem Ecol (2015) 41:1137–1147 1143

to be more specific to M. paradoxus than to the wasps werethe linear alkanes and alkenes, which all loaded strongly neg-atively on PC1 (Table S1). However, when the PCAwas per-formed with only the 21 methylalkane variables (Fig. 3),M. paradoxus and V. vulgaris plotted together on the one sideand V. germanica on the other, showed that the parasite andV. vulgaris are rather similar in their methylalkane profile, andthat the chemical difference between the parasite and its hostlay mostly in the linear alkanes and alkenes. Indeed, whenusing only linear alkanes in the PCA, the largest variationwas that between beetles on the one side and the two waspspecies on the other (Fig. 3). This was due mostly to n-C25 andn-C26 being relatively more characteristic for the wasps,whereas n-C29 was more characteristic for the beetles(Table S1). In the alkene-only analysis, the predominant dif-ferentiation was that between male beetles and the rest, due tomale beetles having a high relative concentration ofpentacosene (x-C25:1) on their cuticles (Table S1), whereasfemale beetles and wasps were more characterized bynonacosene (x-C29:1) and hentriacontene (x-C31:1).
Isolation Experiment A PCA of the CHC data of thebeetles (N=14) and V. vulgaris workers (N=31) collectedfrom natural colonies (i.e., non-isolated), and isolatedbeetles (N=16), showed that the largest variation wasthat between beetles and wasps (Fig. 4; Table 1). Themajor cause of this difference again was that beetleswere characterized generally by a higher proportion oflinear alkanes and alkenes in their profile, while waspprofiles had higher relative abundances of methylalkanes
(Table S1; Table 1). The CHC profiles of isolated beetleswere further away from the wasps than those of non-isolated beetles (GLM: estimate PC1 score±SE: isolated:−6.26±0.55, non-isolated: −3.83±0.40, workers: 8.78±0.48; isolated vs. non-isolated, t=−4.43, P<0.001;workers vs. non-isolated, t=18.15, P<0.001). However,what stands out in Fig. 4 is that this isolation effect wasapparent only in female beetles (GLM: M. paradoxusindividuals only, PC1 score~ isolation×sex, isolation:F1,26=41.45, P<0.001, sex: F1,26=22.51, P<0.001, iso-lation× sex: F1,26 = 9.49, P<0.01). When compoundgroups were analyzed separately, a similar pattern wasfound for the methylalkanes but not for the linear al-kanes, in which isolated and non-isolated beetles didnot differ in their PC1 score (isolated: −2.24±0.29,non-isolated: −2.50±0.21, isolated vs. non-isolated, t=0.91, P=0.368), nor for the alkenes, in which again itwas mostly the male beetles that separated from the restof the individuals (Fig. 4; Table S1).
Hydrocarbon Recycling The analysis on the CHC data ofbeetles from different brood cell types (N=28), i.e., that fedon and developed in the brood cells of larvae belonging todifferent castes (workers, queens, and males), and the data ofnewly emerged wasps belonging to different castes (N=37),showed that, apart from the larger pattern of separation be-tween the parasite and its host, there was a more fine-scaledpattern that separated between individuals originating fromdifferent types of brood cells (Fig. S1; Table 2).
Fig. 3 Principal componentanalysis plots of cuticularhydrocarbon data of Metoecusparadoxus individuals, Vespulavulgaris workers, andV. germanica workers, allcollected from natural nests.Panels from left to right: based onidentified cuticular compoundsshared by all three species (34variables), based only on thelinear alkane subset (9 variables),based only on methylalkanesubset (21 variables), based onlyon alkene subset (4 variables).Grey circles=M. paradoxusfemales, white circles=M. paradoxus males, blackcircles=V. vulgaris workers,white squares=V. germanicaworkers
1144 J Chem Ecol (2015) 41:1137–1147

Discussion
Our study demonstrates that the parasitic beetleM. paradoxusfrequently parasitizes V. vulgaris colonies, with 66.3 % of allour collected colonies containing one or more beetles. Bycontrast, no beetles were detected in V. germanica colonies.Hence, even though the beetle has occasionally been reportedin V. germanica nests (Carl and Wagner 1982; Heitmans and
Peeters 1996), it appears thatM. paradoxus uses V. vulgaris asits main host. Our chemical analyses showed that the CHCprofiles of the beetles resembled the profiles of the commonwasp more than those of the congeneric German wasp, al-though mimicry was not perfect. In particular, we found thatthe beetles shared 51 CHCs with their common wasp host,with a further 5 unique compounds on their cuticle, but sharedonly 34 compounds with the German wasp. Quantitatively,
Fig. 4 Principal componentanalysis plots of cuticularhydrocarbon data of isolated andnon-isolated Metoecusparadoxus, and Vespula vulgarisworkers. Panels from left to right:based on all identified cuticularcompounds (51 variables), basedonly on the linear alkanes (11variables), based only onmethylalkanes (35 variables),based only on alkenes (5variables). Red circles: non-isolated M. paradoxus females,blue circles: non-isolatedM. paradoxus males, Greycircles=non-isolatedM. paradoxus females, whitecircles=non-isolatedM. paradoxus males, greysquares=isolated M. paradoxusfemales, white squares=isolatedM. paradoxus males, blackcircles=V. vulgaris workers
Table 2 Results of MANOVAsof cuticular hydrocarbons usingthe first two principal componentsas dependent variables, andspecies and brood cell type asexplanatory variables (see also,Fig. 4). The original principalcomponent analyses have beenperformed either using allcompounds, linear alkanes only,methylalkanes only, or alkenesonly
All compounds df Wilks’ λ approx. F num df den df P
Species 1 0.070 396.94 2 60 <0.001 ***
Brood cell type 2 0.692 6.06 4 120 <0.001 ***
Residuals 61
Linear alkanes df Wilks’ λ approx. F num df den df P
Species 1 0.207 114.60 2 60 <0.001 ***
Brood cell type 2 0.858 2.39 4 120 0.055
Residuals 61
Methylalkanes df Wilks’ λ approx. F num df den df P
Species 1 0.149 171.81 2 60 <0.001 ***
Brood cell type 2 0.594 8.94 4 120 <0.001 ***
Residuals 61
Alkenes df Wilks’ λ approx. F num df den df P
Species 1 0.284 75.50 2 60 <0.001 ***
Brood cell type 2 0.855 2.44 4 120 0.051
Residuals 61
df degrees of freedom, num df numerator degrees of freedom, den df denominator degrees of freedom
***significance at P<0.001
J Chem Ecol (2015) 41:1137–1147 1145

the beetle’s methylalkane profile, which is generally consid-ered to be important in nestmate recognition (van Zweden andd’Ettorre 2010), resembled that of V. vulgaris and not that ofV. germanica. The behavioral assays showed that commonwasp host workers are also less aggressive toward the beetlesthan are German wasp workers, suggesting that the chemical(CHC) mimicry is effective enough to reduce host aggression.We cannot exclude, however, that other appeasing or repellingsubstances also may play a role in preventing host attacks.
The similarity in CHC profiles betweenM. paradoxus andV. vulgaris raises the question of whether M. paradoxus pro-duces host-specific hydrocarbons de novo, by acquiring themthrough contact with the workers, or by recycling the com-pounds from its prey? Results in the isolation experiment in-dicate that acquisition through contact with adult workers is ofminor importance in obtaining the host-like CHC profile,since isolated beetles showed very similar profiles to non-isolated beetles. Moreover, only female beetles appeared tobe affected by the isolation, in the sense that their profilesplotted further away from the wasp hosts, showing that thiswas not universally true for all beetles but was sex-specific.The isolation effect that we observed also may be confoundedby age, since we kept the beetles in isolation alive for morethan 5 days, and we do not know the exact age of the beetleswe collected from natural colonies, although we assume thatthey resided inside the nest for a maximum of a couple of days(cf. Heitmans and Peeters 1996). Hence, this change in CHCprofile may be linked to sexual maturity or fertility of females.A striking example of the acquisition of host-specific com-pounds through contact with a host nest is found in the myr-mecophilous beetle Myrmecaphodius excavaticollis, a para-site in Solenopsis richteri ant nests, where the beetles loseall host-specific cuticular compounds after an isolation periodof 2 weeks, leaving a cuticular profile innate to the beetle(Vander Meer and Wojcik 1982). Similarly, Varroa destructormites, which are parasites of the honey bee Apis mellifera,acquire pupa-specific methylalkanes within 3–9 h of exposureto the host, and they can largely lose their profile within 18 hof isolation (Kather et al. 2015). The results of our isolationexperiment with M. paradoxus appear to show the exact op-posite, in that the CHC profile remained principally un-changed after isolation from the wasp nest. This is more sim-ilar to, for example, parasitic bumble bees that have remainedisolated over winter but still produce a host-specific odor pro-file (Martin et al. 2010).
These results may be explained when considering the bee-tle’s life cycle, as the time that adult beetles spend inside thehost nest is rather limited, and the observed apparent imperfectmimicry of host CHC profiles may be enough to exit the nestin the adult state. Our observation that beetles were lessattacked by workers from their own host colony indicates thatthere is some colony-specificity in their odor that helps toavoid their being attacked, suggesting CHCs as likely
candidate cues. Nonetheless, the beetles may also use otherappeasing or repelling substances that lower received aggres-sion, such as has been observed in several myrmecophilousparasites (d’Ettorre et al. 2000; Lenoir et al. 2001; Nash andBoomsma 2008).
The results indicate that the CHC profile is, at least partial-ly, recycled from the host larvae on which the beetles feedduring the larval stage, since the CHC profiles of beetles var-ied according to the caste to which their prey larvae belonged.One possibility is that the caste-specific CHC profiles werealready present on the wasp larvae, and that the parasitic beetlelarvae either directly obtained these hydrocarbons from thelarva or indirectly from the cell walls. However, the findingthat the beetle’s odor profile remained virtually unchangedafter several days of isolation suggests an active form of mim-icry, unlike the passive mimicry of Varroamites (Kather et al.2015). Alternatively, they could obtain the building blocks inthe right proportions to produce a caste-specific CHC profilein adult life. Similar results were reported in the myrmecoph-ilous spider Cosmophasis bitaeniata that obtains its host-specific hydrocarbon profile through predation of ant larvae(Elgar and Allan 2004), although there, too, the exact mecha-nism is unknown. Such a system of CHC recycling likely isbeneficial and selected for in parasites that feed on larvae, asthey already resemble their hosts to a certain extent once theyemerge from their cocoons, but also is likely partly explainedby the fact that hydrocarbons are usually not assimilated bythe digestive system, but instead are expressed on the cuticleof the individual (Guerrieri et al. 2009).
Finally, both the beetles and queens show high relativeproportions of linear alkanes compared to worker wasps.In particular, n-C29 seems to be overproduced by the bee-tles, and is one of three n-alkanes (n-C27, n-C28, and n-C29) that have recently been identified as worker sterility-inducing queen pheromones (Van Oystaeyen et al. 2014),suggesting that this could be an additional strategy toavoid attacks by workers. Chemical mimicry of queensalso has been reported in social parasites, such as Apismellifera capensis workers, which produce a series ofqueen pheromones allowing them to infiltrate and repro-duce successfully in Apis mellifera scutellata colonies(Wossler 2002). It still is unclear, however, whetherM. paradoxus produces high amounts of V. vulgaris queenpheromone to avoid aggressive behavior by workers, andthis requires further testing.
In summary, our results demonstrate that the parasitoidbeetle M. paradoxus is chemically adapted to its typicalhost, the common wasp V. vulgaris, although the mimic-ry is not perfect. We show that host-specific hydrocar-bons are not primarily acquired through contact with theadult host, but more likely through contact with the lar-val cell walls or through recycling hydrocarbons fromconsumed larvae.
1146 J Chem Ecol (2015) 41:1137–1147

Acknowledgments AVO, HH, and TW were supported by ResearchFoundation Flanders (FWO) and the KU Leuven Centre of ExcellencePF/2010/007, JSvZ was supported by a postdoctoral fellowship of FWO(grant 12Q7615N).
Author Contributions AVO, HH, WB and TW designed the experi-ments. AVO and HH collected wasp nests and carried out experiments.AVO, JSvZ, HH and FD analyzed the data. AVO, JSvZ, and TW wrotethe manuscript.
References
Aitchison J (1986) The statistical analysis of compositional data. TheBlackburn Press, Caldwell
Akino T, Knapp JJ, Thomas JA, Elmes GW (1999) Chemical mimicryand host specificity in the butterfly Maculinea rebeli, a social para-site of Myrmica ant colonies. Proc R Soc B 266:1419
Akino T, Yamamura K, Wakamura S, Yamaoka R (2004) Direct behav-ioral evidence for hydrocarbons as nestmate recognition cues inFormica japonica (Hymenoptera: Formicidae). Appl EntomolZool 39:381–387
Bagnères A-G, Lorenzi MC (2010) Chemical deception/mimicry usingcuticular hydrocarbons. In: Bagnères A-G, Blomquist GJ (eds)Insect hydrocarbons: biology, biochemistry, and chemical ecology.Cambridge University Press, Cambridge, pp 282–324
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Carl, KP, Wagner, A (1982) Investigations on Sphecophaga vesparumCurtis (Ichneumonidae) and Metoecus paradoxus L.(Rhipiphoidae)for the biological control of Vespula germanica F. (Vespidae) in NewZealand. Silwood Park, Commonwealth Institute of Biological con-trol, Working Report, UK
Dani FR, Jones GR, Destri S, Spencer SH, Turillazzi S (2001)Deciphering the recognition signature within the cuticular chemicalprofile of paper wasps. Anim Behav 62:165–171
Dettner K, Liepert C (1994) Chemical mimicry and camouflage. AnnuRev Entomol 39:129–154
d’Ettorre P, Errard C, Ibarra F, Francke W, Hefetz A (2000) Sneak in orrepel your enemy: Dufour’s gland repellent as a strategy forsucessful usurpation in the slave-maker Polyergus rufescens.Chemoecology 10:135–142
R Development Core Team (2011) R: a language and environment forstatistical computing. Vienna, Austria
Edwards, R (1980) Social wasps. Their biology and control. Rentokil LtdElgar MA, Allan RA (2004) Predatory spider mimics acquire colony-
specific cuticular hydrocarbons from their ant model prey.Naturwissenschaften 91:143–147
GamboaGJ (2004) Kin recognition in eusocial wasps. Ann Zool Fenn 41:789–808
Guerrieri FJ, Nehring V, Jørgensen CG,Nielsen J, Galizia CG, d’Ettorre P(2009) Ants recognize foes and not friends. Proc R Soc B 276:2461–2468
Halang WA, Langlais R, Kugler E (1978) Cubic spline interpolation forthe calculation of retention indices in temperature-programmed gas–liquid chromatography. Anal Chem 50:1829–1832
Heitmans WRB, Peeters TMJ (1996) Metoecus paradoxus inThe Netherlands (Coleoptera: Rhipiphoridae). EntomologischeBerichten 56:109–117
Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochem-ical aspects of insect hydrocarbons. Annu Rev Entomol 50:371–393
Howard RW, McDaniel CA, Blomquist GJ (1980) Chemical mimicry asan integrating mechanism: cuticular hydrocarbons of a termitophileand its host. Science 210:431–433
Howard RW, Stanley-Samuelson DW, Akre RD (1990) Biosynthesis andchemical mimicry of cuticular hydrocarbons from the obligate pred-ator, Microdon albicomatus Novak (Diptera: Syrphidae) and its antprey, Myrmica incompleta Provancher (Hymenoptera: Formicidae).J Kans Entomol Soc 63:437–443
Kather R, Drijfhout FP, Shemilt S, Martin SJ (2015) Evidence for passivechemical camouflage in the parasitic mite Varroa destructor. J ChemEcol 41:178–186
Lenoir A, Fresneau D, Errard C, Hefetz A (1999) Individuality and colo-nial identity in ants: the emergence of the social representation con-cept. In: Detrain C, Deneubourg J-L, Pasteels JM (eds) Informationprocessing in social insects. Birkhäuser Verlag, Basel, pp 219–237
Lenoir A, d’Ettorre P, Errard C, Hefetz A (2001) Chemical ecology andsocial parasitism in ants. Annu Rev Entomol 46:573–599
Lorenzi M, Bagnères A (2002) Concealing identity and mimicking hosts:a dual chemical strategy for a single social parasite? (Polistesatrimandibularis, hymenoptera: vespidae). Parasitology 125:507–512
Lorenzi MC, Bagnères A-G, Clement J-L (1996) The role of cuticularhydrocarbons in social insects: Is it the same in paper-wasps? In:Turillazzi S, West-Eberhard MJ (eds) Natural history and evolutionof paper wasps. Oxford University Press, Oxford, pp 178–189
Martin SJ, Carruthers JM, Williams PH, Drijfhout FP (2010) Host spe-cific social parasites (Psithyrus) indicate chemical recognition sys-tem in bumblebees. J Chem Ecol 36:855–863
Messadi D, Helaimia F, Ali-Mokhnache S, Boumahraz M (1990)Accurate determination of retention indices in programmedtemperature gas chromatography. Chromatographia 29:429–434
Nash DR, Boomsma JJ (2008) Communication between hosts and socialparasites. In: d’Ettorre P, Hughes DP (eds) Sociobiology of commu-nication: an interdisciplinary perspective. Oxford University Press,Oxford, pp 55–79
Singer TL (1998) Roles of hydrocarbons in the recognition systems ofinsects. Am Zool 38:394–405
Soroker V, Vienne C, Hefetz A (1995) Hydrocarbon dynamics within andbetween nestmates in Cataglyphis niger (Hymenoptera:Formicidae). J Chem Ecol 21:365–378
Spradbery JP (1973) Wasps. An account of the biology and natural his-tory of social and solitary wasps, with particular reference to those ofthe British Isles. Sidgwick & Jackson, London
Van Oystaeyen A, Oliveira RC, Holman L, van Zweden JS,Romero C, Oi CA, d’Ettorre P, Khalesi M, Billen J,Wäckers F, Millar JG, Wenseleers T (2014) Conserved classof queen pheromones stops social insect workers from repro-ducing. Science 342:287–290
van Zweden JS, d’Ettorre P (2010) Nestmate recognition in social insectsand the role of hydrocarbons. In: Blomquist GJ, Bagnères A-G (eds)Insect hydrocarbons: Biology, biochemistry and chemical ecology.Cambridge University Press, Cambridge, pp 222–243
van Zweden JS, Brask JB, Christensen JH, Boomsma JJ, Linksvayer TA,d’Ettorre P (2010) Blending of heritable recognition cues among antnestmates creates distinct colony gestalt odours but prevents within-colony nepotism. J Evol Biol 23:1498–1508
Vander Meer RK,Wojcik DP (1982) Chemical mimicry in the myrmecoph-ilous beetle Myrmecaphodius excavaticollis. Science 218:806–808
Wossler TC (2002) Pheromone mimicry byApis mellifera capensis socialparasites leads to reproductive anarchy in host Apis melliferascutellata colonies. Apidologie 33:139–163
J Chem Ecol (2015) 41:1137–1147 1147