Chemical Evolution of Life
Transcript of Chemical Evolution of Life

8/3/2019 Chemical Evolution of Life
http://slidepdf.com/reader/full/chemical-evolution-of-life 1/5
CHEMICAL EVOLUTION
Just as life has evolved into a plethora of forms over an extended time period, the chemical elements
which are the building blocks of matter have also, in a sense, evolved since the origin of the universe.
Chemical evolution is simply the process by which increasingly complex elements, molecules and
compounds developed from the simpler chemical elements that were created in the Big Bang.
Recent astronomical observations have discovered that chemical evolution has even led to the synthesis
of complex organic molecules in space, a discovery that could have serious implications on current
theories of how life developed. But before we discuss the implications of organic compounds being
formed in space, we must first outline the chemical history of the universe.
The chemical history of the universe began with the generation of the simple chemicals in the Big Bang.
Hydrogen and Helium, the two lightest chemical elements, were and still are the most abundant
elements in the universe. All stars derive their energy through then nuclear fusion of these light
elements into heavier elements. Once stars have exhausted their energy supply by converting all of their
hydrogen to helium, the star cools and contracts, with the increased pressure from the contraction
initiating the fusion of helium to form carbon.
Depending on the size and the density of the star, the fusion reactions can end with the formation of
carbon or they can continue on to form all the elements up to iron. Eventually, the successive fusion
reactions of heavy elements trigger the collapse and explosion, or supernova, of the star. These
supernova explosions release the newly-formed elements into space, where they play roles in the
formation of interstellar dust, meteoroids, planets and galaxies.
By examining the composition of stars at various stages of their life, scientists can trace the chemical
evolution of galaxies and get a clear picture of the early conditions of the universe. Techniques such
optical spectroscopy (which measures the intensities and wavelengths of light) gives these scientists the
ability to peer back along the chemical evolutionary timeline and see some of the oldest material in the
universe. In doing so, they have made some remarkable findings.
Astronomers recently discovered the existence of complex organic molecules called aromatic
hydrocarbons in space. Small organic molecules were found to have evolved into complex aromatic
molecules over a period of several thousand years. Although many scientists have long thought that life
arose on primitive Earth from simple inorganic molecules, a theory supported by the Miller-Urey
experiment, the discovery that organic molecules can be synthesized in space could serve to change
popular theories for the development of life on earth. Because the elements and molecules synthesized
by stars are eventually ejected into space, there is a chance that some of these molecules could have
landed on Earth and triggered the development of life.

8/3/2019 Chemical Evolution of Life
http://slidepdf.com/reader/full/chemical-evolution-of-life 2/5
Chemical evolution is an exciting topic of study because it yields insight into the processes which lead to
the generation of the chemical materials essential for the development of life. If the chemical evolution
of organic molecules is a universal process, life is unlikely to be a uniquely terrestrial phenomenon and is
instead likely to be found wherever the essential chemical ingredients are found.
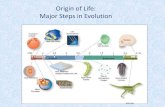
Naturalistic theories concerning lifes origin began to take shape in 1953. Watson and Crick unraveled
the structure of DNA, and Stanley Miller performed an experiment showing that amino acids can be
produced in a spark chamber. Most scientists of the day assumed that the mystery of lifes origin would
be solved in a few years.
The early pioneers in this field realized that a complete living organism, like the bacteria in figure 9.1,
could not spontaneously appear in a spark chamber or in any other environment governed by purely
naturalistic laws. The pioneers needed the first form of life to be simpler than anything that is present
on earth today.
Initial theories hypothesized that the first living thing was a protein. This assumption seemed reasonable
at the time because many of the building blocks of proteins, amino acids, are easily synthesized under
plausible prebiotic conditions. Because proteins regulate and control almost all of the activities
necessary for life, the living protein theory quickly gained widespread acceptance, but soon scientists
realized that there was a major flaw with the protein theory.
Proteins cannot self replicate, so the first living protein would not be able to reproduce itself, and
without replication there can be no natural selection; therefore, the first living protein would have no
way to evolve.
This issue led to the demise of the protein theory. In its place, emerged the RNA theory. This theory
gained substantial momentum when it was found that just like proteins some RNA molecules can
catalyze chemical reactions. Recently this theory has also fallen out of favor because it has its own set of
problems which will be discussed later. Today the most popular theory involves a self replicating pre-
RNA molecule.
Self replicating molecules are probably not the best theory to pursue, because such molecules cannot
reproduce for any length of time without running into serious problems with the second law.
Nevertheless, many researchers in the origins field are absolutely sure that the first living thing was a
self replicating chemical, and their point of view is understandable. There is simply no chance that a
complete bacterium spontaneously formed from the chemicals in a puddle four billion years ago. In
many ways, a self replicating molecule that violates the second law is a better choice.
Nevertheless, the second law should not be casually dismissed because its existence explains why
investigators have not been able to create a self replicating molecule in the lab. Unless a self replicator

8/3/2019 Chemical Evolution of Life
http://slidepdf.com/reader/full/chemical-evolution-of-life 3/5
has the knowledge and ability to harness the power of sunlight (or some other abundant energy source)
and use this energy to drive its own replication, then its lifetime will be short lived and its existence
forbidden by the laws of physics.
The origin of self replication requires a solution to three problems:
y Chemical evolution must create a protein, an RNA molecule or an RNA like molecule.
y This molecule must possess the molecular knowledge that enables self replication. It must also
be able to implement this knowledge.
y The molecule must possess the molecular knowledge needed to harness an energy source to do
useful work, and it must also be able to implement this knowledge in such a way that the energy
source drives replication.
Experiments investigating the origin of life have for the most part ignored the last issue. This is
understandable because until a molecule that can at least replicate itself for a little while can be found,
there is no need to try to find one that can replicate itself indefinitely. This chapter will investigate the
prebiotic synthesis of RNA and proteins. The next chapter will investigate self replication.
The pioneers in chemical evolution expected to show that the primordial ocean was full of biological
molecules. These researchers suggested that the early atmosphere contained no free oxygen, and that
under these conditions, the required biological precursors should be plentiful. The remainder of this
chapter will evaluate the validity of this hypothesis.
It is difficult to synthesize the relevant molecules, and today this difficulty has led most to conclude that
the primitive ocean contained a very limited supply of biological precursors. This finding does not mean
that the primordial soup did not exist. It does mean that the primitive ocean was not the primordial
soup because any relevant molecules in it would be too dilute.
It is possible to imagine environments that will concentrate biological precursors, but this leads to
further problems. It limits the soup in such a way that the conditions necessary for its existence rarely
exist and leads to the perhaps alarming conclusion that even given 5 billion years the soup may not have
existed.
Origin of life theories often speculate that hydrothermal vents like the ones shown in the pictures that I
took in Dominica are responsible for the origin of life. Since these vents exist today, plenty of oxygen in
the atmosphere would prevent the formation of useful prebiotic precursors that might give rise to life.
The first picture is in the valley of desolation on the trip to the boiling lake and the second is in about 30
feet of water at a dive site called Champagne. The bubles are created by hydrothermal vents.
* * *

8/3/2019 Chemical Evolution of Life
http://slidepdf.com/reader/full/chemical-evolution-of-life 4/5
In an attempt to explain the origin of carbon-based life on earth, conventional naturalistic theories of
chemical evolution propose two stages in the transformation of lifeless chemicals into life: 1) the
formation of small organic molecules, which then combine to form larger biomolecules; 2) the self-
organization of these molecules into a primitive living organism.
For each stage, scientists are learning that what is required for life seems much greater than what is
possible by natural process. The huge difference has motivated scientists to creatively construct new
theories for reducing requirements and enhancing possibilities, but none of these ideas has progressed
from speculation to plausibility.
Despite initial optimism following the famous Miller-Urey experiments in 1953, closer investigations
have revealed major problems that have not been solved (and perhaps cannot be solved) in both stages
of the proposed scenario:
In the first stage, chemical equilibria are usually unfavorable (they are "energetically uphill") for the
formation of small biomolecules and also for their synthesis into larger biomolecules. During
experiments in which there is a realistic simulation of the atmosphere on an early earth using the
probable starting molecules (H2O, plus N2 and CO2 which are stable and unreactive) instead of the
improbable starting materials (H2O, plus reactive NH3 and "explosive" H2 and CH4) in the reducing
atmosphere used for the Miller-Urey experiments the yields of essential biomolecules are extremely
low.
Even if biomolecules could form in Stage 1, these lifeless chemicals would have reached only the starting
point for the most challenging part of their journey toward life in Stage 2. The simplest "living system"
we can imagine, involving hundreds of components interacting in an organized way to achieve energy
production and self-replication, would be extremely difficult to assemble by undirected natural process.
And all of this self-organization would have to occur before Darwinian natural selection (which depends
on self-replication) was available.
Basically, what scientists are learning is that the complexity required for life (in terms of biomolecule
formation and self-organization) seems to be much greater than the complexity available by natural
process (beginning with lifeless matter). This huge difference has motivated scientists to stretch their
imaginations, to creatively construct new theories for reducing requirements and enhancing
possibilities.
For example, in an effort to avoid a "chicken and egg" problem in modern cells, DNA is required for
protein synthesis, but protein is required for DNA synthesis scientists have proposed that RNA (which
combines the replicating ability of DNA and the catalytic activity of proteins) was the key life-producing
molecule in the earliest cells. But this "RNA World" theory now seems implausible due to the apparent
impossibility of pre-biological RNA synthesis, and because the catalytic activities of RNA have not
matched initial optimistic hopes. In response, scientists are now proposing "pre-RNA World" theories

8/3/2019 Chemical Evolution of Life
http://slidepdf.com/reader/full/chemical-evolution-of-life 5/5
with key functional roles played by other molecules, and with metabolic energy sources that would be
easier to use.
Other alternatives include variations on the classic "soup" scenario, with new environments such as a
semi-evaporated pond, a seafloor hydrothermal vent, the surface of a clay-like mineral, or even another
planet. Or the first life in the universe might have been different than familiar carbon-based life on
earth.
Scientists are trying to develop principles for a prebiological selection of molecules, analogous to the
biological selection of genes in living organisms. And they are continuing to explore the self-organizing
properties of complex chemical systems, and to search for ways of reducing the minimal complexity
required for a living system.