Characterization and identification of Libyan olive...
Transcript of Characterization and identification of Libyan olive...

Libyan Journal of Science & Technology 9:2 (2019) 129141
Characterization and identification of Libyan olive diversity using microsatellite markers
Salem Abdul Sadeg*, a, Gayle Volkc, Christopher Richardsc, and Harrison Hughesb1
aBiology Department Elmergib University, Masallatah, Libya,
bHorticulture & Landscape Architecture, Colorado State University, Fort Collins, Colorado
cNational center for genetic resource preservation, Fort Collins, Colorado.
Highlights
Combination of morphological traits and molecular data were highly useful to separate closely related genotypes within Libyan olive landraces.
The denominations of homonyms and synonyms or mislabeling were more frequently within landraces than other cultivated and wild types.
The wild types were more closely related to the introduced genotypes than Libyan olive landraces.
A R T I C L E I N F O A B S T R A C T
Article history: Received 10 June 2017 Revised 03 March 2019 Accepted 10 March 2019 Available online 16 June 2019
Ten microsatellite markers were used to differentiate and evaluate the relationships among a to-tal of 91 olive genotypes (39 local cultivated, 36 introduced cultivars and 16 wild types) collected in Libya. A total of 109 alleles were identified, with the number of alleles per locus ranging from 4 to 20 alleles. Three loci (UDO43, DCA16 and GAPU101) had the most alleles across all loci with 20, 18 and 16, respectively. The wild types and introduced cultivars had greater numbers of alleles than the local cultivars. Six cases of duplicated genotypes, two cases of synonymy, and thirteen homonyms that were genetically distinct were observed in the Libyan collection. UPGMA cluster-ing classified the accessions into two main distinct groups. The first group consisted of local gen-otypes and the second group included introduced and wild type accessions. Admixture analysis also clearly distinguished between local ancient landraces and wild genotypes. In general, using molecular data enables to separate the Libyan olive accessions based on their origin but not fruit use.
Keywords: SSR Markers, Olive tree, Microsatellite mark-ers, and Genetic diversity.
* Corresponding author: E-mail address: [email protected] S. Abdul
1. Introduction
Libyan olives (Olea europaea subsp. europaea var. sativa or syl-vestris) have traditionally been evaluated by leaf, fruit, and seed morphological as well as phonological characteristics. It has been difficult to properly manage and conserve olive germplasm be-cause of the problems associated with clearly distinguishing among cultivars. Further complicating identification of cultivars is the ob-servation that wild populations have likely introgressed with lo-cally adapted cultivars. There are more than 100 named olive cul-tivars are grown along the coastal region of Libya. Some of these cultivars are likely to be identical due to the historical renaming of material. This has led to the perception that numerous cultivars ex-ist when in fact, they are actually synonyms or homonyms Morpho-logical differences associated with specific environmental effects have also lead to a mistaken identification of the cultivars. The level of knowledge about cultivar origin, selection and molecular varia-bility is limited because the identification of Libyan olive acces-sions has previously been based on phenotypic traits. Recently, morphological descriptions have improved, and are now consid-ered to be complementary tools to molecular marker, aiding in ol-ive cultivar identification. Using both morphological and molecular descriptors of genotypes within other crops (Corrado et al., 2009). This combination of techniques leads to more robust results (Leon et al., 2005). To date, SSR markers have not been used in combina-tion with morphological data to evaluate and improve the collec-tion of Libyan olive accessions as a genetic resource. In this work, SSR markers were used to differentiate and classify Libyan olive accessions.
1 1 2019 University of Benghazi. All rights reserved.1ISSN 2663-1407; National Library of Libya, Legal number: 390/2018
2. Materials and methods
2.1 Collection sites and plant materials
Accessions were classified into three categories: 42 local culti-vated varieties, 41 introduced cultivars of Olea europaea and 16 wild Olea europaea var. sylvestris. Leaf tissue was collected in 2009 and 2012. Most of the local cultivars (Libyan landraces) were col-lected from orchards of Masallatah city while the introduced culti-vars were collected from Tharouna and Gharian government col-lections as well as from farmers in the Zaltin and Tripoli regions. The wild type accessions were collected from four different sites (S1, S2, S3 and S4) in the Green Mountain region (Fig. 1). Leaf sam-ples were collected then immediately stored in containers with dry ice to prevent DNA degradation. They were then transferred to the National Medical Research Center in Tripoli where they were washed with double distilled water and freeze-dried. Samples were then transported to the Horticulture Laboratory at Colorado State University in Fort Collins, CO. USA where they were stored at –80°C until DNA use.
2.2 Processing samples
Total genomic DNA was extracted from 100-200 mg lyophilized of tissue using the method of large-scale CTAB extraction was per-formed according to (Mace et al., 2003). This protocol was a modi-fication of the CTAB procedure for obtaining purified genomic DNA. Twelve sets of primer pairs were selected (Table 1) because of their high resolution in discriminating polymorphism previous use in the identification of olive genotypes (Ercisli et al., 2011; Ercisli et al., 2012; Sefc et al., 2000; Baldoni et al., 2009; Sarri et al., 2006;

Salem et al./Libyan Journal of Science & Technology 9:2 (2019) 129141
130
Carriero et al., 2002; Cipriani et al., 2002; Belaj et al., 2003; De La Rosa et al., 2002). These were multiplexed using multiplex man-ager 1.2 software (Guichoux et al., 2011) to minimize overlap among the markers and to maximize similarity in the annealing temperature of each primer combination to reduce the variation
and a total number of PCR reactions. Each cycle of multiplex PCR amplification was performed with combinations of three different primers labeled with specific fluorescent dyes that incorporated during multiplex PCR amplification giving a specific color tag to each PCR product (Table 1).
Fig. 1. Map of Libya that illustrates the collection sites of cultivated and wild olive.
Table 1
Locus names, Forward primers including nucleotide sequences and company references.
Locus Name Forward dye
label Company Primer sequence labeled with fluorescent probe (5' –3')
EMO-90-F 56-FAM IDT 5'-/56-FAM/CAT CCG GAT TTC TTG CTT TT-3'
EMO-90-R PIGtail IDT 5'-GTT TCT T/AG CGA ATG TAG CTT TGC ATG T-3'
DCA3-F 56-FAM IDT 5'-/56-FAM/CCC AAG CGG AGG TGT ATA TTG TTA C-3'
DCA3-R PIGtail IDT 5'-GTT TCT T/TG CTT TTG TCG TGT TTG AGA TGT TG-3'
DCA14-F 56-FAM IDT 5'-/56-FAM/AAT TTT TTA ATG CAC TAT AAT TTA C-3'
DCA14-R PIGtail IDT 5'-GTT TCT T/TT GAG GTC TCT ATA TCT CCC AGG GG-3'
GAPU101-F 56-FAM IDT 5'-/56-FAM/CAT GAA AGG AGG GGG ACA TA-3’
GAPU101-R PIGtail IDT 5'-GTT TCT T/GG CAC TTG TTG TGC AGA TTG-3'
DCA18-F VIC AB 5'-/VIC/AAG AAA GAA AAA GGC AGA ATT AAG C-3'
DCA18-R PIGtail IDT 5'-GTT TCT T/GT TTT CGT CTC TCT ACA TAA GTG AC-3'
DCA16-F VIC AB 5'-/VIC/TTA GGT GGG ATT CTG TAG ATG GTT G-3'
DCA16-R PIGtail IDT 5'-GTT TCT T/TT TTA GGT GAG TTC ATA GAA TTA GC-3'
DCA5-F VIC AB 5'-/VIC/AAC AAA TCC CAT ACG AAC TGC C-3'
DCA5-R PIGtail IDT 5'-GTT TCT T/CG TGT TGC TGT GAA GAA AAT CG-3'
DCA17-F VIC AB 5'-/VIC/GAT CAA ATT CTA CCA AAA ATA TA-3'
DCA17-R PIGtail IDT 5'-GTT TCT T/TA AAT TTT TGG CAC GTA GTA TTG G-3'
GAPU103A-F PET AB 5'-/PET/TGA ATT TAA CTT TAA ACC CAC ACA-3'
GAPU103A-R PIGtail IDT 5'-GTT TCT T/GC ATC GCT CGA TTT TTA TCC-3'
GAPU71B-F PET AB 5'-/PET/GAT CAA AGG AAG AAG GGG ATA AA-3’
GAPU71B-R PIGtail IDT 5'-GTT TCT T/AC AAC AAA TCC GTA CGC TTG-3'
UDO-043-F PET AB 5'-/PET/TCG GCT TTA CAA CCC ATT TC-3'
UDO-043-R PIGtail IDT 5'-GTT TCT T/TG CCA ATT ATG GGG CTA ACT-3'
DCA9-F PET AB 5'-/PET/AAT CAA AGT CTT CCT TCT CAT TTC G-3'
DCA9-R PIGtail IDT 5'-GTT TCT T/GA TCC TTC CAA AAG TAT AAC CTC TC-3'

Salem et al./Libyan Journal of Science & Technology 9:2 (2019) 129141
131
Primers EMO90-F, DCA3-F, DCA14-F, and GAPU101-F were la-beled with fluorescent dye (56-FAM) attached to the 5’-end of oli-gonucleotides from Integrated DNA Technologies (IDT) (IDT, Cor-alville, IA). The forward primers DCA18-F, DCA16-F, DCA5-F, and DCA17-F were attached with a green fluorescent dye (VIC) while GAPU103A-F, GAPU71B-F, UDO-043-F, and DCA9-F were attached with a fluorescent dye (PET) (both labeled groups were synthe-sized by Applied Biosystems (AB) (Foster City, CA). The reverse primers for all sets of 12 primer pairs were unlabeled and were ob-tained from Integrated DNA Technologies (IDT).
A small-tailed oligonucleotide or PIG-tail sequence (GTTTCTT) was added to all the unlabeled reverse primers to promote specific priming, full adenylation and reduce stutter bands (Brownstein et al., 1996). PCR amplifications were carried out in a final volume of 10μL in 2 mL 8-strip PCR tubes with 2 µM. The solution mix for PCR reactions consisted of the following: 2.0 μL of (20 ng/μL) genomic DNA; 3 μL of (Type-it microsatellite PCR –Maste mix; QIAGEN, USA); 2.0 μL of (2.0 μM) primer mix; and 3.0 μL of deionized water. All amplifications of multiplex PCR were performed in a 96-well thermocycler (Applied Biosystems, USA) under the following con-ditions of touchdown annealing temperature profile (Viljoen et al., 2005): 2 min at 94°C; 10 cycles of 45 sec at 94°C, 1 min at 65°C (annealing temperature was reduced 1°C after every cycle), and 1 min and 30 sec at 72°C; 35 cycles of 45 s at 94°C, 1 min at 55°C, and 1 min and 30 s at 72°C; and a final extension step of 5 min at 72°C. The touchdown procedure was used to reduce non-specific prim-ing during PCR amplification.
After successful amplification of the target region of isolated DNA, PCR samples were combined with LIZ 600 internal size stand-ards. Fragment analyses were performed on an Applied Biosys-tems 3130 xL. The fragment data were scored using ‘GeneMapper’ software v.3.7 to size and genotype the alleles. Once allele sizes were determined (allele calling), the data set was formatted such that it could be converted to the various formats required by the software packages (Convert program Version) (Glaubitz, 2004).
2.3 Analytical methods
2.3.1 Quality control
Quality control was performed using a set of procedures to en-sure the integrity, stability and consistency of SSR results. All am-plifications of PCR for each sample three times. Negative and posi-tive standard controls were applied. Quality was evaluated prior to exporting the results of the genotype samples as matrix data. Gen-otypes that have the same gene fragment to minimize the error es-timation of genotyping. Filtering loci set to eliminate markers that have a missing data across all genotypes.
2.3.2 Population genetic analyses
Descriptive statistics were performed using FSTAT software version 2.9.3.2 (Goudet, 2002) and GDA software version 1.1. (Ob-served alleles, observed fragment size, private alleles, the probabil-ity of identity and power of discrimination) were estimated for each individual locus (Table 3) (probability of identity, power of discrimination, allele richness, expected heterozygosity, observed heterozygosity and population inbreeding coefficient).
2.4 Diversity and differentiation
2.4.1 Estimation of population structure and diversity
To estimate the dissimilarity or similarity of genetic data based on their populations or type of genotype. The pairwise distance matrix of SSR data was implemented as a (.txt) input file of allelic data in DARwin software v 5.0.158 (Raman et al., 2014). The con-structed tree from DARwin software applied into the Fig Tree soft-ware v1.4.0 (Rambaut, 2012) to describe the relationship among olive samples using genetic distance as a tree based on (UPGMA) with the support of bootstrapped dissimilarities number of (1000) to assess the uncertainty of the tree structure.
2.4.2 Estimation of partition by assignment
Structure analysis was used to estimate genetic data to assign genotypes to specific groups without any prior information. The probability of membership into 1-4 K groups was determined by multiple runs (10 times) using STRUCTURE software Version 2.3.4 by (Pritchard et al., 2003). The STRUCTURE HARVESTER program (Earl, 2012) collects results generated by STRUCTURE program. This method allows assessment and visualizes the likelihood scores of multiple values of K, to evaluate the most likely level of genetic group subdivision. The probability of identity (IP) for each locus and all SSR loci set (accumulated IP) was calculated by means of the CLUster Matching and Permutation Program (CLUMPP) ver-sion 1.1.2 (Jakobsson and Rosenberg, 2007). This program assigns individuals on the basis of optimal membership coefficients within clusters. Molecular data were combined together with morpholog-ical data of stable phenotypic traits that were blocked by results of structure assignment of molecular data to evaluate the relationship between phenotypic and genotypic data.
3. Results
A matrix of 12 SSR primers by 99 individuals (Table 1) was used to evaluate the genetic relationships among genotypes of local cultivated, introduced cultivars and wild types. As a result of filter-ing loci and genotypes that have missing data, allelic data of DCA17 and DCA9 were removed from the dataset due to high failure rate. Eight duplicated accessions, based on their identical genotypes, were also excluded (Table 2). Consequently, a total of 10 SSR loci and 91 genotypes (39 local, 36 introduced and 16 wild) remained in the genetic data matrix.
3.1 Identification of duplicated genotypes
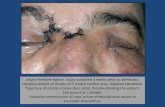
Ten SSRs loci (Table 3) were used to determine if duplicate ol-ive cultivar samples were present in the dataset. Twelve genotypes (6 pairs) had the same names and were genetically identical as true duplicates (Table 2 and Fig. 2). Two sets of cultivars had different names but identical genotypes and were therefore considered to be (synonyms) (Table 2 and Fig. 2). One cultivar from each of these eight pairs was excluded from further analyses. A review of their morphological data and associated images indicated similarity in phenotypic traits (Fig. 3).
Fig. 2. A Neighbor-joining tree of 23 duplicated olive genotypes; each tip represents a single individual genotype with all pairs of duplicated geno-types similar.

Salem et al./Libyan Journal of Science & Technology 9:2 (2019) 129141
132
Fig. 3. Phenotypic traits of the duplicated olive genotypes that illustrate similarity of genotypes.

Salem et al./Libyan Journal of Science & Technology 9:2 (2019) 129141
133
Table 2
The same fragment sizes were considered to be duplicated or synonyms.
Variety name Local location Relationship Bootstrap val-
ues (1000) Variety name
Local loca-tion
Relationship Bootstrap val-
ues (1000)
Chemlkussabat Tharouna Duplicated 99 Maurino Tharouna Duplicated 100
Chemlkussabat Mesalata Duplicated 99 Maurino Gharian Duplicated 100
Khaddira Mesalata Synonyms 100 Zalmati Zaltin Duplicated 59
Khaddra Mesalata Synonyms 100 Zalmati Gharian Duplicated 59
Ouslati Tharouna Duplicated 100 Frantoio Tharouna Duplicated 96
Ouslati Gharian Duplicated 100 Frantoio Gharian Duplicated 96
Leccino Tharouna Duplicated 100 Chemlali Zaltin Synonyms 98
Leccino Gharian Duplicated 100 Gargashi Tharouna Synonyms 98
3.2 Descriptive statistics of loci
A total of 109 alleles were identified, and the number of alleles per locus ranged from 4 alleles at the DCA5 locus to 20 alleles at the UDO043 locus, with an average of approximately 11 alleles per lo-cus (Table 3). The combined discrimination power for all 10 loci
was calculated with an average of (0.70) indicating that there is a moderate to high discrimination of the markers that were used, so there is a high probability that two individuals have different gen-otypes for each locus. The average probability of identity for all loci was low, indicating that there is a low (0.30) probability of acces-sions matching by chance.
Table 3
Descriptive statistics of 10 loci based on genetic data from 91 individual olive genotypes collected in Libya.
Locus Sample size Observed alleles (A) Observed frag-
ment size Private alleles Probability of iden-
tity (PI) Power of discrimi-
nation (PD)
DCA14 90 10 168-188 3 0.22 0.78
DCA16 85 18 121-193 10 0.24 0.76
DCA18 92 10 154-180 3 0.20 0.80
DCA3 84 9 229-252 4 0.49 0.51
DCA5 83 4 194-206 1 0.85 0.15
EMO90 92 5 180-193 0 0.30 0.70
GAPU101 81 16 164-215 6 0.12 0.88
GAPU103A 88 11 134-189 4 0.21 0.79
GAPU71B 92 6 117-140 1 0.23 0.77
UDO043 69 20 154-227 9 0.10 0.90
All 85.6 10.9 161-198 4.1 0.30 0.70
3.3 Descriptive statistics of populations
Descriptive analysis of populations using GDA analysis (Table 4) revealed a higher inbreeding coefficient in the wild population (0.36) than the two sets of individuals, introduced (0.23) and local (0.24). The private allele frequency in the wild types was relatively higher than the other two populations. However, the discrimina-tion power (PD) of private alleles in local and introduced genotypes
was relatively high (0.99 and 0.98) respectively (Table 4). Results from the descriptive statistics of these populations provided in-sights into observed and expected heterozygosity. The value of ex-pected heterozygosity (He) was higher than the value of observed heterozygosity for all three sets of individuals (Table 4) indicating there is more chance of heterozygosity at each population and they have some outbreeding resulting in disassortative mating and dis-similar traits.

Salem et al./Libyan Journal of Science & Technology 9:2 (2019) 129141
134
Table 4
Descriptive statistics of three sets of individuals (Introduced, local and wild) collected from six locations in Libya
Sets of individ-uals
Sample size Number of Pri-
vate alleles Probability of identity (PI)
Power of dis-crimination
(PD) Allele richness He Ho
Population in-breeding coef-
ficient
Introduced 36 19 0.02 0.98 5.89 0.71 0.55 0.23
Local 39 4 0.002 0.99 4.88 0.68 0.52 0.24
Wild 16 18 0.13 0.87 5.88 0.64 0.41 0.36
Overall 30.33 13.67 0.05 0.95 5.55 0.68 0.49 0.28
z Six locations located as identified in Fig. 1.
In general, allelic richness was higher in wild and introduced
genotypes (5.89 and 5.88) respectively than in local genotypes (4.88) (Table 4). There were more private alleles (observed once) in the introduced genotypes (19 private alleles), than in the wild (18 alleles) and local genotypes (4 alleles) (Table 4). Overall, all of the 41 private alleles were considered to be highly polymorphic across locations and could be used to assign individuals into a spe-cific population based on their origins (Table 4). A total of 42 mon-omorphic alleles were estimated in all three different populations. These could not be used to assign any genotype to a specific popu-lation. Common alleles were most often observed in wild and intro-duced genotypes.
F-stats for the three sets of individuals (Introduced, local and wild) were estimated by performing a bootstrap analysis across loci to create 95% confidence intervals (Table 5). The pairwise Fst for the three sets of individuals were significantly different. Genetic differentiation of Fit, Fst and Fis was estimated by bootstrap test over all loci, and it was significant among all loci.
Table 5
Genetic differentiation as estimated by Fst with confidence intervals of 95% overall loci and three different locations.
Source Fst Fst confidence interval
Loci 0.025 0.025-0.077
Sets of individuals 0.030 0.030-0.080
3.4 Estimation of diversity and differentiation
3.4.1 Identification of mislabeled genotypes
Neighbor-joining relationships revealed that the 10 loci failed to distinguish a total of seven cultivars appeared to be similar when the molecular data were evaluated. These genotypes were Krusi, Pendolino, Tombarella, Ouslatikussabat, Accession53, Nepal and Aceession46. However, all seven cultivars had missing data for two loci (Table 6). A review of their morphological data and associated images (Fig. 4) indicated large differences in phenotypic traits across all of these cultivars.
Table 6
The seven cultivars had missing data that were considered to be mislabeled genotypes.
POP = Introduced DCA18 DCA18 UDO043 UDO043 GAPU101 GAPU101 DCA3 DCA3 DCA5 DCA5 KrusiG 168 168 227 227 ? ? 240 240 202 202
GargashiT 168 168 ? ? 187 193 240 240 202 202
PendolinoG 174 174 204 204 189 203 240 240 202 202 TombarellaG 174 174 ? ? ? ? 240 240 202 202
Ac#53 174 174 168 168 189 195 240 240 202 202 OuslatikussabatT 174 174 168 168 189 195 ? ? ? ?
Ac#46 ? ? ? ? 181 195 240 240 202 202 NepalTri 174 174 177 177 181 195 ? ? ? ?
POP = Introduced DCA14 DCA14 GAPU103A GAPU103A DCA16 DCA16 GAPU71B GAPU71B EMO90 EMO90 KrusiG 182 186 159 159 147 160 124 127 184 184
GargashiT 182 186 159 159 147 160 124 127 184 184 PendolinoG 186 186 150 150 147 147 121 127 183 189
TombarellaG 186 186 150 150 147 147 121 127 183 189
Ac#53 168 186 159 159 ? ? ? ? 183 184 OuslatikussabatT 168 186 159 159 147 183 121 140 183 184
Ac#46 178 186 162 174 147 147 121 140 183 189 NepalTri 178 186 162 174 147 147 121 140 183 189
3.4.2 Identification of homonyms genotypes
There were 13 samples that had the same cultivar names but did not have matching genotypes (Table 7 & Fig. 5). This suggests that most of them (Chemlali-M, Chemlalisfax-T, Chemlalisfax-G, Coratina-T, Coratina-G, Jabbugi-T, Jabbugi-M, Mbuti-T, Mbuti-M, Mignolo-T, Mignolo-G, Moraiolo-T, Moraiolo-G, Rasli-T, Rasli-M, Zaafrani-T, Zaafrani-M, Zarrasi-M and Zarrasi-T) considered to be homonyms and were given the same names by human error, some of the labeled cultivars were misidentified because they matched
other cultivars (Gargashi-T match Chemlali –Za, 53% bootstrap). Whereas other four cultivars (Hammudi-M, Hammudi-T and Mar-rari-M, Marrari-T) considered being close clones and were differ-ent from each other by 3 and 2 different alleles respectively. Com-parisons of morphology images of each duplicate pair of genotypes showed distinct differences and supported the genetic results of polymorphism (Fig. 6). The problems associated with cultivar iden-tification likely in landrace types than in introduced cultivars or wild type olives.

Salem et al./Libyan Journal of Science & Technology 9:2 (2019) 129141
135
Table 7
Duplicated cultivars were considered to be mislabeled or homonyms genotypes
Variety name Local location Relationship Variety name Local location Relationship
Chemlali Masallatah Homonyms Mbuti Masallatah Homonyms
Chemlali Zaltin Homonyms Mbuti Tharouna Homonyms
Chemlalisfax Gharian Homonyms Mignolo Gharian Homonyms
Chemlalisfax Tharouna Homonyms Mignolo Tharouna Homonyms
Coratina Gharian Homonyms Moraiolo Gharian Homonyms
Coratina Tharouna Homonyms Moraiolo Tharouna Homonyms
Gargashi Masallatah Homonyms Rasli Masallatah Homonyms
Gargashi Tharouna Homonyms Rasli Tharouna Homonyms
Hammudi Masallatah Homonyms Zaafrani Masallatah Homonyms
Hammudi Tharouna Homonyms Zaafrani Tharouna Homonyms
Jabbugi Masallatah Homonyms Zarrasi Masallatah Homonyms
Jabbugi Tharouna Homonyms Zarrasi Tharouna Homonyms
Marrari Masallatah Homonyms
Marrari Tharouna Homonyms
Fig. 5. Neighbor-joining tree of 13 duplicated pairs of olive genotypes; each tip represents a single individual acces-sion with all pairs of duplicated genotypes different.

Salem et al./Libyan Journal of Science & Technology 9:2 (2019) 129141
136
Fig. 6. Accessions identified by the same name (Homonyms accessions).
An UPGMA neighbor-joining tree (Fig. 7) was constructed to study the genetic relationships among the 91 different olive geno-types that were discriminated by the 10 SSR markers. Two primary clusters of individuals were identified (green color = landraces)
and (intermixed color, red = introduced cultivars and blue = wild types). Most of the wild types were found within the intermixed wild and introduced genotypes (Fig. 7).

Salem et al./Libyan Journal of Science & Technology 9:2 (2019) 129141
137
Fig. 7. Neighbor-joining tree of 91 individuals, each tip represents a single olive genotype and the colors of clades indicate the populations of origin (Local, introduced and wild).
3.5 Estimation of partition by assignment
Structure analysis using the admixture model without prior in-formation was used to identify the genetic relationships of Libyan landraces, wild, and introduced cultivars. It was also used to differ-entiate individuals within each population. The most likely number of clusters inferred by structure software were at K=3. The local genotypes clustered together and two distinct sub-groups were identified. The first group consisted of the 20 most popular local genotypes (blue color) that are used mainly to produce olive oil. The second group consisted of 11 hybrid genotypes (blue and red color) between local and introduced cultivars (Fig. 8). These acces-sions are not widely grown and are not preferred for oil produc-tion. Those cultivars that were primarily local cultivars genetically were ancient ones grown in the Masallatah region where they are widely grown for their valuable oil characteristics. This group in-cludes the main two cultivars Rasli and Gargashi that are used mainly for their oil production under extremely dry climates. There were six genotypes (ZarrasiM, ChemlaliM, MoraioloG, Ac#48, Pen-dolinoG and TombarellaG) that were considered to be local geno-types in neighbor-joining tree cluster (Fig. 7) but based on the structure analysis were included in the introduced genotype
grouping. This is perhaps best explained by saying that they are re-ally introduced genotypes especially given the derivation of the names of 4 of them is not Arabic but Italian. In the case of ZarrasiM relative fruit size is similar to the introduced genotypes that have larger fruit size as compared to the smaller fruit of the local types. The wild and introduced accessions remained unchanged and were clustered the same as the UPGMA of the neighbor-joining tree (Fig. 7). They had an intermixed genetic background (red color) as shown in (Fig. 8). There were 13 genotypes that had a lot of admix-ture and mixed genetic background of all populations (Fig. 8). Most of these genotypes (Beserri-M, Oliarolasalentina-T, Santagostin-T, Mignolo-T, Gragnano-G, Ouslati-T, Nebgemel-M and Kalefy-M) were previously reported to be clustered as individual genotypes with Fig Tree cluster too (Fig. 7), also they have proportions of their membership in three different gene pools. Finally, the results from population structure analyses clearly distinguished the known an-cient local cultivars, introduced cultivars and wild types into spe-cific clusters associated with their origin (local, introduced and wild), but not always due to their use (oil, table and dual purpose) as reported in previous studies (Besnard et al., 2001; Belaj et al., 2010).

Salem et al./Libyan Journal of Science & Technology 9:2 (2019) 129141
138
Fig. 8. Separation of the structure analysis into specific groups; intermixed group between introduce and wild genotypes (red color), Introduced genotypes (green color) and hybrid genotypes (mixed color) and local genotypes (blue color). Every single vertical strain is represented by an individual genotype.
3.6 Genotype-phenotype comparison
We sought to determine if independent stable phenotypic traits could be used to predicate the genetic classification of olive geno-types to verify if there is a strong correlation between the pheno-typic and genotypic traits. Highly significant differentiation
(P<0.0001***) (Fig. 9 A and Fig. 9 B) of stable phenotypic traits were observed when using the average q values of structure mem-bership coefficient (1=local, 2=mixed and 3=introduced) or struc-turama partition assignment (1=mixed, 2=Introduced and 3=Land-races) respectively as a categorical data for all 90 genotypes based on the cultivar origin (introduced or local).
Fig. 9 Discriminant analysis was used to differentiate among all 90 genotypes based on membership q values of structure (p<0.0001***) A, and structurama assignment (p<0.0001***) B.

Salem et al./Libyan Journal of Science & Technology 9:2 (2019) 129141
139
4. Discussion
The SSR markers (Table 1) used in this study were selected based on previously published reports (Baldoni et al., 2009; Erre et al., 2010; Dı´ez et al., 2011 and Ipek et al., 2012). The identification of duplicated, mislabeled or homonymes genotypes (Table 2, 6 and 7) respectively, found within the Libyan olive collection illustrates one of the most important problems associated with olive produc-tion in Libya. This misidentification may growers planting geno-types that are not those of yield potential in their specific area. A source of this misidentification may be due to phenotypic variation (Fig. 3) associated with environmental conditions when grown in diverse locations, to the description of the same genotype with dif-ferent names. Rao et al. (2009) showed that synonyms and homo-nyms occur more frequently among landraces than in common cul-tivars. However, phenotypic data (fruit, seed and leaf) may be im-portant in distinguishing different genotypes when molecular data indicates no differences due to missing or limited data. This is es-pecially true when stable phenotype characteristics indicate differ-ences between genotypes. Seven cultivars (Table 6) were deter-mined to be identical based on the data from eight loci. However, this data was insufficient to discriminate all seven cultivars due to missing data of two additional loci. The combination of phenotypic traits (Fig. 4) clearly indicated that these cultivars were different.
The genetic descriptive analysis identified the most informa-tive with a total of 20 alleles as similarly reported by D ı́ez et al. (2011). In general, loci that have many different alleles were pre-ferred to distinguish between two different individuals. (http://www.mathcs.citadel.edu). The lowest probability of identity (PI) (0.1) was observed for locus UDO43 that was the most in-formative locus the highest discrimination power (0.90). The high-est probability of identity (0.85) observed was for locus DCA5 that had the lowest power of discrimination (PD) (0.15) with (Table 3).
Overall, loci probability were generally low (0.30), particularly at loci that have a high allelic number as noted also in previous re-sults (Roubos et al., 2010). Overall, the values observed for the ex-pected and observed heterozygosity, for all three sets of individu-als (0.68 and 0.49), respectively, were somewhat higher than re-ported by the authors using similar sets of SSR markers (Erre et al., 2010; Belaj et al., 2010; Muzzalupo et al., 2010; Baldoni et al., 2009; Zaher et al., 2011 and Erre et al., 2010). Reason for the number of alleles observed in the study could be due to the use of a large num-ber of exotic genotypes.
Wild types have a higher inbreeding coefficient (0.36) than the two cultivated populations, introduced (0.23) and local (0.24). This may be the result of continued breeding of closely related individ-uals since the area in which the wild genotypes grow is far away from cultivated genotypes. In addition, it has the highest number of private alleles and the highest level of genetic diversity found in this area in spite of the low number of wild types. This may be use-ful information for the preservation traits of the wild type in the same genetic pool. The result is that the wild type may then be a source of some genes for potential improvement of local cultivars. Genetic diversity studies of the local ancient olive cultivars (Banilas et al., 2003 and Baldoni et al., 2006) have revealed that only a few of these landraces matched current olive cultivars grown today. These studies comparable to our results, which clearly indicate large differences observed in the Libyan collection.
Distinct groups of local landraces differed from introduced and wild genotypes as indicated in both the neighbor-joining tree (Fig. 7) and the admixture analysis (Fig. 8). This was also noted by (Zaher et al., 2011) distinct clustering of the landraces from the same region a unique genetic background and did not have match-ing genotypes form the other two sets of individuals. In contrast, early (Hannachi et al., 2010) that ‘Roumi’ could be a progeny of ‘Chemlali’, but our results from the dendrogram the major propor-tion of ancient Libyan local landraces did not match any other in-troduced or wild olive genotypes. The local Libyan cultivars may represent early stages of olive cultivation (D ́ıez et al., 2011 and Belaj et al., 2010) that remain as unexploited genetic diversity and
therefore important germplasm resources Among the three sets of individuals (local, introduced and wild) that were assumed to be different not as different as expected. Neighbor-joining tree (Fig. 7) and STRUCTURE analysis (Fig. 8) demonstrated a strong between wild and introduced genotypes. The wild types were genetically more closely related to the introduced. This was unexpected since one would most commonly assume that the local cultivars were de-scended from the native wild types. However, samples of wild-32 and wild -48 were an exception they were phenotypically and ge-netically related to the landraces than the wild type. This may be due to errors of the propagation process. Therefore, the idea of Lib-yan ancient local cultivars maybe descendants from the wild types not supported by either neighbor-joining tree or the structural analyses. This is likely due to the result of gene flow based on geo-graphical proximity over the years. Our results are comparable with previous studies (Hannachi et al., 2008 and Hannachi et al., 2010) that showed there are close genetic relationships between oleaster types and cultivated genotypes using SSR data with NJ method. Although some oleaster types were intermixed within cul-tivated genotypes, others only clustered from wild types alone.
Most of the wild type accessions were collected from the East-ern side of Libya (Fig. 1), which is closer to Europe from which in-troduced genotypes came to Libya in 1954 during the years of col-onization by Italy. D ı́ez (2011) noted the exchange genetic mate-rial between North Africa and Europe took place during the Arab expansion through Andalusia between the eighth and fourteenth centuries. This offers the archaeological evidence to support the gene flow of olives with human migration. Wild olive genotypes are currently thought to have a common gene pool in the entire Medi-terranean Basin (Kole, 2011). This may be why the wild Libyan ac-cessions are closely related to the introduced lines from Europe. Several morphological traits can differentiate between wild and cultivated olive (Hannachi et al., 2008). Phenotypic traits not as in-formative as molecular data and limited in discriminatory power to evaluate the relatedness and the level of genetic similarity (Cor-rado et al., 2009 and Hannachi et al., 2008). In addition, Rao et al. (2009) reported that biometry values alone were unable to differ-entiate between similar genotypes that were evaluated by morpho-logical traits.
It seems, there is a strong correlation of comparison between the genotype and phenotype data (Fig. 9 A and Fig. 9 B) that were based on independent phenotypic stable traits and blocked by structure membership coefficient (1=local, 2=mixed and 3=intro-duced) or structurama partition assignment (1=mixed, 2=intro-duced and 3=local) (Fig. 9 A and Fig. 9 B) respectively. The results showed that stable phenotypic data could be used the same as ge-netic data to assign each individual to a specific group of cultivars based on their origin (local, introduced or wild). The resemblance between molecular and morphological relationships within olive varieties expected when there is a little effect of genetic and envi-ronment interaction observed. Our results are relevant to the most recent olive. Recently, both morphological and molecular aspects have been combined to clarify the identity of genotypes within other crops (Corrado et al., 2009; Hannachi et al., 2010; D ́ıez et al., 2011 and Belaj et al., 2012).
5. Conclusion
The study of local ancient cultivars and wild types of the Libyan collection is increasingly important in order to conserve those gen-otypes as a potential genetic resource; they may have valuable genes that could provide a novel and useful phenotypic traits for advanced plant breeding. This study provides useful information a general molecular database of Libyan olive cultivars. There is a high heterozygosity within the Libyan collection studied, which identified all genotypes with limited similarity. The current set of 10 SSR loci amplified the corresponding microsatellite fragments in all 91 genotypes; also, it can be used to genotype the Libyan olive collection and to assign each individual into a genetic relatedness group. In this study, molecular data led to the clear identification

Salem et al./Libyan Journal of Science & Technology 9:2 (2019) 129141
140
of 91 distinct genotypes (39 local, 36 introduced and 16 wild) out of the 99 accessions included in this study, also it revealed the ex-istence of a high level of genetic variability among Libyan collec-tion. It is interesting that changes of the denominations are more frequently within landraces than other cultivated and wild types. Identification of additional new candidate loci with the use of a ref-erence sample could lead to a more robust molecular database, which could be used to characterize the Libya olive collection. This may then be used to optimize the management strategy of the Lib-yan olive germplasm. The combination based on morphological traits and molecular data were highly useful to separate closely re-lated genotypes and facilitate genetic differentiation among olive genotypes.
Reference
Baldoni, L., Cultrera, N.G., Mariotti, R., Ricciolini, C., Arcioni, S., Ven-dramin, G.G., Buonamici, A., Porceddu, A., Sarri, V., Ojeda, M.A., Trujillo, I., Rallo, I., Belaj, A., Perri, E., Salimonti, A., Muzzalupo,I., Casagrande,A., Lain,O., Messina,R., and Testolin, R. (2009) ‘A consensus list of microsatellite markers for olive genotyping ’, Mol Breeding, 24, pp.213–231.
Baldoni, L., Tosti, N., Ricciolini, C., Belaj, A., Arcioni, S., Pannell, G., Germana, M. A., Mulas, M., and Porceddu, A. (2006) ‘Genetic structure of wild and cultivated olives in the central Mediterra-nean Basin ’, Annals of Botany, 98, pp.935–942.
Banilas, G., Minas, J., Gregoriou, C., Demoliou, C., Kourti, A., and Hatzopoulos, P. (2003) ‘Genetic diversity among accessions of an ancient olive variety of Cyprus ’, Genome 46, pp.370–376.
Belaj, A., del Carmen Dominguez-García, M., Atienza, S. G., Urdíroz, N. M., De la Rosa, R., Satovic, Z., and Del Río, C. (2012) ‘Develop-ing a core collection of olive (Olea europaea L.) based on molec-ular markers (DArTs, SSRs, and SNPs) and agronomic traits ’, Tree Genetics & Genomes, 8(2), pp.365-378.
Belaj, A., Munoz-Diez, C., Baldoni, L., Satovic, Z., and Barranco, D. (2010). ‘Genetic diversity and relationships of wild and culti-vated olives at regional level in Spain ’, Scientia Horti culturae, 124, pp.323–330.
Belaj, A., Satovic, Z., Cipriani, G., Baldoni, L., Testolin, R., Rallo, L., and Trujillo, I. (2003) ‘Comparative study of the discriminating capacity of RAPD, AFLP and SSR markers and of their effective-ness in establishing genetic relationships in olive ’, TheorAppl Genet. 107, pp.736–744.
Besnard, G., Baradat, P., and Berville. A. (2001) ‘Genetic relation-ships in the olive (Olea europaea L.) reflect multifocal selection of cultivars ’, Theor. Appl. Genet. , 102, pp.251–258.
Brownstein, M. J., Carpten, J. D., and Smith, J. R. (1996) ‘Modulation of non-templated nucleotide addition by Taq DNA polymerase: primer modifications that facilitate genotyping ’, Biotechniques, 20(6), pp.1004 -6, 1008-10.
Carriero, F., Fontanazza, G., Cellini, F., and Giori, G. (2002) ‘Identifi-cation of simple sequence repeats (SSRs) in olive (Olea euro-paea L) ’, Theor Appl Genet, 104, pp.301–307.
Cipriani, G., Marrazzo, M.T., Marconi, R., Cimato, A., and Testolin, R. (2002) ‘Microsatellite markers isolated in olive (Olea europaea L.) are suitable for individual fingerprinting and reveal poly-morphism within ancient cultivars ’, Theor. Appl. Genet. 104, pp.223–228.
Corrado, G., La Mura, M., Ambrosino, O., Pugliano, G., Varricchio, P., and Rao, R. (2009) ‘Relationships of companion olive cultivars: comparative analysis of molecular and phenotypic data ’, Ge-nome, 52, pp.692–700.
De La Rosa, R., James, C. M., and Tobutt, K. R. (2002) ‘Isolation and characterization of polymorphic microsatellites in olive (Olea europaea L.) and their transferability to other genera in the Ole-aceae ’, Molecular Ecology Notes, 2, pp .265–267.
D íez, C. M., Trujillo, I., Barrio, E., Belaj, A., Barranco, D., and Rallo, L. (2011) ‘Centennial olive trees as a reservoir of genetic diver-sity. Annals of Botany, 108(5), pp.797-807.
Earl, D. A. (2012) ‘STRUCTURE HARVESTER: a website and pro-gram for visualizing STRUCTURE output and implementing the Evanno method ’, Conservation Genetics Resources, 4(2), pp.359-361.
Ercisli, S., Bencic, D., Ipek, A., Barut, E., and Liber, Z. (2012) ‘Genetic relationships among olive (Olea europaea L.) cultivars native to Croatia and Turkey ’, Journal of Applied Botany and Food Qual-ity, 85(2), pp.144-149.
Ercisli, S., Ipek, A., and Barut, E. (2011) ‘SSR marker-based DNA fin-gerprinting and cultivar identification of olives (Olea europaea L.) ’, Biochem Genet. 49(9-10):555-61.
Erre, P., Chessa, I., Muñoz-Diez, C., Belaj, A., Rallo, L., and Trujillo, I. (2010) ‘Genetic diversity and relationships between wild and cultivated olives (Olea europaea L.) in Sardinia as assessed by SSR markers ’, Genetic Resources and Crop Evolution, 57(1), pp.41-54.
Glaubitz, J. C. (2004) ‘Convert: A user-friendly program to reformat diploid genotypic data for commonly used population genetic software packages ’, Molecular Ecology Notes, 4(2), pp.309-310.
Goudet, J. (2002) ‘Fstat Vision (1.2): A Computer Program to Calcu-late F-Statistics ’, Journal of Heredity, 86, 485-486.
Guichoux, E., Lagache, L., Wagner, S., Chaumeil, P., Léger, P., Lepais, O., and Petit, R. J. (2011) ‘Current trends in microsatellite geno-typing ’, Molecular ecology resources, 11(4), pp.591-611.
Hannachi, H., Breton, C., Msallem, M., Ben El Hadj, S., El Gazzah,M., and Berville, A. (2010) ‘Genetic relationships between culti-vated and wild olive trees (Olea europaea L. var. europaea and var. sylvestris) based on nuclear and chloroplast SSR markers’, Natural Resources, 1, pp. 95-103.
Hannachi, H., Breton, C., Msallem, M., Ben El Hadj, S., El Gazzah, M., and Berville, A. (2008) ‘Differences between native and in-troduced olive cultivars as revealed by morphology of drupes, oil composition and SSR polymorphisms: A case study in Tuni-sia’, Scientia Horti culturae, 116(3), pp.280–290.
http://www.mathcs.citadel.edu/trautmand/stuff/dnapapers/lit-tle.htm
Ipek, A., Barut, E., Gulen, H., and Ipek, M. (2012) ‘Assessment of in-ter-and intra-cultivar variations in olive using SSR markers’, Scientia Agricola, 69(5), pp.327-335.
Jakobsson, M., and Rosenberg, N. A. (2007) ‘CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population struc-ture’, Bioinformatics, 23, pp.1801–1806.
Kole, C. (2011) ‘Wild crop relatives: Genomic and breeding re-sources: Temperate fruits. Springer Heidelberg Dordrecht Lon-don, New York’, Vegetables, pp.217-246.
Leon, L., Garrido, V. A., and Downey, G. (2005) ‘Near infrared spec-troscopy (NIRS) as a promising selection tool in olive breeding programs. FRUTIC, 05, pp.12-16.
Mace, E. S., Hutokshi K. Buhariwalla, H., and Crouch, J. H. (2003) ‘A high-throughput DNA extraction protocol for tropical molecu-lar breeding programs’, Plant Molecular Biology Reporter, 21, pp.459a–459h.
Mariotti, R.; Cultrera, N. G. M.; Munoz-Diez, C.; Baldoni, L. and Ru-bini, A. (2010). ‘Identification of new polymorphic regions and differentiation of cultivated olives (Olea europaea L.) through platome sequence comparison’, BMC Plant Biol, 10, pp. 211.
Muzzalupo, I., Chiappetta, A., Benincasa, C., and Perri, E. (2010) ‘In-tra-cultivar variability of three major olive cultivars grown in different areas of central-southern Italy and studied using mi-crosatellite markers’, Scientia Horticulturae, 126(3), pp.324-329.

Salem et al./Libyan Journal of Science & Technology 9:2 (2019) 129141
141
Pritchard, J. K., Wen, W., and Falush, D. (2003) ‘Documentation for STRUCTURE software: version 2’, http://pritch.bsd.uchi-cago.edu
Raman, R., Cowley, R. B., Raman, H., and Luckett, D. J. (2014) ‘Anal-yses Using SSR and DArT Molecular Markers Reveal that Ethio-pian Accessions of White Lupin (Lupinus albus L.) Represent a Unique Genepool’, Open Journal of Genetics, 4, pp.87-98.
Rambaut, A. (2012) ‘FigTree version 1.4. 0. Computer program dis-tributed by the author’, http://tree. bio. ed. ac. uk/soft-ware/figtree.
Rao, R., La Mura, M., Corrado, G., Ambrosino, O., Foroni, I., Perri, E., and Pugliano, G. (2009) ‘Molecular diversity and genetic rela-tionships of southern Italian olive cultivars as depicted by AFLP and morphological traits’, J. Hortic. Sci. Biotechnol, 84(3), pp.261-266.
Roubos, K., Moustakas, M., and Aravanopoulos, F. A. (2010) ‘Molec-ular identification of Greek olive (Olea europaea) cultivars based on microsatellite loci’, Genet. Mol. Res, 9, pp.1865-1876.
Sarri, V., Baldoni, L., Porceddu, A., Cultrera, N.G.M., Contento, A., Frediani, M., Belaj, A., Trujillo, I., and Cionini, P.G. (2006) ‘Mi-crosatellite markers are powerful tools for discriminating among olive cultivars and assigning them to geographically de-fined populations’, Genome, 49, pp.1606-1615.
Sefc, K. M., Lopes, S., Mendonca, D., Dos Santos, M. R., Machado, M. L. D., and Machado, A. D. (2000) Identification of microsatellite loci in olive (Olea europaea) and their characterizationin Italian and Iberian olive trees’, Mol. Ecol. 9, pp.1171–1173.
Viljoen, G. J., Nel, L. H., and Crowther, J. R. (2005) ‘Molecular diag-nostic PCR handbook Dordrecht’, Springer, pp. 13-14.
Zaher, H., Boulouha, B., Baaziz, M., Sikaoui, L., Gaboun, F., and Udupa, S. M. (2011) ‘Morphological and genetic diversity in ol-ive (Olea europaea subsp. europaea L.) clones and varieties’, Plant Omics Journal, 4(7), pp.370-376.