Chapter 2 Matter and Change 2.1 Properties of Matter 2.2 Mixtures 2.3 Elements and Compounds 2.4...
-
Upload
baldwin-hood -
Category
Documents
-
view
224 -
download
5
Transcript of Chapter 2 Matter and Change 2.1 Properties of Matter 2.2 Mixtures 2.3 Elements and Compounds 2.4...

Chapter 2Matter and Change
2.1 Properties of Matter2.2 Mixtures2.3 Elements and Compounds2.4 Chemical Reactions

2.1 Properties of Matter
Objectives:Identify properties of matter as extensive and intensiveDefine physical propertiesDifferentiate between the 3 states of matter

KEY TERMS
Mass
Volume
Extensive Property
Intensive Property
Substance
Physical Property
Solid
Liquid
Gas
Vapor
Physical Change

Definitions
Mass (m) – the amount of matter an object contains (a numerical value of its inertia)Volume(V) – the amount of space an object occupiesExtensive property - a property that depends on the amount of matter an object containsIntensive property – a property that depends on type of matter an object containsSubstance – matter with uniform propertiesPhysical property - a quality or condition of a substance that can be observed without changing the composition of the substance

Definitions cont.
Physical change - a change that do not affect the composition of a substanceChemical property – a quality or condition of a substance that can be observed with changing the composition of the substance

In this lesson, you will learn how properties can be used to classify and identify matter.
CHEMISTRY & YOUCHEMISTRY & YOU
Why are windows made of glass?

Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved..
What you observe when you look at a particular sample of matter is its properties.
Is a solid shiny or dull? Does a liquid flow quickly or slowly? Is a gas odorless, or does it have a smell?
Describing Matter

Some Criteria for the Classification of Matter
Properties
State (solid, liquid, gas)
Composition

Intensive and Extensive Properties
Sulfur

Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved..
Recall that matter is anything that has mass and takes up space.
The mass of an object is a measure of the amount of matter the object contains.
Extensive Properties
– The mass of a basketball is greater than the mass of a golf ball.

Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved..
The volume of an object is a measure of the space occupied by the object.
The volume of a basketball is greater than the volume of a golf ball.
Extensive Properties

Who has a greater volume?

Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved..
Mass and volume are both examples of extensive properties.
An extensive property is a property that depends on the amount of matter in a sample.
Examples: mass and volume
Extensive Properties

Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved..
An intensive property is a property that depends on the type of matter in a sample, not the amount of matter.
Examples include:
- Hardness of an object -Color- Softness -Boiling point- Absorbency -Odor
Intensive Properties

Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved..
Matter that has a uniform and definite composition is called a substance.
Aluminum and copper are examples of substances, which are also referred to as pure substances.
Identifying a Substance

Examples of Physical Properties
A physical property is a quality or condition of a substance that can be observed or measured without changing the substance’s composition.
Examples: Color, odor, hardness, density, melting point, boiling point, state, solubility.

Substance State Color Melting
Point (C°)
Boiling Point (C°)
Density (g/cm3)
Oxygen O2 Gas Colorless -218 -183 0.0014
Mercury Hg Liquid Silvery-white
-39 357 13.5
Bromine Br2 Liquid Red-brown -7 59 3.12
Water H2O Liquid Colorless 0 100 1.00
Sodium Chloride
NaCl Solid White 801 1413 2.17
Example: Physical Properties

What are the 3 states of matter?
• Solid
• Liquid
• Gas

States of Matter
Property Solid Liquid Gas
Definite mass Yes Yes Yes
Definite volume Yes Yes No
Definite shape Yes No No
Compressible No No Yes
Molecule Drawing
Examples rock, cookie, gold bar
Water, oil Air, helium, nitrogen

States of Matter
Solid fixed shape and volume,
incompressibleLiquid
fixed volume, takes the shape of its container
Gas takes the volume and shape of its
container


Bromine
Gas (Vapor)
Liquid

Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved..
The melting point of gallium metal is 30˚C. The figure at left shows how the heat from a person’s hand can melt a sample of gallium.

Change of Phase
Melting solid liquidCondensation gas liquid
Freezing liquid solid
Deposition gas solid
Evaporation liquid gasSublimation solid gas
*Boiling: Evaporation occurring beneath the liquid’s surface.

Is changing phase a physical or chemical change?

Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved..
During a physical change, some properties of a material change, but the composition of the material does not change.
Melting is a physical change. Words such as boil, freeze, melt, and condense
are used to describe physical changes. So are words such as break, split, grind, cut,
and crush.
Physical Changes

Do Now
Name the phase change you see in the images.

Name the phase change

Name the phase change



Video - https://www.youtube.com/watch?v=wln6WSv-cro

Chemical Properties
Chemical property – a quality or condition of a substance that can be observed with changing the composition of the substance
Examples:- Flammability- rusting - reactivity- corrosion

2.2 MIXTURES
Objectives: Categorize samples of matter into substance or mixture Differentiate between homogeneous and heterogeneous Describe two ways that components of mixtures can be
separated

KEY TERMS Mixture - physical blend of 2 or more substances Heterogeneous – mixture in which the composition is not uniform
throughout Homogeneous – mixture with a uniform composition throughout Phase – a part of a mixture with uniform composition Solution – another name for homogeneous mixture Filtration - a process that separates a solid from a liquid Distillation - a process that separates a solid dissolved in a liquid/
separation of 2 miscible liquids with different boiling
temperatures Miscible - 2 or more liquids that can form a homogeneous mixture

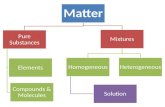
(Pure) Substance
Matter that has a uniform and definite composition.
Elements
Compounds

Classification of Matter(by composition)

MixtureA physical blend of two or more
substances.

Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved.
.
Describing MatterDescribing Matter
Most samples of matter are mixtures.
• Some mixtures are easier to recognize than others.
• You can easily recognize chicken noodle soup as a mixture of chicken, noodles, and broth.

Copper II Sulfate and its solution in water.

Mixtures can be classified as Homogenous mixtures
Heterogeneous mixtures

A homogeneous mixture is a mixture in which the composition is the same throughout.
Examples: olive oil, lemonade, coffee, air, alloys

Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved.
.
Describing MatterDescribing Matter
Homogeneous Mixtures
The substances in the olive oil are evenly distributed throughout the mixture.
• So, olive oil doesn’t look like a mixture.

Example: Stainless Steel
A homogeneous mixture of:
-Iron (Fe)
-Chromium (Cr)
-Nickel (Ni)

heterogeneous mixture: a mixture that is not uniform in composition; components are not evenly distributed throughout the mixture
Examples:
Oil and vinegar (mixed together)
Salad
Chicken soup
Note: Mixtures can be physically separated

Separation Methods
Use differences in the physical properties of the components of the mixture.

Example: Separate iron filings from sulfur using a magnet.

Filtration: separates a solid from a liquid in a heterogeneous mixture

Distillation: -separate dissolved solids from a liquid -uses boiling and condensation.

Distillation of Crude Oil (Refining)
Crude Oil is a mixture of Hydrocarbons

Distillation of Crude Oil

2.3 Elements and Compounds

Elements
The simplest substances.
Can not be separated into simpler
substances.
Building blocks of all matter.
More than 100 known elements.
Represented by chemical symbols.

Chemical Symbols of Elements
System started by Jons Berzelius (Sweden, 1779-1848)
One or two first letters of name of the element.
Many elements names have roots from: Latin, Greek, mythology, geography, names of scientists.

Examples:
Americium, Am
Einsteinium, Es
Bromine, Br
Helium, He
Lead(Plumbum), Pb
Niobium, Nb
Iron (Ferrum), Fe
Mendelevium, Md

Compound A substance that contains two or more
elements chemically combined.
Can be broken down into simpler substances
Compounds have different properties from the individual substances.
(Ex: H2O -> liquid O2 -> gas H2 -> gas)

When the elements sodium and chlorine combine chemically to form sodium chloride, there is a change in composition and a change in properties.
Properties of Compounds
• Sodium chloride (commonly known as table salt) is a white solid.
Distinguishing ElementsDistinguishing Elementsand Compoundsand Compounds

Breaking down Compounds
Breaking down NaCl
•Sodium is a soft gray metal.
Distinguishing ElementsDistinguishing Elementsand Compoundsand Compounds

Breaking down NaCl
Breaking down Compounds
• Chlorine is a pale yellow poisonous gas.
Distinguishing ElementsDistinguishing Elementsand Compoundsand Compounds

Substance or mixture?
If composition is fixed and may not change
substance


2.4 Chemical Properties and Chemical Changes

H2O composition is fixed- compound
Gaseous Phase Liquid Phase

Chemical Properties
Chemical property - a quality or condition of a substance that can be observed with changing the composition of the substance
Example: Magnesium reacts with oxygen to form magnesium oxide.

Magnesium Mg

Burning of Magnesium2Mg+ O2 2MgO

Physical Change vs Chemical Change
Physical change - Composition does not change.
May be reversible or irreversible.Example of reversible:
Liquid water -> steamSteam -> liquid water

Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved.
.
Chemical ChangesChemical Changes
When charcoal is broken into smaller pieces, the change is a physical change.
• During a physical change, the composition of the matter never changes.

Chemical Change
Chemical change - a change that produces matter with a different composition than the original matter.Atoms rearrange themselves into new combinations.

Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved.
.
Chemical ChangesChemical Changes
- Burn - decompose
- rot - ferment
- rust - corrode
- explode
Words that describe chemical changes:Words that describe chemical changes:

Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved.
.
Chemical ChangesChemical Changes
When the charcoal is heated and burned, a chemical change occurs.
• The substances in charcoal react with oxygen in the air to form other substances.

Burning of MethaneCH4 +2O2 CO2 + 2H2O

Burning of MethaneCH4 + 2O2 CO2 + 2H2O

Recognizing a Chemical Change
energy exchange production of a gascolor changeformation of a precipitate

Formation of a Precipitate
Cu(OH)2
Precipitate

The Law of Conservation of Mass (Antoine Lavoisier)
In any chemical or physical change, mass is neither created or destroyed
Mass is CONSTANT

END OF SHOW

Example: H2O