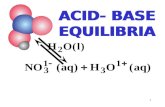Chapter 18.3 : Equilibria of Acids, Bases, and Salts
-
Upload
chris-foltz -
Category
Education
-
view
1.439 -
download
1
description
Transcript of Chapter 18.3 : Equilibria of Acids, Bases, and Salts

Chapter 18.3
Equilibria of Acids, Bases, and Salts

Objectives:
1.Explain the concept of acid ionization constants, and write acid ionization equilibrium expressions.
2.Review the ionization constant of water.
3.Explain buffering.
4.Compare cation and anion hydrolysis.

Ionization Constant of a Weak Acid• The term Ka is called the acid ionization constant.
• The acid ionization constant, Ka , is constant for a specified temperature but has a new value for each new temperature.
• Acetic acid, CH3COOH, is a weak acid and most of the CH3COOH molecules remain unionized.
• An acetic solution contains CH3COOH molecules, H3O+ ions, and acetate ions, CH3COO.

• The equilibrium equation for the ionization of acetic acid is
–
3 2 3 3CH COOH H O H O CH COO + +
–3 3
a3
[H O ][CH COO ]K
[CH COOH]
• Because water is the solvent, one can assume that the molar concentration of H2O molecules remains constant. The concentration of water is not included in the equilibrium expression.
• The equation for Ka is

• Ionization data and constants for some dilute acetic acid solutions at 25°C are given below.
• The numerical value of Ka is almost identical for each solution molarity shown.

• At constant temperature, an increase in the concentration of CH3COO− ions through the addition of sodium acetate, NaCH3COO, disturbs the equilibrium.
+ + 3 2 3 3CH COOH H O H O CH COO
• This disturbance causes a decrease in [H3O+] and an increase in [CH3COOH].
• The equilibrium is reestablished with the same value of Ka. But there is a higher concentration of nonionized acetic acid molecules and a lower concentration of H3O+ ions.• Changes in the hydronium ion concentration
affect pH.

BuffersBuffered solutions resist changes in pH.
• Buffered solutions contains both a weak acid and a salt of the weak acid
• example: CH3COOH and NaCH3COO–
• Buffered solution can react with either an acid or a base. When small amounts of acids or bases are added, the pH of the solution remains nearly constant.


Buffered Vs. Nonbuffered Solutions

• If a small amount of acid is added to the acetic acid–sodium acetate solution, acetate ions react with most of the added hydronium ions to form nonionized acetic acid molecules.
aq + aq aq + l –3 3 3 2H O ( ) CH COO ( ) CH COOH( ) H O( )
• The hydronium ion concentration and the pH of the solution remain practically unchanged.

• If a small amount of a base is added, the OH− ions of the base react with and remove hydronium ions to form nonionized water molecules. Acetic acid molecules then ionize and mostly replace the hydronium ions neutralized by the added OH− ions.
+ + 3 2 3 3CH COOH H O H O CH COO
• The hydronium ion concentration and the pH of the solution remain practically unchanged.

• A solution of a weak base containing a salt of the base also behaves as a buffered solution.
• Buffer action has many important applications in chemistry and physiology.
• Human blood is naturally buffered to maintain a pH of between 7.3 and 7.5.

Ionization Constant of Water• The self-ionization of water is an equilibrium
reaction.
l + l aq + aq2 2 3H O( ) H O( ) H O ( ) OH ( )
• Equilibrium is established with a very low concentration of H3O+ and OH− ions.
Kw=[H3O+][OH–] = 1.0 10-14

Hydrolysis of Salt• Salts are formed during the neutralization
reaction between a Brønsted acid and a Brønsted base.
• When a salt dissolves in water, it produces• positive ions (cations) of the base from which it was
formed
• negative ions (anions) of the acid from which it was formed
• If the ions formed are from weak acids or bases, they react chemically with the water molecules, and the pH of the solution will have a value other than 7.

• A reaction between water molecules and ions of a dissolved salt is hydrolysis.
• If the anions react with water, the process is anion hydrolysis and results in a more basic solution.
• If the cations react with water molecules, the process is cation hydrolysis and results in a more acidic solution.

Anion Hydrolysis• In the Brønsted sense, the anion of the salt
is the conjugate base of the acid from which it was formed. • It is also a proton acceptor.
• If the acid is weak, its conjugate base (the anion) will be strong enough to remove protons from some of the water molecules.
• An equilibrium is established in which the net effect of the anion hydrolysis is an increase in the hydroxide ion concentration, [OH−], of the solution.

• The equilibrium equation for a typical weak acid in water, HA, is aq + aq aq + aq2 3HA( ) H O( ) H O ( ) A ( )
aK 3[H O ][A ]
[HA]
• The general equilibrium equation is
• The hydrolysis reaction between water and the anion, A−, that is produced by the dissociation of the weak acid, HA, is
aq + aq aq + aq2A ( ) H O( ) HA( ) OH ( )
• The extent of OH− ion formation and the position of the equilibrium depends on the relative strength of the anion, A−.
• The lower the Ka value of HA, the weaker the acid, HA, the stronger its conjugate base, A−

• Aqueous solutions of sodium carbonate are strongly basic.
• The carbonate ions react as a Brønsted base. aq + aq aq + aq23 2 3CO ( ) H O( ) HCO ( ) OH ( )
• The OH− ion concentration increases until equilibrium is established.

Cation Hydrolysis• In the Brønsted sense, the cation of the salt is
the conjugate acid of the base from which it was formed.
• It is also a proton donor.
• If the base is weak, the cation is an acid strong enough to donate a proton to a water molecule to form H3O+ ions.
• An equilibrium is established in which the net effect of the cation hydrolysis is an increase in the [H3O+] of the solution.

• The equilibrium equation for a typical weak base, B, is
–2B( ) H O( ) BH ( ) OH ( )aq + aq aq + aq
bK–[BH ][OH ]
[B]
• Kb, the base dissociation constant is
• The hydrolysis reaction between water and the cation, BH+, produced by the dissociation of the weak base, B, is 2 3BH ( ) H O( ) H O ( ) B( )aq + aq aq + aq
• The extent of H3O+ ion formation and the position of the equilibrium depend on the relative strength of the cation, BH+.
• The lower the Kb value of B, the weaker the base, the stronger its conjugate acid will be.

Hydrolysis in Acid-Base Reactions• Hydrolysis can help explain why the end
point of a neutralization reaction can occur at a pH other than 7.
• Salts can be placed in four general categories, depending on their hydrolysis properties: • strong acid–strong base• strong acid–weak base• weak acid–strong base• weak acid–weak base

• Salts of strong acids and strong bases produce neutral solutions.• Neither the cation of a strong base nor the anion
of a strong acid hydrolyzes appreciably in aqueous solutions.
• The aqueous solutions of salts formed from reactions between weak acids and strong bases are basic.• Anions of the dissolved salt are hydrolyzed by the
water molecules, and the hydroxide-ion concentration increases. This raises the pH of the solution.

Neutralization Curve for a Weak Acid and a Strong Base

• Salts of strong acids and weak bases produce acidic aqueous solutions. • Cations of the dissolved salt are hydrolyzed in the
water solvent, and hydronium ion concentration increases. The pH of the solution is lowered.
• Salts of weak acids and weak bases can produce either acidic, neutral, or basic aqueous solutions, depending on the salt dissolved. • Both ions of the dissolved salt are hydrolyzed
extensively.

Neutralization Curve for a Strong Acid and a Weak Base

Equilibrium and pH Calculations