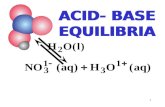Chapter 17 Acid-Base Equilibria
-
Upload
caldwell-newton -
Category
Documents
-
view
54 -
download
2
description
Transcript of Chapter 17 Acid-Base Equilibria

Chapter 17Acid-Base Equilibria

Brønsted-Lowry Acids
HNO3 + H2O → H3O+ + NO3-
Acid

Brønsted-Lowry Bases
+ -( aq) ( l) ( aq) ( aq)3 2 4
BaseNH +H O →NH +OH
Base

Arrhenius
Acids and Bases
NH3+ H2O → NH4+ + OH-
HCl + H2O → H3O+ + Cl-

Acids and Bases
LewisBH3 + NH3 → BH3NH3
Acid Base
BH H
HN H
H
H
: B N H
H
H
H
H
H

Examples of Lewis Acid and Bases
Acid Base

Conjugate Acid-Base Pairs

Conjugate Acid-Base Pairs

Multiprotic acids
A B
A B

Multiprotic acids

Ionization Constants for Acids

Ionization Constants for Bases
] + -
- +2 b( aq) K( aq) ( l) ( l)+
[HB ][OHB +H O OH HB =
[B]

Molecular basis of acid/base strength
Increasing acid strength
HF HCl HBr HI
H–A bond strength (kJ/mol) 569 431 368 297
Electron affinity of A (kJ/mol) 328 349 325 295

Electron affinity
Cl O H Cl O HO+-
Cl O HO
O-
Cl O HO
O
O-
-
--
2+3+
:..
::..
..
..:
..
..
..
..
..
..:
..
..
..
..
..
..:
..
..
....
..
..::
::
hypochlorous acid chlorous acid chloric acid perchloric acid

Summary
3.2 × 10 5

Concepts from Chapter 17
Definitions of acids and bases (Lewis, Brønsted and Arrhenius)
Conjugate acid-base pairs
Ionization constants (Ka, Kb, Kw) and ionization constant expressions
pH, pOH, pKw,
Factors contributing to acidity
Solvent leveling
The strong acids and strong bases (names and formulæ)
Acid-base reactions (strong-strong, strong-weak, weak-strong, and weak-weak)
Calculations
Method of iterative approximation