ICE CH. 5 Thermochemistry of Fuel & Stoichiometric By Dr ...
Ch 17 Thermochemistry(First Class)
-
Upload
smita-sahoo -
Category
Documents
-
view
218 -
download
0
Transcript of Ch 17 Thermochemistry(First Class)
-
7/27/2019 Ch 17 Thermochemistry(First Class)
1/37
Chapter 17
Thermochemistry
-
7/27/2019 Ch 17 Thermochemistry(First Class)
2/37
Thermochemistry:
Study ofenergy changes that occur
during chemical reactions andchanges in state
Section 17.1: The flow of energy
-
7/27/2019 Ch 17 Thermochemistry(First Class)
3/37
energy changes can either occurthrough heat transfer or work
heat (q), energy is transferredfrom a warmer object to a coolerobject (always)
Adding heat increasestemperature
Section 17.1: The flow of energy
-
7/27/2019 Ch 17 Thermochemistry(First Class)
4/37
Kinetic energy vs.potential energy
17.1: Endothermic & Exothermic processes
energy due tomotion
energy due to a substance'schemical composition
potential energy is determined by the strength of repulsive
and attractive forces between atoms
In a chemical reaction, atoms are recombined into new
arrangements that have different potential energies
change in potential energy: due to absorption and release of
energy to and from the surroundings
-
7/27/2019 Ch 17 Thermochemistry(First Class)
5/37
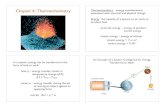
Two parameters crucial in Thermochemistry:
a) system--part of the universe
attention is focused
b) surroundings--everything else in the
universe
System + surrounding = universe
Fundamental goal of Thermochem.: study the
heat flow between the system and its
surroundings
17.1: Endothermic & Exothermic processes
-
7/27/2019 Ch 17 Thermochemistry(First Class)
6/37
IfSystem (energy) Surrounding(energy) by the same amount
the total energy of the universe does notchange
Law of conservation of energy
17.1: Endothermic & Exothermic processes
-
7/27/2019 Ch 17 Thermochemistry(First Class)
7/37
17.1: Endothermic & Exothermic processes
Direction of heat flow is given from the point
of view of the system
So, endothermic process: system absorbs heat
from the surroundings (system heats up)
Heat flowing into the system = +q
Exothermic process: heat is released into thesurroundings (system cools down, -q)
-
7/27/2019 Ch 17 Thermochemistry(First Class)
8/37
17.1: Endothermic & Exothermic processes
Example 1:
A container of melted paraffin wax is allowed tostand at room temperature (r.t.) until the waxsolidifies. What is the direction of heat flow asthe liquid wax solidifies? Is the process
exothermic or endothermic?
Answer: Heat flows from the system (paraffin) tothe surroundings (air)
Process: exothermic
-
7/27/2019 Ch 17 Thermochemistry(First Class)
9/37
17.1: Endothermic & Exothermic processes
Example 2:
When solid Ba(OH)28H2O is mixed in a beakerwith solid NH4SCN, a reaction occurs. Thebeaker quickly becomes very cold. Is thereaction exothermic or endothermic?
Answer: Endothermic
surrounding = beaker and air
System = chemicals within beaker
-
7/27/2019 Ch 17 Thermochemistry(First Class)
10/37
17.1: Units of heat flow
Two units used:
a) calorie (cal)amount of heat required to
raise the temperature of 1g of pure water by1oC
b) joule (j)1 joule of heat raises thetemperature of 1 g of pure water 0.2390oC
Joule = SI unit of energy
-
7/27/2019 Ch 17 Thermochemistry(First Class)
11/37
17.1 Heat capacity & specific heat
Heat capacity is the quantity of heat needed to
raise the temperature of an object exactly 1oC
Heat capacity depends on:
a) mass
b) chemical composition
So, the greater the mass the greater the heat
capacity
eg.: cup of water vs. a drop of water
(cup of water = greater heat capacity)
-
7/27/2019 Ch 17 Thermochemistry(First Class)
12/37
17.1: Heat capacity & specific heat
Specific heat: amount of heat required to raise
the temperature of 1g of a substance by 1oC
Table 17.1 (p.508): List of specific heats of
substances
-
7/27/2019 Ch 17 Thermochemistry(First Class)
13/37
Specific heat calculation
C = q = heat (joules/calories)
m * T mass (g) * Temp. (oC)
T = TfTi (Tf= final temperature)
(Ti = initial temperature)
C = j or cal
(g * oC) (g * oC)
-
7/27/2019 Ch 17 Thermochemistry(First Class)
14/37
Example 1
1. The temperature of a 95.4 g piece of copper
increases from 25.0 oC to 48.0 oC when thecopper absorbs 849 j of heat. What is the
specific heat of copper?
unknown: Ccu
Know:
mass copper = 95.4 g
T = TfTi = (48.0 oC25.0 oC)
= 23.0 oC
q = 849 j
-
7/27/2019 Ch 17 Thermochemistry(First Class)
15/37
Example1
C = q
m * T
C = 849 j
95.4 g * 23.0 oC
C = 0.387 j/g * oC
Sample problem 17.1, page 510
-
7/27/2019 Ch 17 Thermochemistry(First Class)
16/37
Example 2
2. How much heat is required to raise the
temperature of 250.0 g of mercury (Hg) 52oC?
unknown: q
Know:
mass Hg = 250.0 gT = 52.0 oC
CHg = 0.14 j/(g *oC)
-
7/27/2019 Ch 17 Thermochemistry(First Class)
17/37
Example 2
C = qm * T
q = CHg
* m * T
q = 0.14 (j/g * oC) * 250.0 g * 52 oC
q = 1.8 x 103 j (1.8 kj)
Problem #4 page 510
-
7/27/2019 Ch 17 Thermochemistry(First Class)
18/37
Section 17.2
Measuring Enthalpy Changes
-
7/27/2019 Ch 17 Thermochemistry(First Class)
19/37
-
7/27/2019 Ch 17 Thermochemistry(First Class)
20/37
Two types of calorimeters:
a) Constant-Pressure calorimeter (eg. foam cups)
As most reactions occur at constant pressure we can saythat:
A change in enthalpy (H) = heat supplied (q)
So, a release of heat (exothermic) corresponds to adecrease in enthalpy (at constant pressure)
An absorption of heat (endothermic) corresponds to anincrease in enthalpy (constant pressure)
17.2: Enthalpy (measuring heat flow)
-
7/27/2019 Ch 17 Thermochemistry(First Class)
21/37
17.2: Enthalpy (measuring heat flow)
b) Constant-Volume Calorimeters (eg. bomb
calorimeters)
Substance is burned (in the presence of O2) inside a
chamber surrounded by water (high pressure)
Heat released warms the water
Figure 17.6 (p. 512) Bomb calorimeter.
-
7/27/2019 Ch 17 Thermochemistry(First Class)
22/37
17.2: Thermochemical equations
CaO(s) + H2O(l) Ca(OH)2 + 65.2 kJ
Heat released
A chemical equation that includes the enthalpy
change is called
a thermochemical equation
Reactants and products at their usual physical
state (at 25 oC) given at standard pressure (101.3
kPa)
So, the heat of reaction (orH) for the above
equation is -65.2 kJ
-
7/27/2019 Ch 17 Thermochemistry(First Class)
23/37
17.2: Thermochemical equations
So, rewrite the equation as
follows:
CaO(s) + H2O(l) Ca(OH)2(s) 65.2 kJ
Other reactions absorb heat from the surroundings, eg.:
Rewrite to show heat of reaction
2 NaHCO3(s) Na2CO3(s) + H2O(g) + CO2(g)+ 129 kJ
kNaHCO3(s) Na2CO3(s) + H2O(g) + CO2(g)2
-
7/27/2019 Ch 17 Thermochemistry(First Class)
24/37
Amount of heat released/absorbed during a
reaction depends on the number of moles of
reactants involved
eg.:
17.2: Thermochemical equations
kJNaHCO3(s) Na2CO3(s) + H2O(g) + CO2(g)2
258 kJNaHCO3(s) Na2CO3(s) + H2O(g) + CO2(g)4
-
7/27/2019 Ch 17 Thermochemistry(First Class)
25/37
Enthalpy Diagrams
CaO(s) + H2O(l)
H = -65.2 kJ
Ca(OH)2(s)
Exothermic Reaction
Na2CO3(s) + H2O(l) + CO2(g)
H = 129 kJ
2 NaHCO3(s)
Endothermic Reaction
Diagram A:
Enthalpy of reactants greater
than of products
Diagram B:
Enthalpy of reactant less
than of products
-
7/27/2019 Ch 17 Thermochemistry(First Class)
26/37
Physical states of reactants and products must be
stated:
17.2: Thermochemical equations
H2O(l) H2(g)+ 1 O2(g)
2285.8 kJ
H2O(g) H2(g)+ 1 O2(g)
2241.8 kJ
Difference = 44.0 kJ
Vaporization of H2O(l) requires more heat (44.0 kJ)
l 1
-
7/27/2019 Ch 17 Thermochemistry(First Class)
27/37
Example1
kJNaHCO3(s) Na2CO3(s) + H2O(g) + CO2(g)2
1. Calculate the amount of heat in (kJ) required to decompose 2.24 mol
NaHCO3(S)
Known:
2.24 mol NaHCO3 decomposes
H= 129 kJ (2 mol NaHCO3)
Unknown:
H= ?
Solve:
129 kJ = H
2 mol NaHCO3(s) 2.24 mol NaHCO3(s)
H = (129 kJ) * 2.24 mol NaHCO3(s)2 mol NaHCO3(s)
H = 144 kJ
Sample problem 17.3; p. 516
E l 2
-
7/27/2019 Ch 17 Thermochemistry(First Class)
28/37
Example 22. When carbon disulfide is formed from its
elements, heat is absorbed. Calculate theamount of heat in (kJ) absorbed when 5.66 g of
carbon disulfide is formed.
C(s) + 2 S(s) CS2(l) H = 89.3 kJ
Known:
5.66 g CS2 is formed
H= 89.3 kJ (1 mol CS2(l)
)
Molar mass: CS2(l): C = 12.0 g/mol
2 *S = 32.1 g/mol = 64.2 g/mol
76.2 g/mol
Unknown:
H= ?
-
7/27/2019 Ch 17 Thermochemistry(First Class)
29/37
Example 2
Solve:
1. Moles CS2(l) = 5.66g CS2 = 0.0743 mol CS2(l)76.2 g/mol CS2(l)
2. 89.3 kJ = H
1 mol CS2(l) 0.074 mol CS2(l)
H = (89.3 kJ) * 0.074 mol CS2(l)
1 mol CS2(l)
H = 6.63 kJ
-
7/27/2019 Ch 17 Thermochemistry(First Class)
30/37
17.3: Heat in changes of state
Objective:
-Heats of Fusion and Solidification
-Heats of Vaporization and Condensation
-Heat of solution
-
7/27/2019 Ch 17 Thermochemistry(First Class)
31/37
The temperature remains constant when achange of state occurs via a gain/loss of energy
Heat absorbed by 1 mole of a solid during
melting (constant temperature) is the molarheat of fusion (Hfus)
Molar heat of solidification (Hsolid) is the heat
lost by 1 mole of liquid as it solidifies(constant temperature)
So, Hfus = Hsolid
17.3: Heat of fusion and solidification
-
7/27/2019 Ch 17 Thermochemistry(First Class)
32/37
Figure 17.9: Enthalpy changes and changes of
state
17.3: Heat of fusion and solidification
-
7/27/2019 Ch 17 Thermochemistry(First Class)
33/37
H2O(s) fus.01 kJ/molH2O(l)
H2O(l) solid6.01 kJ/molH2O(s)
17.3: Heat of fusion and solidification
-
7/27/2019 Ch 17 Thermochemistry(First Class)
34/37
17.3: Heats of Vaporization and Condensation
Molar heat of vaporization (Hvap): Amount of
heat required to vaporize one mole of a liquid
at the liquids normal boiling point
H2O(l) vap kJ/molH2O(g)
Molar heat of condensation (Hcond): heat released
when 1 mole of vapor condenses
So, Hvap = -Hcond
H2O(l) cond kJ/molH2O(g)
-
7/27/2019 Ch 17 Thermochemistry(First Class)
35/37
Figure 17.10: Heating curve of water
17.3: Heat of vaporization and
condensation
-
7/27/2019 Ch 17 Thermochemistry(First Class)
36/37
17.3: Heat of solution
There is heat released/gained when a solute
dissolves in a solvent
The enthalpy change due to 1 mole of a
substance dissolving: molar heat of solution
(Hsoln)
NaOH(s) soln5.1 kJ/molNa+
(aq) + OH-(aq)
H2O(l)
-
7/27/2019 Ch 17 Thermochemistry(First Class)
37/37
Applications: hot/cold packs
Hot pack:
17.3: Heat of solution
CaCl2(s) soln82.8 kJ/molCa2+
(aq) + 2Cl-(aq)
H2O(l)
Cold pack:
NH4NO3(s) soln25.7 kJ/molNH4+
(aq) + NO3-(aq)
H2O(l)