Breast Patho Lect
-
Upload
rose-ann-mecija -
Category
Documents
-
view
30 -
download
0
description
Transcript of Breast Patho Lect
-
BREAST PATHOLOGY
Edna May Go, MD
-
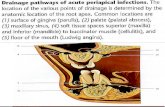
The Female Breast
-
Life Cycle Changes
Prepubertal breast
Consists of duct
system ending in
terminal ducts with
minimal lobule
Beginning of
Menarche
Terminal ducts give
rise to lobules
Interlobular stroma
increases in volume
Paucity of adipose
tissue
-
Life Cycle Changes
Follicular phase
Lobules are
quiescent
After ovulation
Estrogen and
progesterone
Cell proliferation
increases
Vacuolization of
epithelial cells
Intralobular stroma
becomes markedly
edematous
-
Life Cycle Chnages
Pregnancy Lobules increase
number and size
Reversal of stromal-epithelial relationship
End of pregnancy Breast is composed
almost entirely of lobules separated by a relatively scant amount of stroma
By third trimester, secretory vacuoles of lipid material found within epithelial cells of TDLU
After Birth Breast produces
colostrum Changes to milk (higher
fat calories) within first 10 days as progesterone drops
After cessation of lactation Lobules regress and
atrophy
-
Life Cycle Changes
Third decade
Lobules and stroma
start to involute
Old Age
Lobules may totally
disappear, leaving only
ducts to create a
morphologic pattern
similar to male breast
-
Normal breast
extreme
-
Terminal duct lobular unit
-
SMA
-
p63
-
Normal postpubertal female breast, non-lactating. Arrow heads
delineate the lobule. The terminal ductule (short arrow) leads
from the lobule to the duct system (larger arrows). Note how the
pink fibrillar extracellular matrix material (mostly collagen) tends
to wrap concentrically around the ducts and lobules. r Figure 2.
-
Fine needle aspiration
cytology True-Cut biopsy
Examination of frozen section
Mammography
Diagnostic methods
-
woman presents at a clinic with a breast lump, a
needle can be inserted into the area and cells
aspirated without the need for even a local
anaesthetic.
After smearing and staining, the cells are
examined by a pathologist, and if the specimen
is adequate a diagnosis can be made.
Fine needle aspiration cytology
-
Figure 13 Quick-core cutting needle (Cook Medical Inc, Bloomington, IN, USA)
used to obtain core biopsies of soft tissue. (a) Photograph shows the needle set
that has a handle, which enables one-handed control and a spring-loaded
trigger with a rapid-firing mechanism. (b) Close-up photograph of the needle tip
shows the bevelled-point stylet that enables easy penetration into the lesion with
minimal trauma to the surrounding tissue. Firing of the sharp cutting edge of the
cannula facilitates obtaining an intact core tissue sample within the slotted
stylet.
-
Mammography
X-raying of the breasts is used to help in the
diagnosis of both palpable and impalpable
lesions.
the basis of screening programmes, which try to
detect impalpable small breast cancers. i.e.
early tumours.
It is important that the pathologist carefully
examines the tissue to ensure that the lesion
has been removed.
-
Another approach which can be used in the clinic is Tru-Cut biopsy, in which a
core of tissue is removed using a biopsy needle.
Examination of frozen section
A further approach is that of examining the breast lesion
very rapidly by frozen section at the time of surgery.
A small sample is frozen, and sections are cut, stained
and interpreted by a pathologist within a few minutes.
Tru-Cut biopsy
-
http://clinlabs.path.queensu.ca/kgh/pathology/process-1.jpg
-
http://clinlabs.path.queensu.ca/kgh/pathology/photos/frozencutting.jpg
-
Disorder of Development
Milkline remnants
Accessory axillary bresat tissue
Congenital nipple inversion
Spontaneously corrected during pregnancy
Macromastia
Reconstruction or augmentation
Most common complication is formation of thick
fibrous capsule causing cosmetic deformity
-
Site for fibroadenoma and carcinoma
http://www.brooksidepress.org/Products/Military_OBGYN/Textbook/Breast/supnipple2.jpg
-
http://en.wikipedia.org/wiki/Image:Invertednipple.jpg
-
Clinical Presentations of Breast
Disease
Mastalgia or mastodynia
Most common symptom
Palpable mass
Not become palpable until 2cm in diameter
Nipple discharge
Galactorrhea is seen in increased production of
prolactin (pituitary adenoma), hypothyroidism,
endocrine anovulatory syndromes
-
Mammography
Screening recommended at age 40
Principal mammographic signs of breast
cancer
Densities
Invasive carcinoma, fibroadenoma, cysts
Calcifications
Associated with secretory material, necrotic debris,
hyalinized stroma
DCIS is most common malignancy associated with
calcifications
-
Inflammations
Acute mastitis
During early weeks of nursing
Vulnerable to bacterial infection because of
development of cracks and fissures in the nipple
Staphylococcus aureus
Most common
Single or multiple abscesses
Streptococci
Less common
Diffuse spreading infection
-
INFLAMMATIONS
ACUTE MASTITIS
+ BREAST FEEDING
+ CRACKS FISSURES IN NIPPLES,
+ STAPH, STREP
+ LOCALIZED ACUTE
INFLAMMATION
-
Inflammations
Periductal mastitis
Recurrent subareolar abscess, squamous metaplasia of lactiferous ducts, Zuska disease
Seen in smokers (90%)
Vitamin A deficiency
Toxic substances alter differentation of ductal epithelium
Recurrent disease
Fistula tract
Inverted nipple secondary to fibrosis and scarring
Keratin is trapped within ductal system causing dilation and rupture
-
PERIDUCTAL MASTITIS
+ SQUAMOUS METAPLASIA OF
LACTIFEROUS DUCTS
+ RECURRENT SUBAREOLAR
ABSCESS
+ MORE THAN 90% ARE SMOKERS
+ KERATINIZING SQUAMOUS
EPITHELIUM INTO THE ORIFICES
OF THE LACTIFEROUS DUCTS/
GRANULOMATOUS REACTIONS
-
Inflammations
Mammary duct
ectasia
Fifth-sixth decade
Multiparous women
Dilation of ducts,
inspissation of breast
secretions, marked
periductal and
interstitial chronic
granulomatous
inflammation reaction
-
Duct ectasia
-
Mamfoamy histiocytes and the periductal tissue
is infiltrated mary duct ectasia. The dilated duct
contains by lymphocytes
-
Inflammations
Fat necrosis
History of trauma
Hemorrhage (early stage), central liquefactive
necrosis of fat (later), pregressive fibroblastic
proliferation and increase vascularization and
lymphocytic inflitration
Foreign body giant cells, calcification,
hemosiderin
-
This is fat necrosis of the breast. The most common
etiology is trauma. It can be a localized, firm area with
scarring that can mimic a breast carcinoma.
Microscopically, however, fat necrosis consists of irregular
steatocytes with no peripheral nuclei and intervening pink
amorphous necrotic material and inflammatory cells,
including foreign body giant cells responding to the
necrotic fat cells.
-
Inflammations
Lymphocytic mastopathy
Sclerosing lymphocytic lobulitis
Collagenized stroma surrounding atrophic ducts
and lobules
Epithelial basement membrane is thickened
Prominent lymphocytic infiltrate surrounds
epithelium and blood vessels
Most common in women with type 1 diabetes or
autoimmune thyroid disease
-
Lymphocytic mastitis/diabetic
mastopathy characterized by keloid-like fibrosis and prominent lymphocytic infiltrate surrounding breast ducts and
lobules.
-
Inflammations
Granulomatous mastitis
Systemic Wegener granulomatosis, sarcoidosis
Infections Mycobacterial, fungal
Granulomatous lobular mastitis Uncommon breast-limited disease distinguished by
grnulomas involving lobular epithelium
Only affects parous women
Hypersensitivity reaction mediated by alterations in lobular epithelium during lactation
-
Benign Epithelial Lesions
Nonproliferative breast changes (Fibrocystic changes)
Benign morphologic changes
Cysts are most common cause of palpable mass and alarming if solitary, firm and unyielding
Patterns
Cysts
May have apocrine metaplasia
Fibrosis
Caused by cyst rupture
Adenosis
Increase in number of acini per lobule
-
Benign Epithelial Lesions
Lactational adenoma
Palpable masses in pregnant or lactating women
Normal-appearing breast tissue with physiologic
adenosis and epithelial lactational changes
Exaggerated focal response to hormonal
influences
-
PROLIFERATIVE BREAST DISEASE WITHOUT
ATYPIA
+ PROLIFERATION OF DUCTAL EPITHELIUM AND / OR STROMA
WITHOUT EPITHELIAL ABNORMALITY
-
+ MODERATE OR FLORID EPITHELIAL HYPERPLASIA
+ SCLEROSING ADENOSIS
+ COMPLEX SCLEROSING LESIONS
+ PAPILLOMAS
+ FIBROADENOMAS WITH COMPLEX FEATURES
-
Benign Epithelial Lesions
Epithelial
Hyperplasia
Defined as more
than two cell layers
Moderate to florid
More then four cell
layers
-
Benign Epithelial Lesions
Sclerosing adenosis
Number of acini per terminal duct is ingreased to at least twice the number found in uninvolved lobules
Normal lobular arrangement is maintained
Myoepithelial cells are prominent
-
Benign Epithelial Lesions
Complex Sclerosing
Lesion (Radial Scar)
Stellate lesions
characterized by a
central nidus of
entrapped glands in
a hyalinized stroma
Not associated with
prior trauma or
surgery
-
Benign Epithelial Lesions
Papillomas Composed of multiple
branching fibrovascular cores, each having a connective tissue axis lined by luminal and myoepithelial cells
Epithelial hyperplasia and apocrine metaplasia may be present
Small duct papillomas showincreased risk of subsequent carcinoma
-
Proliferative Breast Disease with
Atypia
Atypical ductal
hyperplasia
Histologically
resemble DCIS
Characteristically
limited in extent,
cells are not
completely
monomorphic, fail to
completely fill ductal
spaces
Atypical lobular
hyperplasia
Cells identical to LCIS
Cells do not fill or
distend more than
50% of the acini
within a lobule
May extend into ducts
Increased risk of
developing invasive
carcinoma
-
Breast Lesions and Relative Risk of Developing Invasive
Carcinoma
Pathologic lesion Relative risk (Absolute
lifetime risk)
Breast at risk Modifiers of risk
Nonproliferative breast changes 1.0 (3%) neither
Proliferative disease without
atypia
1.5 to 2.0
(5-7%)
both Increased risk if there is
family history
Decreased risk 10 years
after biopsy
Proliferative disease with atypia
(ADH, ALH)
4.0 to 5.0
(13-17%)
both Increased risk if there is
familty history
Increase risk if
premenopausal
Decreased risk 10 years
after biopsy for ALH
LCIS 8.0 to 10.0
(25-30%)
both Treatment
DCIS 8.0 to 10.0
(25-30%)
ipsilateral Treatment
-
Risk Factors in Carcinoma of the Breast
Age
Rarely found before
25y/o
77% occur in
women50 y/o up
Average age is 64
Age at menarche
Women who reach
menarche
whenyounger than
11 y/o have 20%
increased risk
-
Risk Factors in Carcinoma of the Breast
First live birth
First full term
pregnancy at
younger than 20 y/o
have half the risk of
nulliparous women
or women over the
age of 35 at their
first birth
First-degree
relatives with
breast cancer
Risk of breast
cancer increase
with the number of
affected first
degree relatives
(mother, sister,
daughter)
-
Risk Factors in Carcinoma of the
Breast
Breast biopsies
Increased risk associated with prior breast biopsies showing atypical hyperplasia
Race
Lower in black women but presents at more advanced stage and increased mortality compared to white women
Caucasian women generally have the highest rate of breast cancer
-
Risk Factors in Carcinoma of the
Breast
Estrogen exposure
Postmenopausal hormone replacement therapy slightly increases the risk of breast cancer
Estrogen with progesterone increases the risk more than estrogen alone
Radiation exposure
Threapeutic radiation or radiation after atom bomb exposure increases risk
-
Risk Factors in Carcinoma of the
Breast
Carcinoma of the
contralateral breast
or endometrium
Increases risk
Geographic
influence
US and EU are 4x to
7x higher than other
countries
-
Risk Factors in Carcinoma of the Breast
Diet
Moderate or heavy alcohol consumption increases risk
Due to higher estrogen levels and lower folate levels?
Obesity
Decreased risk in obese women younger than 40 years
Due to anovulatory cycles and lower progesterone levels
Increased risk in postmenopausal obese women
Due to synthesis of estrogen in fat
-
Risk Factors in Carcinoma of the Breast
Exercise
Decreased risk of
breast cancer in
premenopausal
women who
exercise
Breast-feeding
The longer women
breast-feed, the
greater is the
reduction in the risk
of breast cancer
-
Risk Factors in Carcinoma of the Breast
Environmental
toxins
Organochlorine
pesticides have
estrogenic effects
Tobacco
Not associated with
breast cancer
-
Etiology and Pathogenesis
The major risk factors for the development of breast cancer are hormonal and genetic (family history)
About 25% of familial cancers (or around 3%of all breast cancers) can be attributed to 2 highly penetrant autosomal dominant genes: BRCA1 and BRCA2
The general lifetime breast cancer risk for female carriers is 60% to 85%, the median age at diagnosis is about 20 years earlier compared to women without these mutations
-
Etiology and Pathogenesis
Mutated BRCA1 also increases the risk of developing ovarian carcinoma
Male breast cancers are markedly increased only in familites carrying BRCA2 mutations
BRCA1-associated breast cancers are more commonly poorly differentiated, have a syncitial growth pattern with pushing margins, have a lymphocytic response, and do not express hormone receptors or overexpress HER2/neu epidermal growth factor receptor
-
Most common Single Gene Mutations Associated
with Hereditary Breast Cancer
BRCA1 (17q21)
Syndrome: Familial breast and ovarian cancer
Incidence: 1 in 860
~2% of all breast cancers
Function: tumor suppressor, transcriptional
regulation, repair of double-stranded DNA breaks
Breast carcinomas are commonly poorly
differntiated and triple negative (basal-like),
and have p53 mutations
-
Most common Single Gene Mutations Associated
with Hereditary Breast Cancer
BRCA2 (13q12-13)
Syndrome: Familial breast and ovarian cancer
Incidence: 1 in 740
~1% of all breast cancers
Function: tumor suppressor, transcriptional
regulation, repair of double-stranded DNA breaks
Biallelic germline mutations cause a rare form of
Fanconi anemia
-
Most common Single Gene Mutations Associated
with Hereditary Breast Cancer
P53 (17p13.1)
Syndrome: Li-Fraumeni
Incidence: 1 in 5000
-
Most common Single Gene Mutations Associated
with Hereditary Breast Cancer
CHEK2 (22q12.1)
Syndrome: Li-Fraumeni variant
Incidence: 1 in 100
~1% of all breast cancers
Function: cell cycle checkpoint kinase, recognition and repair of DNA damage, activates BRCA1 and p53 by phosphorylation
May increase risk for breast cancer after radiation exposure
Also seen in cancers of prostate, thyroid, kidney, colon
-
Etiology and Pathogenesis
In sporadic tumors, about 50% have decreased or absent expression of BRCA1
Major risk factors for sporadic breast cancer are related to hormone exposure: gender, age at menarche and menopause, reproductive history, breast feeding, and exogenous estrogens
Majority of sporadic tumors occur in postmenopausal women and overexpressed ER
-
Proposed precursor-carcinoma sequence in breast cancer
-
Ductal Carcioma in Situ
Intraductal carcinoma
5 architectural subtypes
Comedocarcinoma Solid sheets of
pleomorphic cells with high-grade nuclei and central necrosis
Necrotic cell membranes commonly calcify
-
Ductal Carcinoma in Situ
Noncomedo DCIS
Monomorphic population of cells with nuclear grades ranging from low to high
Cribriform DCIS Intraepithelial
spaces are evenly distributed and regular in shape (cookie cutter like)
Papillary DCIS Grows into spaces and
lines fibrovascular cores typically lacking the normal myoepithelial cell layer
Micropapillary DCIS Bulbous protrusions
without a fibrovascular core, forming complex intraductal patterns
-
Ductal Carcinoma in Situ
-
Ductal Carcinoma in Situ
-
Lobular Carcinoma in Situ
Not associated with calcifications or a stromal reaction that would form a density
1-6% of all carcinomas
Bilateral in 20-40%
Cells are identical with invasive lobular carcinoma and atypical lobular hyperplasia
LCIS and ILC lack expression of e-cadherin (transmembrane protein responsible for epithelial cell adhesion)
-
Invasive Ductal Carcinoma
Invasive carcinoma, no special type (NST) Cannot be classified as
any other type
70-80% of all breast cancer
Gross: firm to hard, irregular border, small pinpoint foci or streaks of chalky white elastotic stroma at the center, calcifaction may be present
Microscopic: well differentiated tumors consists of tubules lined by minimally atypical cells (typically do not overexpress HER2/neu),
others are composed of anastomosing sheets of pleomorphic cells (typically overexpress HER2/neu)
-
Invasive Ductal Carcinoma
-
Invasive Ductal Carcinoma
-
Invasive Lobular Carcinoma
Usually present like IDC as palpable mass or mammographic density
Reported to have greater incidence of bilaterality (biased)
Incidence increasing in postmenopausal women probably due to hormone replacement therapy
Histologic hallmark is the pattern of single infiltrating tumor cells , often only once cell width (Indian filing)
Lack hormone receptors, aneuploid, may overexpress HER2/neu
Has different metastasis pattern: peritoneum, retroperitoneum, leptomeninges, GIT, ovaries and uterus
-
Invasive Lobular Carcinoma
-
Medullary Carcinoma
Well circumscribed mass
History of rapid growth
Consists of solid synctium-like sheets (occupying more than 75% of the tumor) of large cells with vesicular, pleomorphic nuclei, containing prominent nucleoli and frequent mitoses
Has moderate to marked lymphoplasmacytic infiltrates
Has a pushing (non-infiltrative) border
All medullary carcinoma are poorly differentiated
Lymphovascular invasion is never seen
Has slightly better prognosis than IDC
Aneuploid, absence of hormone receptors, HER2/neu overexpression is not observed
13% carry BRCA1 gene but most are not associated with BRCA1 mutation
-
Medullary Carcinoma
-
Mucinous Carcinoma
1-6% of all breast
cancers
Presents as
circumscribed mass
Seen in older women
Grow slowly
Tumor cells are seen
as clusters and small
islands of cells within
large lakes of mucin
Prognosis is slightly
better than IDC
Incidence slightly
higher in women with
BRACA1 mutation
-
Mucinous Carcinoma
-
Tubular Carcinoma
2% of all breast cancers
10% of all breast cancers with less than 1cm diameter
Women in late forties
Multifocal within one breast (10-56%), bilateral in 9-38%
Axillary metastasis occur in less than 10%
Consists exclusively of well-formed tubules with absent myoeptihelial cell layer
Apocrine snouts are typical, calcifications
95% are diploid and express hormone receptors
Excellent prognosis
-
Tubular Carcinoma
-
Invasive Papillary Carcinoma
~1% of all invasive breast cancers
Invasive carcinoma with papillary
architecture
Overall prognosis is better than IDC
Invasive papillary carcinomas are usually ER
positive and have favorable prognosis
Invasive micropapillary carcinomas are more
likely ER negative and HER2 positive with
lymph node metastates and poorer prognosis
-
Metaplastic Carcinoma
-
Major Prognostic Factors
Invasive carcinoma or in situ disease
In situ by definition are confined to ductal system and cannot metastasize
Distant metastasis
Once distant metastases are present, cure is unlikely
Lymph node metastases
Axillary lymph node status is the most important prognostic factor for invasive carcinoma in the absence of distant metastases
Sentinel node is highly predictive of the status of the remaining nodes
Macrometastases (>0.2cm) are of proven prognostic importance
~10-20% without axillary lymph node metastases have a recurrence outside of breast
Metastasis occur via internal mammary lymph nodes or hematogenously
-
Major Prognostic Factors
Tumor size Second most important
prognostic factor and is independent from lymph node status
Women with node-negative carcinomas under 1cm in diameter have a prognosis approaching that of women without breast cancer (10 year survival rate is 90% without treatment)
Locally advance disease Tumors invading into skin
or skeletal muscle are frequently associated with concurrent or subsequent distant disease
Inflammatory carcinoma Breast cancers presenting
with breast swelling and skin thickening due to dermal lymphatic involvement have a particularly poor prognosis
-
Minor Prognostic Factors
Histologic subtypes Tubular, mucinous,
medullary, lobular, papillary
Tumor grade Nottingham Histologic
Score (Scarf-Bloom-Richardson)
Combines nuclear grade, tubule formation and mitotic rate
Estrogen and Progesterone receptors Hormone receptor-
positive cancers have a slightly better prognosis than hormone receptor-negative cancers
ER-positive cancers are less likely to respond to chemotherapy
-
Minor Prognostic Factors
HER2/neu
Human epidermal growth factor receptor 2, c-erb B2, neu)
Transmembrane glycoprotein involved in cell growth control
Overexpression is associated with amplification of the gene on 17q21
Overexpression is associated with poorer survival but its main importance is as a predictor of response to agents that target it [e.g. Trastuzumab (Herceptin) or lapatinib]
Lymphovascular invasion
Strongly associated with the presence of lymph node metastases
Poor prognostic factor in women without lymph node metastases
Risk factor for local recurrence
-
Minor Prognostic Factors
Proliferative rate Tumors with high
proliferation rates have a worse prognosis
Methods to asses prolifetation Mitotic count
Immunohistochemical detection of cellular proteins produced during cell cycle (cyclins, Ki-67)
Flow cytometry as the S-phase fraction
Thymidine labeling index
DNA content Determined by flow
cytometry or image tissue analysis
Tumors with a DNA index of 1 have the same total amount of DNA as normal diploid cell
Aneuploid tumors are those with abnormal DNA indices and have a slightly worse prognosis
-
Minor Prognostic Factors
Response to
neoadjuvant therapy
Alternative approach
wherein patient is
treated before
surgery
Most likely to respond
well are poorly
differentiated, ER
negative tumors with
necrosis
Gene expression
profiling
Can predict survival
and recurrence-free
interval
Identifies patient who
are most likely to
benefit from
particular type of
chemotherapy
-
Stromal Tumors
Fibroadenoma
Most common benign tumor of the female breast
Epithelium is hormonally responsive
Characteristic large lobulated (popcorn) calcifications on mammography
Well circumscribed, rubbery mass
Stroma is usually delicate, cellular and often myxoid, enclosing glandular and cystic spaces lined by epithelium
-
Stromal Tumors
Phyllodes Tumor Arise fromintralobular
stroma like fibroadenoma
Cystosarcoma phllodes
Phyllodes (Greek, leaflike)
Varies in size, larger lesions often have bulbous protrusions
Distinguished fromfibroadenoma on the basis of cellularity, mitotic rate, nuclear pleomorphism, stromal overgrowth and infiltrative borders
-
Stromal Tumors
Benign stromal lesions
Pseudoangiomatous stromal hyperplasia
Fibrous tumors
Myofibroblastoma
Lipoma
Hamartoma
Fibromatosis
Clonal proliferation of fibroblasts and myofibroblasts
Locally aggressive but do not metastasize
Sarcomas
Malignant tumors of the extrinsic connective tissue of the breast
Angiosarcoma, rhabdomyosarcoma, liposarcoma, leiomyosarcoma, chondrosarcoma, osteosarcoma
Sarcomatous differentiation is seen in phyllodes tumor and metaplastic carcinomas
-
Other malignant tumors
Lymphomas
Mostly of large cell type of B-cell origin
Young women with Burkitt lymphoma may present with massive bilateral breast involvement and are often pregnant or lactating
Metastases
Most frequent nonmammary metastases are melanomas and lung cancer
-
Male Breast
Gynecomastia Enlargement of male
breast Proliferation of dense
collagenous connective tissue
Marked micropapillary hyperplasia of ductal linings
Seen in puberty, very aged, hyperestrinism (esp. in liver cirrhosis), Klinefelter syndrome (XXY), functioning testicular neoplasms
-
Male Breast Cancer
Frequency ratio to female breast cancer is less than 1:100
Risk factors similar to those in women
First degree relatives with breast cancer, decreased testicular function, exposure to exogenous estrogens, increasing age, infertility, obesity, prior benign breast disease, exposure to ionizing radiation, residency in Western countries
Gynecomastia is not a risk factor
4-14% are attributed to germ line BRCA2 mutations
ER positivity is more common in male breast cancer (81%)
Because breast epithelium in men is limited to large ducts near the nipple, cancer usually present as a palpable subareolar mass, 2-3cm in diameter
Prognostic factor are similar in men and women
-
Molecular classification
Studies of breast cancers using gene
expression profiling have identified several
major breast cancer subtypes
.The best characterized of these have been
designated :
luminal A, luminal B, HER2 and basal-like
-
basal-like breast cancers as a distinct group.
These tumors are invasive ductal carcinomas
that feature high-histologic grade,
solid architecture,
absence of tubule formation,
high mitotic rate,
a stromal lymphocytic infiltrate,
a pushing border,
geographic zones of necrosis
and/or a central fibrotic focus, and little or
no associated DCIS
-
-these tumors are typically ER-, PR-, and HER2-
negative (triple negative)
and show expression of basal cytokeratins,
EGFR, and other basal-related genes
-approximately 80% of BRCA1-associated breast
cancers cluster with the basal-like group
-
Luminal HER2 Basal
Gene Expression Patterns High expression of hormone receptors
and associated genes (luminal A>
luminal B)
High expression of HER2 and other
genes in amplicon
Low expression of ER and associated
genes
High expression of basal epithelial genes,
basal cytokeratins
Low expression of ER and associated
genes
Low expression of HER2
Clinical Features ~70% of invasive breast cancers
ER/PR positive
Luminal B tend to be higher-histologic
grade than luminal A
Some overexpress com-HER2 (luminal
B)
~15% of invasive breast cancers
ER/PR negative
More likely to be high grade and node-
positive HER2 overexpression and gene
amplification
~15% of invasive breast cancers
ER/PR/HER2 negative
BRCA1-associated cancers
Particularly common in African
American women
Treatment Response and Outcome Respond to endocrine therapy (response
may be different for luminal A and
luminal B)
Response to chemotherapy variable
(luminal B> luminal A)
Favorable prognosis
Respond to trastuzumab (Herceptin)
Respond to anthracycline-based
chemotherapy
Poor prognosis
No response to endocrine therapy or
trastuzumab (Herceptin)
?Response to platinum-based
chemotherapy
Poor prognosis
Table 10.6 Major Molecular Categories of Breast Cancer Determined by
Gene Expression Profiling
-
At the present time, the clinical value of characterizing invasive breast
cancers beyond routine histopathologic type and ER, PR, and HER2
status has not been established.
Molecular Category
Luminal A Luminal B HER2 Basal-like*
ER + + - -
PR + + - -
HER2 - + + -
*Additionally performing immunostains for cytokeratin (CK)5/6 and epidermal growth factor receptor (EGFR) helps to
define more precisely tumors in the basal-like group which in addition to being ER-, PR-, and HER2-negative are positive
for CK5/6 and/or EGFR.
Table 10.7 Immunophenotyping as a Surrogate for Molecular
Category Using Estrogen Receptor, Progesterone Receptor
and HER2 Status
-
Thank you.