B di II:Bonding II: Molecular Geometry and Bonding Theories · The Bonding π2p and antibonding,...
Transcript of B di II:Bonding II: Molecular Geometry and Bonding Theories · The Bonding π2p and antibonding,...

27-Nov-11
1
Chapter 9
B di II:Bonding II:Molecular Geometry and Bonding Theories
Dr. A. Al-Saadi 1
Molecular Orbital Theory
Molecular orbital theory : Atomic orbitals (AO)
Chapter 9 Section 6
combine to form new molecular orbitals (MO) which are spread out over the entire molecule.
Molecular orbitals (MO) describe the properties of the entire molecule, and not the properties of individual atoms.
Dr. A. Al-Saadi 2
Molecular orbitals (wave functions) result from adding and/or subtracting atomic orbitals (wave functions).

27-Nov-11
2
Molecular Orbital Theory
Molecular orbitals:
Chapter 9 Section 6
have specific shapes and energies.
can have a maximum of two electrons with opposite spins.
are equal to the number of atomic orbitals they have been composed from “the number
Dr. A. Al-Saadi 3
y pof orbitals is conserved”.
Our study about MO theory is restricted to diatomic molecules.
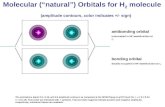
Molecular Orbitals from s Atomic Orbitals
Chapter 9 Section 6
H + H Hydrogen l l
H-H 2
1s1 1s1
bonding orbital
antibonding orbital
*s1difference
molecule
ergy
1s2
Dr. A. Al-Saadi 4
g
s11s 1s
H atomic orbitals H2 molecular orbitals
sum
En
e

27-Nov-11
3
σ1s Molecular Orbital
Chapter 9 Section 6
Hydrogen l l
“Bonding” molecular orbital.
molecule
Dr. A. Al-Saadi 5
g
Constructive combination of “ns” AOs.
High electron density “high probability” between the two nuclei.
Lower energy and more stable than the AOs that were added.
σ*1s Molecular Orbital
Chapter 9 Section 6
Hydrogen l l
“Antibonding” molecular orbital.
Destructive combination of “ns” AOs
molecule
node
Dr. A. Al-Saadi 6
Destructive combination of ns AOs.
Very low electron density “low probability” between the two nuclei. The electron density pulls the two nuclei in opposite directions.
Higher energy and less stable than the AOs that were subtracted.

27-Nov-11
4
σ1s and σ*1s Molecular Orbitals
Chapter 9 Section 6
Hydrogen l l
Showing all the MOs in a molecule can result in a
molecule
Dr. A. Al-Saadi 7
gcomplicated picture.
Energy diagrams / energy levels are often used to represent MOs in the molecule.
MOs in the Hydrogen Molecule
Chapter 9 Section 6
Dr. A. Al-Saadi 8
Energy diagrams / energy levels are often used to represent MOs in the molecule.

27-Nov-11
5
Bond Order
Chapter 9 Section 6
Dr. A. Al-Saadi 9
bond order = 1/2 (2 – 0) = 1single bond
bond order = 1/2 (2 – 2) = 0no bond
Bond Order
The higher the value of the bond order, the more stable the molecule and the stronger the bond.
Chapter 9 Section 6
g
Dr. A. Al-Saadi 10
bond order = 1/2 (2 – 0) = 1single bond
bond order = 1/2 (2 – 2) = 0no bond
MO theory predicts Li2 to be a stable molecule whereas Be2 to be not.

27-Nov-11
6
Molecular Orbitals from p Atomic Orbitals
Chapter 9 Section 6
Some molecules, such as B2, combine their pAOs to generate new MOs. (B: 1s22s22p1).g ( p )
Recall the shape of the 2p orbitals.
Dr. A. Al-Saadi 11
Molecular Orbitals from p Atomic Orbitals
Chapter 9 Section 6
Two pairs of parallel p orbitals can overlap sideways, and the third pair can overlap head-on.y , p p
σ2p
Dr. A. Al-Saadi 12
π2p

27-Nov-11
7
Head-On Overlap for p MOs
Chapter 9 Section 6
σ*2
node
Th bi l h l h d d MO
σ2p
σ 2p
Ene
rgy
Dr. A. Al-Saadi 13
The two p orbitals that overlap head-on produce two σ MOs:
one bonding, σ2p (constructive combination), and
one antibonding, σ*2p, (destructive combination).
Head-On Overlap for p MOs
Chapter 9 Section 6
Dr. A. Al-Saadi 14
Both bonding σ2p and antibonding σ*2p MOs can be shown
together in one diagram. They would look kind of complicated.

27-Nov-11
8
Sideway Overlap for p MOs
Chapter 9 Section 6
node
π*2p
π2p
2p
Ene
rgy
Dr. A. Al-Saadi 15
Two p orbitals that lie parallel overlap to produce two π MOs:
one bonding , π2p (constructive combination), and
one antibonding, π*2p (destructive combination).
Sideway Overlap for p MOs
Chapter 9 Section 6
The Bonding π2p and antibonding, π*2p MOs can be shown
together in one diagram They would look kind of
Dr. A. Al-Saadi 16
together in one diagram. They would look kind of complicated.
Remember that you have two Bonding π2p MOs (along the yand z-axes) and two antibonding, π*
2p MOs (also along the yand z-axes), giving four π MOs in total.

27-Nov-11
9
Molecular Orbital Diagram
Chapter 9 Section 6
MOs generated from the combination of pthe combination of pAOs are always higher in energy than MOs generated form the combination of sAOs.
Antibonding MOs
Dr. A. Al-Saadi 17
Antibonding MOs are higher in energy than bonding MOs.
Molecular Orbital Diagram
Chapter 9 Section 6
The order of MO energies assumes noenergies assumes no interaction taking place between p and sorbitals.
In atoms of smaller nuclear charges (Li, B C and N) the s
Dr. A. Al-Saadi 18
B, C and N) the sorbitals are held less tightly by the nucleus and some s-pinteraction takes place. O2, F2, Ne2 Li2, B2, C2 , N2
s and p orbitals do not interact
s and p orbitals do interact

27-Nov-11
10
Molecular Orbital Diagram of O2
Chapter 9 Section 6
When filling the MO levels, you have to:y
Count the number of valence electrons,
Start with the lower energy orbitals first,
Follow Hund’s rule, and
P t t th t
Dr. A. Al-Saadi 19
Put not more than two electrons in one MO.
Molecular Orbital Diagram of N2
Chapter 9 Section 6
The order of these two MOs are switched
Dr. A. Al-Saadi 20

27-Nov-11
11
Paramagnetism and Diamagnetism
Paramagnetism causes the substance to be attracted to a
Chapter 9 Section 6
magnetic field.
Diamagnetism causes the substance to be repelled from a magnetic field.
Paramagnetism is
Dr. A. Al-Saadi 21
Paramagnetism is associated with unpairedelectrons.
Diamagnetism is associated with paired electrons.
Liquid oxygen, O2(l), is attracted to the poles of a
magnet because O2 is paramagnetic.
Molecular Orbital Diagram
Molecular orbital theory helps
Chapter 9 Section 6
Molecular orbital theory helps you predict several important properties of the substance.
Bond order (bond length and bond strength).
Bond enthalpy (bond energy)
Dr. A. Al-Saadi 22
Magnetic properties.
MO diagram of O2

27-Nov-11
12
Molecular Orbital Diagrams
Chapter 9 Section 6
Dr. A. Al-Saadi 23
Exercises
Chapter 9 Section 6
Predict the bond order and magnetism of Ne2.
Bond order
= (8 – 8) / 2 = 0
According to the MO theory, Ne2 doesn’t exist.
Ne2
Dr. A. Al-Saadi 24

27-Nov-11
13
Exercises
Chapter 9 Section 6
Give the electron configurations and bond orders for O2, O2
+, and O2–.
O2 O2+ O2
–
Dr. A. Al-Saadi 25
Exercises
Chapter 9 Section 6
Predict the bond order and magnetism of P2.
Bond order
= (8 – 2) / 2 = 3
According to the MO theory, P2 exists and it is diamagnetic.
P2
Dr. A. Al-Saadi 26

27-Nov-11
14
Bonding in Heteronuclear Diatomic Molecules
Chapter 9 Section 6
MO model can be expanded to diatomic pmolecules with two different nuclei whose electronic natures are not so different.
Carbon monoxide (CO) is expected from
Dr. A. Al-Saadi 27
MO theory to be diamagnetic and to have a bond order of:
(8 – 2) / 2 = 3The MO energy-level
diagram of the CO molecule
Descriptions of Molecules with Delocalized Bonding
Localized electron model assumes that the bonding electron pair is being shared between two atoms (localized).
Chapter 9 Section 7
p g ( )
In several cases, such as O3 and NO3–, the π-electron
bonding pairs are delocalized and are not present at a specific location in the molecule.
Dr. A. Al-Saadi 28

27-Nov-11
15
Descriptions of Molecules with Delocalized Bonding
In molecules with resonance structures,
Chapter 9 Section 7
σ bonds can be viewed to be localized, and
π bonds can be considered to be delocalized.
Dr. A. Al-Saadi 29
Each C atom has 3electron domains
Example: Benzene (C6H6)
Chapter 9 Section 7
electron domains
Hybridization: sp2
Dr. A. Al-Saadi 30













![Bostan'e Saadi [Urdu]](https://static.fdocuments.net/doc/165x107/577c7a831a28abe05495724e/bostane-saadi-urdu.jpg)


