Autoregulation and neurovascular coupling in the optic ...jarciero/Publication_22.pdf ·...
Transcript of Autoregulation and neurovascular coupling in the optic ...jarciero/Publication_22.pdf ·...

ww.sciencedirect.com
s u r v e y o f o p h t h a lmo l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6
Available online at w
ScienceDirect
journal homepage: www.elsevier .com/locate/survophthal
Major review
Autoregulation and neurovascular couplingin the optic nerve head
Daniele Prada, PhDa,*, Alon Harris, MS, PhD, FARVOb,Giovanna Guidoboni, PhDa,b,c, Brent Siesky, PhDb, Amelia M. Huang, BSb,Julia Arciero, PhDa
aDepartment of Mathematical Sciences, Indiana University-Purdue University Indianapolis (IUPUI), Indianapolis,
Indiana, USAbDepartment of Ophthalmology, Indiana University School of Medicine, Indianapolis, Indiana, USAc LabEx IRMIA, University of Strasbourg, Strasbourg, Alsace, France
a r t i c l e i n f o
Article history:
Received 8 May 2015
Received in revised form
2 October 2015
Accepted 2 October 2015
Available online 20 October 2015
Keywords:
autoregulation in the optic nerve
head
neurovascular coupling
ocular biomechanics and
hemodynamics
mechanical influences on
autoregulation
metabolic autoregulation
effects of impaired blood flow
regulation
optic neuropathies
glaucoma
blood flow regulation modeling
* Corresponding author: Mr. Daniele Pradaanapolis (IUPUI), 402 North Blackford Street,
E-mail address: [email protected] (D. Pra0039-6257/$ e see front matter ª 2016 Elsevhttp://dx.doi.org/10.1016/j.survophthal.2015.
a b s t r a c t
Impairments of autoregulation and neurovascular coupling in the optic nerve head play a
critical role in ocular pathologies, especially glaucomatous optic neuropathy. We critically
review the literature in the field, integrating results obtained in clinical, experimental, and
theoretical studies. We address the mechanisms of autoregulation and neurovascular
coupling in the optic nerve head, the current methods used to assess autor-
egulationdincluding measurements of optic nerve head blood flow (or volume and
velocity)dblood flow data collected in the optic nerve head as pressure or metabolic
demand is varied in healthy and pathologic conditions, and the current status and
potential of mathematical modeling work to further the understanding of the relationship
between ocular blood flow mechanisms and diseases such as glaucoma.
ª 2016 Elsevier Inc. All rights reserved.
, PhD, Department of Mathematical Sciences, Indiana University-Purdue University Indi-LD 270, Indianapolis, IN 46202, USA. Tel.: þ1 317 274 3460.da).ier Inc. All rights reserved.10.004

s u r v e y o f o p h t h a lm o l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6 165
1. Introduction Table 1 e Table with abbreviations
Abbreviation Full name
20-HETE 20-hydroxy-eicosatetraenoic acid
BP Blood pressure
CCD Charge coupled device
CDI Color Doppler imaging
CO2 Carbon dioxide
CRA Central retinal artery
CRV Central retinal vein
CSFp Cerebrospinal fluid pressure
dAR Dynamic autoregulation
EC Endothelial cell
EDV End diastolic velocity
EET Epoxyeicosatrienoic acid
ET Endothelin
IOP Intraocular pressure
LDF Laser Doppler flowmetry
LSFG Laser speckle flowgraphy
MAP Mean arterial pressure
NB Normalized blur
NO Nitric oxide
NOS Nitric oxide synthase
NTG Normal tension glaucoma
OCT Optical coherence tomography
ONH Optic nerve head
OPP Ocular perfusion pressure
PCA Posterior ciliary artery
PGE2 Prostaglandin E2PI Pulsatility index
PO2 Oxygen partial pressure
POAG Primary open-angle glaucoma
PSV Peak systolic velocity
RGC Retinal ganglion cell
RI Resistive index
RLTp Retro laminar tissue pressure
ROS Reactive oxygen species
sAR Static autoregulation
SBR Square blur ratio
SNFL Superficial nerve fiber layer
SSADA Split spectrum amplitude
decorrelation angiography
Glaucoma is an optic neuropathy characterized by progressive
death of retinal ganglion cells (RGCs) and irreversible visual loss.
Glaucoma is the second leading cause of blindness world-
wide,177 and yet its etiology and treatment remain unclear. The
main modifiable risk factor in glaucoma patients is elevated
intraocular pressure (IOP)1,45,110,118,121; however, a high percent-
age of individuals with elevated IOP (a condition called ocular
hypertension) never develop glaucoma,103 and many glaucoma
patients continue to experience disease progression despite
lowering IOP to target levels or have nohistory of elevated IOPea
condition called normal tension glaucoma (NTG).203
Several studies suggest correlations between impaired
ocular blood flow and glaucoma.56,60,67,84,85,90,241 In healthy
conditions, vascular beds exhibit an intrinsic ability to main-
tain relatively constant blood flowover a large range of arterial
pressures. This autoregulatory behavior is recognized in most
vascular bedsdincluding the eye,3,169 brain,162 heart,21 kid-
ney,178 skeletal muscle,61 and gut129dbut the effectiveness of
autoregulation differs among these vascular beds according to
importance of function. For example, the brain and kidney
receive stable flow over a range of arterial pressure,32,162
whereas autoregulation in other beds such as the gut is less
effective. In the eye, the retinal and optic nerve head (ONH)
vascular beds are known to exhibit autoregulation, though to
differing extents. Details and experimental measures of
autoregulation are better established in the retina than in the
ONH. In experiments assessing hemodynamic responses to
light stimulation,68,69,184,186 blood flow in the retina and ONH
seems to be highly correlated to increased neural activity. This
phenomenon is called neurovascular coupling.130
In glaucoma the location of damage to nerve cells is
hypothesized to be predominantly in the ONH,176 and thus a
clearer understanding of the factors affecting the blood supply
to the ONH is necessary to determine how this may be
compromised and potentially contribute to the pathophysi-
ology of glaucoma.
The aim of this review is to 1) summarize the mechanisms
of autoregulation and neurovascular coupling that function in
the ONH; 2) describe the current ability to assess autor-
egulation in the ONH using methodologies capable of deter-
mining ONH blood flow (or volume and velocity); 3) compare
data on blood flow for varying pressure or metabolic needs in
the ONH to assess autoregulation in healthy and pathologic
conditions; and 4) describe the current status of ophthalmic
research and support the potential of mathematical modeling
to further the understanding of the relationship between
ocular blood flow mechanisms and ocular diseases such as
glaucoma. In order to help the reader, a list of the acronyms
used in this paper is provided in Table 1.
2. Anatomy and vascular supply of the ONH
2.1. Anatomy
The ONH is where RGC axons leave the eye through the scleral
portion of the neural canal, forming bundles separated by
astrocytes, a particular type of glial cell.28 For the purpose of
description, the anatomy and vascular supply of the ONH is
best divided into 4 regions, from anterior to posterior seg-
ments (see Fig. 1).
The most anterior part of the ONH is the superficial nerve
fiber layer (SNFL). Some vascular details of this layer can be
resolved on ophthalmoscopy examination or angiography. A
part of the appearance of the SNFL comes from light back-
scattered from deeper tissue.51 Immediately behind the SNFL
is the “prelaminar region,” which lies adjacent to the peri-
papillary choroid. Posterior to the prelaminar region, the
“laminar region” is composed of the lamina cribrosa, a
structure consisting of fenestrated connective tissue beams
through which the RGC axons pass on their path from the
retina to the optic nerve. Finally, the “retrolaminar region”
lies posterior to the lamina cribrosa. It is marked by the
beginning of axonal myelination and is surrounded by
meninges.
The lamina cribrosa bears the translaminar pressure
difference: the difference between the IOP, which is the

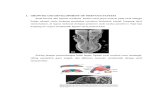
Fig. 1 e Anatomy and vascular supply of the optic nerve head (ONH). The ONH includes the superficial nerve fiber layer, the
prelaminar region, the laminar region, and the retrolaminar region.
s u r v e y o f o p h t h a lmo l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6166
pressure in the intraocular space, and the retrolaminar
tissue pressure, which is the pressure in the retrolaminar
region. The retrolaminar tissue pressure is usually lower
than the IOP and is strongly correlated to the cerebrospinal
fluid pressure and the pressure in the subarachnoid space
of the optic nerve when cerebrospinal fluid pressure >
2 mm Hg (1 mm Hg z 133.3224 Pa).134,142 A hoop stress is
also transferred to the lamina by the sclera.28 There is
evidence that an annulus of collagen fibrils exists around
the scleral canal in the peripapillary sclera. These fibrils
appear to be oriented mostly radially in the periphery of
the lamina.79,188,240 The peripapillary annulus significantly
reduces the IOP-related expansion of the scleral canal and
shields the lamina from high-tensile stress. The radially
oriented fibrils in the lamina periphery reinforce the lam-
ina against transversal shear stresses and reduce laminar
bending deformations.79 The lamina cribrosa remodels
into a thicker, more posterior structure, which in-
corporates more connective tissue after chronic IOP
elevation.80,188
In the prelaminar, laminar, and retrolaminar regions, RGC
axons are surrounded by astrocytes, which are believed
to maintain the homeostasis of the extracellular environ-
ment. In particular, astrocytes remove potassium and gluta-
mate from the extracellular space, provide cellular support to
the axons, and synthesize extracellular matrix macromole-
cules.28,101 In the prelaminar and retrolaminar region, it is
presumed that nutrient delivery to the axons occurs both via
diffusion and advection.28 In the laminar region, the extra-
cellular matrix of laminar beams lies in between capillaries
and astrocytes. Consequently, nutrients likely diffuse from
laminar capillaries, across the endothelial and pericyte
basement membranes, through the extracellular matrix of
the laminar beams, across the basement membranes of
astrocytes. From there, they may go into the astrocytes or
percolate in the extracellular space between them, ultimately
reaching the adjacent axons.
2.2. Vascular supply
The vascular system nourishing the ONH is quite complex89,92
and shows high interindividual and intraindividual vari-
ability.94,95,211 An important anatomic distinction between the
different portions of the ONH is that blood flow to the ONH is
primarily supplied by the posterior ciliary arteries (PCAs),
whereas the SNFL receives oxygenated blood primarily from
retinal arterioles.155 These small vessels, called “epipapillary
vessels,” originate in the peripapillary SNFL and run toward
the center of the ONH (see Fig. 2).
In approximately 30% of all people, a cilioretinal artery
may be present and supply the temporal SNFL. This artery, if
present, may be a direct branch of the ciliary or choroidal
arteries, emerging from the temporal SNFL of the ONH and
extending laterally along the papillomacular bundle. The
retinal arteries and the cilioretinal arteries lack anastomotic
blood exchange in the case of an artery occlusion, leading to
an ischemic infarct in the area supplied by the artery or its
branches.161
The prelaminar region is mainly supplied by direct
branches of the short PCAs and by branches from the circle of
Zinn-Haller (see Fig. 3).
The circle of Zinn-Haller, if present, is a complete or
incomplete ring of arterioles within the perineural sclera
formed by the confluence of branches of the short PCAs. The
arterial circle branches into the prelaminar region, lamina
cribrosa, and retrolaminar pial system and supplies the peri-
papillary choroid. This vascular ring can be recognized in vivo
using indocynanine green videoangiography in highly myopic

Fig. 2 e Superficial nerve fiber layer (SNFL). The SNFL receives oxygenated blood primarily from retinal arterioles. These
small vessels, called epipapillary vessels, originate in the peripapillary SNFL and run toward the center of the optic nerve
head.
s u r v e y o f o p h t h a lm o l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6 167
eyes.153 These vessels exhibit an anastomotic blood
exchange,17 but it is unclear whether this exchange can
counterbalance an insufficiency of a single PCA. There is also
evidence of direct arterial supply to the prelaminar layer
arising from the choroidal vasculature,92 even though the
extent to which it contributes to the perfusion of the region is
still a matter of debate.89 Blood flow to the laminar region is
provided by centripetal branches of the short PCAs (see Fig. 4).
Such a 3-D architecture differs from, without effectively
denying, what is proposed in some histology studies, where
Fig. 3 e Prelaminar region. The prelaminar region is mainly supp
(PCAs) and by branches of the circle of Zinn-Haller. The circle of
arterioles within the perineural sclera formed by the confluenc
the lamina is viewed as a set of stacked perforated sheets
containing vessels, with pores in each sheet aligned to create
tunnel for bundles of nerve fibers to exit from the eye.8,9
Unlike in vivo imaging, histology imaging suffers from
distortions because of the loss of pressure (IOP, intracranial
pressure, and blood pressure), distortions during tissue prep-
aration, and tissue degradation after death. Nevertheless, care
is needed when comparing optical coherence tomography
(OCT) results to histology because of differences in optical
resolution and sampling density. OCT has significantly worse
lied by direct branches of the short posterior ciliary arteries
Zinn-Haller, if present, is a complete or incomplete ring of
e of branches of the short PCAs.

Fig. 4 e Laminar region. Blood flow to the laminar region is provided by centripetal branches of the short PCAs. The
centripetal branches arise either directly from the short PCAs or from the circle of Zinn-Haller. The lamina cribrosa is shown
as a 3-D network, as suggested in recent in vivo imaging studies based on optical coherence tomography
(OCT)106,146,215,235,236 and in finite element modeling studies of the lamina cribrosa microarchitecture.29,55,188
s u r v e y o f o p h t h a lmo l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6168
lateral resolution when compared with electron microscopy
or other forms of microscopy used to study the lamina cri-
brosa, and it likely overemphasizes beams, compared to his-
tology.236 Hence, many questions still need to be answered to
characterize the 3-D geometry of the lamina cribrosa
accurately.209
The centripetal branches arise either directly from the
short PCAs or from the circle of Zinn-Haller. These precapil-
lary branches perforate the outer boundary of the lamina and
then branch into an intraseptal capillary network, which runs
inside the laminar beams. It is still unclear whether there are
anastomoses between the capillary or precapillary bed of the
laminar region, the prelaminar region, and the SNFL region. If
these anastomoses exist, it is unclear whether they play a role
when a sudden (or slowly progressive) vascular occlusion on
the precapillary or intracapillary level happens.161 The retro-
laminar region is supplied by the central retinal artery (CRA)
and the pial system (see Fig. 5). The pial system is an anas-
tomosing network of capillaries located immediately within
the pia mater. The pial system originates from the circle of
Zinn-Haller and may also be fed directly by the short PCAs.
The branches of the pial system extend centripetally to
nourish the axons of the optic nerve. The CRA may supply
several small intraneural branches in the retrolaminar region.
Some of these branches may also anastomose with the pial
system.
In the ONH the capillaries form a continuous network
throughout its entire length, being continuous posteriorly
with those in the rest of the optic nerve and anteriorly with
the adjacent retinal capillaries.7,122 It is unclear whether this
implies that blood flow regulation is similar122 or not92 in
both vascular regions, independent of the arterial source.
Critical questions remain unanswered. The CRA within the
intraorbital optic nerve is innervated, but innervation stops (at
least) anterior to the lamina cribrosa, and it does not follow
the branches of the CRA inside the eye.243 Neurotransmitter
receptors, however, are present on the surface of retinal ves-
sels.58,104 In addition, normal retinal vessels lack fenestra-
tions.161 Hence, vasoactive hormones cannot leak from
capillaries and reach the muscular coat of nearby arterioles
where they can influence blood flow. The branches of the PCA
that feed the intrascleral portion of the optic nerve may or
may not be innervated and/or fenestrated. Such knowledge is
crucial to understand how blood flow is regulated in the ONH.
Venous drainage of the ONH occurs primarily through the
central retinal vein (CRV). In the SNFL, blood is drained directly
into the retinal veins, which then join to form the CRV. In the
prelaminar, laminar, and retrolaminar regions, venous
drainage occurs via the CRV or axial tributaries to the CRV.
3. Techniques for in vivo studies of ONHhemodynamics
As described in section 2, the complex vasculature of the
ONH is comprised of small diameter vessels arranged in
an intricate 3-dimensional geometry. At present, no
technology allows a noninvasive measurement of volu-
metric blood flow in absolute units; however, some he-
modynamic measurement techniques provide surrogates
for ONH blood flow in arbitrary units. Four of these
measurement techniques for in vivo studies of ONH he-
modynamics are discussed and compared in the following
sections. Table 2 summarizes their main features, ad-
vantages, and limitations.

Fig. 5 e Retrolaminar region. The retrolaminar region is supplied by the central retinal artery (CRA) and the pial system. The
pial system is an anastomosing network of capillaries located immediately within the pia mater.
s u r v e y o f o p h t h a lm o l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6 169
3.1. Laser Doppler flowmetry
Laser Doppler flowmetry (LDF) is a noninvasive method of
assessing blood flow and perfusion in the ONH. LDF is based
on the Doppler effect. It measures the shift in frequency that
occurs when light is scattered by the red blood cells moving
through capillaries. LDF uses a fundus camera and a com-
puter system to detect these changes in frequency. This
information is used to calculate 3 hemodynamic parame-
ters: velocity, blood volume, and blood flowwithin the ONH.
Velocity is defined as the average speed of red blood cells
traveling through capillaries and is proportional to the
mean change in Doppler shift frequency. Blood volume is
defined as the number of red blood cells in the given sample.
Blood flow or flux is defined as the flux of red blood cells
Table 2 e Techniques for in vivo studies of ONH hemodynamic
Technique Measurements Location ofmeasurement
Laser Doppler
flowmetry
Velocity, volume, and flow
in arbitrary units
ONH
OCT angiography Flow in arbitrary units ONH
Color Doppler
imaging
Velocity Ophthalmic artery,
CRA, and short PCAs
Laser speckle
flowgraphy
Velocity, flow in arbitrary
units
ONH
CRA, central retinal artery; OCT, optical coherence tomography; ONH, op
through a specific part of a capillary at a given time. The
main advantage of LDF is its ability to measure 3 different
hemodynamic parameters; however, LDF only provides
measurements of blood perfusion in arbitrary (and not ab-
solute) units, which limits its usefulness in a clinical
setting.183 Moreover, LDF measurements depend signifi-
cantly on the depth of the sampled tissue. This depth de-
termines the relative contribution to the Doppler signal of
the superficial layers, the layers supplied by the CRA, and
the deeper layers supplied by the short PCAs. Blood flow
autoregulation may or may not differ within these vascular
beds. In a study on monkey eyes, LDF appeared to be more
heavily influenced by blood flow changes in the more su-
perficial layers of the ONH than in deeper ones, but to what
extent remains uncertain.187
s
Advantages Limitations
Multiple hemodynamic
parameters
No absolute measurements;
no interindividual comparisons
High quality images, fast No absolute measurements;
difficult to localize measurements
Vessel selective, no need for
pupil dilation, clear media, or
fixation
No absolute measurements;
velocity measurements only;
expert operator required
Time evolution of velocity at
the same site of the same eye
No absolute measurements; no
interindividual comparisons
tic nerve head; PCA, posterior ciliary artery.

s u r v e y o f o p h t h a lmo l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6170
3.2. OCT angiography
OCT angiography, a combination of high speed OCT and a new
3D angiography system called split-spectrum amplitude-
decorrelation angiography, is a noninvasive method used to
estimate blood flow in the ONH, especially within the micro-
circulation.108 It computes the flow index, which is a surrogate
for blood flow in arbitrary units.
OCT is a technique that takes cross-sectional images of a
biologic tissue using a low-coherence interferometer. These
cross-section images are captured using a low-coherence
beam directed at the target tissue. The light signals reflect
off of the tissue back to the interferometer, which stacks a
series of longitudinal tomographic b-scans to derive a
3-dimensional image. Doppler OCT, a commonly used subtype
of OCT, can detect the Doppler frequency shift of the reflected
light, providing additional information on blood flow. The
split-spectrum amplitude-decorrelation angiography algo-
rithm allows 3-dimensional angiography to be done 4 times
faster than previous algorithms and also improves blood flow
detection, creates better visualization of the microvascula-
ture, and removes motion errors automatically.105
OCT angiography has many advantages over Doppler
OCT. Doppler OCT can only quantify blood flow in large
superficial vessels of the ONH and cannot visualize the
microvasculature.105 OCT angiography minimizes the pul-
satory bulk motion noise along the axial direction and
optimizes flow detection along the transverse direction.108
As with all measuring techniques, OCT angiography has
limitations. One of the main disadvantages is that blood
flow from superficial layers can be projected to deeper
layers, thereby incorrectly indicating that the imaged blood
flow is a few layers deeper than its location in vivo.108 Also,
split-spectrum amplitude-decorrelation angiography cannot
distinguish between perfusion defects caused by damaged
tissue or ischemia,109 and ONH blood flow estimates are
provided in arbitrary units. Despite these limitations, OCT
angiography is a very useful tool to measure blood flow in
the ONH.
3.3. Color Doppler imaging
Color Doppler imaging (CDI), also known as color Doppler
ultrasound, is a noninvasive procedure that allows the user to
visualize a color-coded image of blood velocity against a gray-
scale image of the surrounding structures. This technique
uses the principle of Doppler frequency shift tomeasure blood
flow velocity in absolute units. Various transducers are used
to measure the Doppler frequency shift, which produces color
pixels.239 The color red represents blood flowing toward the
ultrasound probe, whereas blue represents blood flowing
away from the probe.214
CDI is most commonly used to measure the peak systolic
velocity (PSV) and end diastolic velocity (EDV), which are then
used to measure the resistive index [RI ¼ (PSV�EDV)/PSV] and
pulsatility index [PI ¼ (PSV�EDV)/Tmax, where Tmax is the
time averaged peak velocity]. These values estimate resis-
tance to blood flow caused by the microvasculature distal to
the site of measurement. The RI is particularly suitable for
investigating the low resistance retrobulbar vasculature.239
The major advantages of CDI are that it is noninvasive,
vessel selective, reproducible, and does not require pupil
dilation, clear media, or fixation. CDI is limited in its ability to
measure only velocity (not flow) and calculate vascular
resistance and requires for an experienced operator to obtain
accurate results.87 CDI has particular difficulty imaging and
interpreting small vessels, and the PCAs are close to the size
limit that can be studied.
3.4. Laser speckle flowgraphy
Laser speckle flowgraphy (LSFG) is a noninvasive method of
measuring blood flow and velocity in the ONH. LSFGmeasures
bloodflowbyusing the laser speckle phenomenon,which is an
interference event that occurswhen laser light scatters off of a
diffusing tissue. This creates a speckle pattern that varies in
proportion to thevelocity of redbloodcells and thus represents
capillary blood flow. The faster the velocity of the red blood
cells, the greater the rate of pattern variation. Although the
velocity cannot bemeasured directly, the normalized blur and
square blur ratio values can be calculated as quantitative in-
dicators of blood velocity. Normalized blur values are well
correlated with blood flow measurements simultaneously
taken with the hydrogen gas clearance method, colored mi-
crospheres technique, and other methods in the ONH, iris,
choroid, and retina.218,220e226Thedistributionof bloodflowcan
be displayed in a 2 dimensional color-coded map, which re-
flects the time variation of the speckles at each pixel point.217
This allows for visualization of blood flow in real time.
LSFG uses a diode laser, image sensor, infrared charge-
coupled device camera, and digital charge-coupled device
camera. The diode laser and image sensor are used for laser
speckle measurements. The laser is focused on the image
sensor and creates a speckle pattern, which is scanned at 512
scans/second.217 The digital charge-coupled device camera
measures vessel diameter and takes pictures of the fundus.
The advantages of LSFG are that its results are adequately
reproducible and that the change in velocity at the same site
of the same eye can be followed over time. A major disad-
vantage of LSFG is that themeaning of its measurement is not
clearly understood and does not allow for intereye or inter-
individual comparisons.87
4. Evidence of blood flow autoregulation inthe ONH
Autoregulation is the intrinsic ability of vascular beds to main-
tain relatively constant blood flow over a large range of pressure,
while meeting the metabolic demand of the tissue. Autor-
egulation is evaluated most often on a flow versus pressure
graph, where pressure may be expressed as mean arterial pres-
sure (MAP), IOP, or ocular perfusion pressure (OPP) (see Fig. 6).
OPP refers to the arterovenous pressure difference driving
blood flow through the intraocular vasculature. The intraoc-
ular venous pressure is very close to IOP,15,46,73,81 and thus,
OPP is usually estimated as the difference between the arterial
BP and IOP in the upright position. OPP may be defined as
mean, systolic, or diastolic OPP. Mean OPP is typically calcu-
lated as

OPP
Ste
ady-
stat
e bl
ood
flow
Autoregulationplateau
Fig. 6 e A schematic representation of a static
autoregulation curve describing the relationship between
steady-state blood flow responses in the ONH and OPP.
ONH, optic nerve head; OPP, ocular perfusion pressure.
s u r v e y o f o p h t h a lm o l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6 171
mean OPP ¼ 23MAP� IOP
where
MAP ¼ diastolic BPþ 13ðsystolic BP� diastolic BPÞ:
The factor 2/3 accounts for the drop in BP between the
brachial and ophthalmic artery when the subject is seated182
and the fact that the orbital arteries are further downstream.
In clinical studies, the brachial arterial pressure has been
often considered as representative of systemic BP and is used
as the basis for calculating the ophthalmic arterial pressure
in the calculation of OPP. The pressure in the brachial artery,
however, is not a precise predictor of the pressure in the
ophthalmic artery. It is not clear how accurately the above
formula approximates the difference between the ocular
arterial BP and the brachial arterial pressure because of the
hydrostatic column effect when an individual is sitting.30 We
also cannot assume that the difference between the ocular
and brachial arterial pressures is the same in normal and
diseased vascular beds. Moreover, blood flow is determined
not only by OPP but also by vascular tone. Regulation of blood
flow may occur through changes in vascular resistance
(vasoconstriction or vasodilation, see section 5) indepen-
dently of changes in OPP. Physical exercise may or may not
cause a change both in cardiac output and in the net resis-
tance of the many microvascular pathways in parallel.
Equating venous pressure to IOP is also sometimes erro-
neous. For example, if the cerebrospinal fluid pressure is
higher than IOP, the venous pressure must exceed cerebro-
spinal fluid pressure in the subarachnoid space for the CRV to
remain patent, and thus venous pressure could not be
approximated by IOP. In this way, the estimate for OPP in-
volves systematic errors; however, even if more reliable for-
mulas for computing the OPP have been proposed,46 the
previously mentioned relations are consistently used in
clinical studies. A reliable, direct measure of OPP would of
course be desirable, but without this, care is needed when
interpreting blood flow regulation studies.
4.1. Evidence of ONH autoregulatory capacity duringOPP changes in healthy subjects
In several animal70,186,212 and human studies,166,181 the ONH
vascular bed was shown to maintain autoregulatory capacity
over a wide range of perfusion pressures. Autoregulatory ca-
pacity is conventionally assessed by a “two-point” blood flow
measurement: blood flow or other hemodynamic parameters
are measured before and after the OPP is artificially modified
by a step challenge in either the IOP or the systemic arterial BP.
If blood flow changes significantly from normal after the
pressure challenge, then autoregulation is said to be impaired;
if blood flow remains nearly constant over the pressure
change, autoregulation is said to be intact.
When the pressure step challenge is applied rapidly, it
induces 2 phases of hemodynamic response: 1) an initial
transient, or dynamic, phase lasting a few seconds during
which the vasculature tries to return blood flow to its original
level by adjusting vascular resistance and 2) a steady-state
phase when transient blood flow changes have equilibrated
to a steady state level. High temporal resolution blood flow
measures made during the initial phase following pressure
changes are referred to as dynamic autoregulation (dAR),
whereas those made during the steady-state phase are
referred to as static autoregulation (sAR).120 To date, most
studies assessing autoregulatory capacity in the ONH have
been limited to sAR.
In this section, some important clinical studies that
address dAR and sAR in the ONH in healthy conditions are
reviewed.
4.1.1. Dynamic autoregulation studiesThe dAR refers to the transient vascular changes preceding
the equilibrated steady state. Interestingly, dAR was pointed
out as a more sensitive indicator of cerebral blood flow
autoregulation than sAR since dAR is better correlated to
neuronal activities than sAR.160,191 Moreover, a dynamic blood
flow response contains both time and frequency information
that can effectively reveal potential autoregulation dysfunc-
tion. In fact, dAR measurements have become the standard
method for assessing cerebral blood flow autoregulation in
cerebral diseases.230
A blood flow time course similar to that described in the
brain was observed in the ONH of rabbits220 and nonhuman
primates119 after an acute increase in IOP. The time course of
relative ONH blood flow changes from baseline (IOP ¼ 10 mm
Hg) to elevated IOP (IOP ¼ 30 mm Hg), and then back to
baseline, was tracked for 3 different ranges of BP in mon-
keys.119 In the high-BP group, there was no significant change
in ONH blood flow during the IOP alterations; however, the
same IOP alterations caused a significant ONH blood flow
change in the 2 lower BP groups, suggesting that autor-
egulation of the ONH is deficient in the lower BP groups.
To characterize dAR in the ONH, time-domain parameters
were extracted from high temporal resolution blood flow
measurement obtained using an LSFG device in a group of
monkeys.120 A rapid OPP decrease induced by a sudden IOP
step increase evoked a transient and reproducible dAR in the
ONH. The duration of blood flow decrease was much shorter

s u r v e y o f o p h t h a lmo l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6172
than that of the pressure change. In otherwords,while the IOP
was still increasing, the blood flow had already started
recovering. This observation suggests that autoregulation is
activated immediately after IOP elevation.
Responses of OPP, ONH blood flow, and vascular resistance
to increases in BP were investigated by hand gripping.39 BP
and ONH blood flow parameters were simultaneously and
continuously measured by LDF. Healthy subjects could be
subdivided into 2 groups because of marked differences in the
efficiency and OPP range of autoregulation: in one group,
autoregulation was found to be highly efficient once OPP was
increased by approximately 15% above baseline; in another
group, autoregulation was found to be less efficient. These
findings are in accordance with other studies.24
The dAR studies mentioned above are summarized in
Table 3.
4.1.2. Static autoregulation studiesThe sAR refers to steady-state responses of ONH blood flow to
a wide range of OPP values. These responses constitute a
classic autoregulation curve or pressure-flow relationship (see
Fig. 6). This plateau of the autoregulation curve indicates the
range of OPP where autoregulation is functioning. When the
OPP values are outside the range defined by this plateau,
autoregulation fails and blood flow will gradually decrease or
increase passively as OPP changes.
Microspheres were used to measure blood flow in the
various compartments of the monkey optic nerve following
manometric IOP elevation.70 Small changes in IOP which
reduced OPP as low as 29 mm Hg had small effects on prel-
aminar blood flow. At OPP less than 29 mm Hg, prelaminar
flow was proportional to OPP. Laminar blood flow was nearly
unchanged even at high IOP (low OPP).
OPP was decreased by increasing IOP with a scleral suction
cup in normal human subjects.68,166,181 An LDF probing laser
beamwasdirectedat theONHtissueat temporal andnasaldisk
areas, at least 200 mm from the disk margin and outside the
physiologic cup. Autoregulation was active for OPP values as
low as 15e20 mm Hg (IOP ¼ 40e45 mm Hg),181 or until IOP
reached 45 mm Hg.166 Apparently, the ONH vasculature was
not fully dilatedat this point, becausediffuse luminanceflicker
increased ONH blood flow even more.68 Regional variability in
the degree of autoregulation has been reported.166 Some optic
Table 3 e Summary of dynamic autoregulation studies in heal
Study Methods
Liang et al119 Combined changes in IOP and MAP in monkeys
Liang et al120 Sudden IOP step increase in monkeys
Chiquet et al39 MAP elevation in healthy humans
BP, blood pressure; IOP, intraocular pressure; LDF, Laser Doppler flowmetr
optic nerve head; OPP, ocular perfusion pressure.
disk locations of some individuals appeared unable to regulate
blood flow under even a minimal IOP challenge.
Effects of elevated OPP on the ONH blood flow of healthy
volunteers were investigated by increasing MAP through iso-
metric exercise (squatting).144 An LDF probing laser beamwas
directed at temporal disk areas of the ONH, at least 200 mm
from the diskmargin and outside of the physiologic cup. In the
range of OPP between 56 � 4 and 80 � 5 mm Hg (30% � 8%),
there was no significant variation of mean velocity, volume,
and flux of red blood cells, but vascular resistance increased
by about 50%. These results suggest that the maintenance of
constant blood flow is achieved by an increase in vascular
resistance. Similar results were obtained in another study.171
Regulation of ONH blood flow was investigated during
combined changes in IOP and systemic BP. ONH blood flow
was measured in monkeys using LSFG during artificial
changes in IOP and BP.119 When IOP was increased to 30 mm
Hg and MAP was normal (102 mm Hg), no significant change
was observed in ONH blood flow, indicating that autor-
egulation was functioning. However, when IOP was increased
to 30 mm Hg and MAP was reduced to 56 mm Hg, significant
reductions in ONH blood flow were observed, suggesting that
autoregulation was unable to maintain blood flow at low OPP.
These blood flow results, as well as those obtained in other
studies,234,237 must be interpreted in the context of where and
how they were measured.
ONH blood flow was investigated in 40 healthy subjects
using continuous LDF during a separate increase in IOP and
MAP as well as during their combined elevation.24 The laser
beam was directed toward the neurovascular rim. MAP was
increased by isometric exercise consisting of squatting, and
IOP was raised via suction cup. During both experiments, the
change in ONH blood flow was less pronounced than the
change in OPP, indicating autoregulation.
The sAR studies mentioned in the previous paragraphs are
summarized in Table 4.
In Figure 7, OPP-ONH blood flow relationships reported in a
few studies24,70,144,166,181 have been gathered. Each data set
has been normalized to its corresponding baseline OPP and
ONH blood flow estimate. Care is needed to interpret this
figure correctly. First, different species and different tech-
niques for estimating ONH blood flowwere involved in these 6
studies. Second, in one study,70 monkeys were supine,
thy subjects
Technique Main findings
LSFG The duration of the changes in ONH blood flow
from baseline to a peak and to a steady state was
significantly delayed in subjects with medium or
low, but not high, BP
LSFG While the IOP was still increasing, the blood flow
had already started recovering
LDF Marked differences in the efficiency and OPP
range of autoregulation were found among
subjects, in accordance with another study24
y; LSFG, laser speckle flowgraphy; MAP, mean arterial pressure; ONH,

Table 4 e Summary of static autoregulation studies in healthy subjects
Study Methods Technique Main findings
Geijer et al70 IOP elevation in healthy monkeys Microspheres The laminar and retrolaminar portions of the
ONH showed higher autoregulation capabilities
than the prelaminar portion
Riva et al181 IOP elevation in healthy humans LDF ONH blood flowwas relatively constant to an OPP
as low as 15e20 mm Hg
Pillunat et al166 IOP elevation in healthy humans LDF ONH blood flow was regulated to an IOP up to
45 mm Hg
Movaffaghy et al144 MAP was elevated in healthy humans LDF In the range of OPP between 56� 4 and 80� 5mm
Hg, there was no significant variation of mean
velocity, volume, and flux of red blood cells, but
vascular resistance increased by about 50%
Liang et al119 Combined changes in IOP and MAP in monkeys LSFG When IOP was increased to 30 mm Hg and MAP
was normal (102 mmHg), ONH blood flow did not
change significantly. Instead, blood flow
decreased when IOP was increased to 30 mm Hg
and MAP was reduced to 56 mm Hg
Boltz et al24 Combined and separate IOP and MAP elevation in
healthy humans
LSFG ONH blood flow increased with OPP for OPP
values of 66% above baseline. ONH blood flow
decreased for OPP values of 40% below baseline
Wang et al237 IOP elevation in monkeys LSFG ONH blood flow is effectively regulated for OPPs
of approximately 41 mm Hg and above
IOP, intraocular pressure; LDF, Laser Doppler flowmetry; LSFG, laser speckle flowgraphy; MAP, mean arterial pressure; ONH, optic nerve head;
OPP, ocular perfusion pressure.
s u r v e y o f o p h t h a lm o l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6 173
whereas, in the others, human volunteers were standing.
Third, some data were obtained by increasing IOP, whereas
other data were obtained others by increasing MAP. Last,
displaying all the data in a single graph presumes the use of a
common baseline. Despite these limitations, Figure 7 shows
that the static OPP-blood flow curve in the ONH is consistent
with the curve schematized in Figure 6.
On reviewing a number of clinical studies addressing
dynamic and static blood flow autoregulation in the ONH
in healthy conditions, note the different sensitivity of
OPP (mean % change from baseline)-100 -50 0 50 100
ON
H b
lood
flow
(mea
n %
cha
nge
from
bas
elin
e)
-100
-50
0
50
Geijer (1979), prelaminar regionGeijer (1979), laminar regionPillunat (1997), ONHRiva (1997), ONHMovaffaghy (1998), ONHBoltz (2013), ONH
Fig. 7 e Pressure-flow relationships for ONH blood flow in 6
experimental studies in healthy monkeys (Geijer et al.,70
circles and asterisks), and in healthy humans (Pillunat
et al.,166 squares), (Riva et al.,181 downward-pointing
triangles), (Movaffaghy et al.,144 upward-pointing
triangles), (Boltz et al.,24 crosses). ONH, optic nerve head;
OPP, ocular perfusion pressure.
autoregulation components. Dynamic and static aspects are
2 related, but different, components of the autoregulation
process. Importantly, measuring dAR could succeed in
revealing potential autoregulation dysfunction in situations
where the conventional “two-point” method measured dur-
ing the sAR phase would fail.
4.2. Clinical studies of static blood flow autoregulationin the ONH in pathologic conditions
Underpathologic conditions,ONHbloodflowregulationmaybe
disrupted.5 Impairedautoregulation in theONHassociatedwith
altered blood flow has been observed in experimental dia-
betes204 and hypercholesterolemia.205 In glaucoma, and espe-
cially in NTG, impaired autoregulation in the ONH has been
speculated to be an important risk factor for the progression of
the disease.43,60,75,91,156 Glaucomapatients havebeen suggested
to exhibit abnormal autoregulation especially in response to
acute changes in OPP, as reviewed in the following paragraphs.
The autoregulatory control of retrobulbar blood flow in
response to postural challenge was investigated in NTG
patients in comparison with primary open-angle glaucoma
patientsandhealthyvolunteers.66 PSV, EDV, andRI in the short
PCAs were recorded after a change from sitting upright to a
supine body position using CDI. Ten minutes after postural
change, blood flow velocities in the short PCAs remained un-
changed in controls, whereas a significant increase of PSV and
EDV occurred in both glaucoma groups. The RI in the short
PCAswas significantly lower in theNTGgroupwhencompared
to healthy and primary open-angle glaucoma individuals. The
authors suggested that the unaltered flow velocities in the
short PCAs of healthy controls might indicate tight autor-
egulatory control. In contrast, the accelerated flow in the short

s u r v e y o f o p h t h a lmo l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6174
PCAs exhibited by NTG and primary open-angle glaucoma
patientsmight indicatean insufficient compensatory response
topostural change.However, it isnot clear towhatextent these
interpretations are consistent with the experimental data. It is
unknown how the supine position affects intraocular venous
pressure and, in turn, OPP.46 It is accepted that, in the supine
position, there is adifferent interactionbetweenorbital venous
pressure and intraocular venous pressure than in the upright
position. This interaction is affected by the tissue pressure,
which experiences huge variations due to differences in body
mass and the hydrostatic water column effect on venous
pressure.30 Consequently, it ishard to establishhowthesupine
position impacts OPP and, hence, blood flow.
Chronic unilateral elevation of IOP was induced in mon-
keys by laser treatment to the trabecular meshwork, leading
to glaucomatous damage.234 The authors found compromised
blood flow in the anterior ONH (including SNFL, prelaminar
tissue, and lamina cribrosa) of glaucomatous eyes measured
by both LSFG and the microsphere method. ONH blood flow
was measured for IOP ¼ 40 mm Hg in both control and glau-
comatous eyes. In a parallel study, longitudinal changes in
basal blood flow of the ONH were investigated in the same
monkey model.47 There are a couple of possible explanations
for the reduced flow observed in these 2 studies during the
later stages of glaucoma. If there is a reduced amount of
neural tissue requiring nutrition, the flow would be regulated
to avoid supplying more blood than what is necessary for the
remaining tissue. ONH blood flow could also be reduced if
autoregulation is impaired.
There was no evidence of altered autoregulation in the
ONH in glaucoma in some studies. Regulation of ONH blood
flow in response to an increase of OPP was investigated
through a challenge in systemic BP induced by isometric
exercise in NTG patients and in age-matched healthy volun-
teers.171 ONH blood flow parameters, as determined by LDF,
did not indicate abnormal blood flow regulation in either
healthy subjects or NTG patients; however, NTG patients
showed a greater percent increase in vascular resistance
compared to the normal subjects for a similar percent
increase in OPP in both groups during squatting.
To our knowledge, neither dynamic nor static mechanisms
of ONH blood flow regulation have been quantified in patients
with arterial hypertension. Arterial hypertension can poten-
tially interfere with ONH blood flow in many ways.94 For
example, atherosclerosis resulting from prolonged arterial
hypertension can cause inadequate changes in vessel size in
response to fluctuations in OPP.88 Chronic arterial hyperten-
sion can also cause the range of autoregulation to shift to
higher levels to adapt to high BP.97 Such an adjustmentmakes
the patientmore tolerant to high BP but less tolerant to low BP.
In this situation, a sudden reduction of BP in hypertensive
subjects can cause anterior ischemic optic neuropathy.96,98
Systemic hypertension is associated with pathologic
changes in retinal vasculature.123 Endothelial function of the
retinal vasculature is impaired in early essential hyperten-
sion,50 and hypertensive retinopathy is associated with
endothelial dysfunction111,112 (see section 5 for a discussion
about the role of endothelial cells (ECs) in regulating blood
flow). Hence, since systemic hypertension is linked to a wide
range of major eye diseases, it is incredibly important to
quantify its effects on ONH blood flow regulation.
In a study of the ONH of control and glaucomatousmonkey
eyes,237 static blood flow autoregulation was characterized,
and impaired autoregulation was tested as a potential mech-
anism involved in the reduction of ONH blood flow observed
in previous studies.47,234 Autoregulation curves were created
based on a series of relative ONH blood flow changes, each
measured with an LSFG device in response to an acute OPP
decrease induced by instantaneous IOP elevation monitored
across stages of glaucoma. Within the ONH of the control
eyes, blood flow was effectively regulated within the OPP
range from 41 mm Hg to 115 mm Hg. When OPP was below
41 mm Hg, blood flow declined linearly with OPP. Autor-
egulation curves of control and glaucomatous eyes were not
significantly different from each other. Thus, the authors
argued that sAR is not a predominant factor accounting for the
reduced ONH blood flow observed in the monkey model.47,234
All of these clinical studies addressing autoregulation in
pathologic conditions are summarized in Table 5.
5. Mechanisms of blood flow regulation
Several important response mechanisms combine to cause
changes in vascular tone that lead to blood flow regulation to a
particular tissue. A complete overview of the biochemistry of
all the mediators and modulators involved is beyond the
scope of this review. We will focus on studies relevant for the
control of blood flow in the ONH.
5.1. Mechanical influences
An important class of autoregulation is seemingly more
dependent on mechanical influences. In the myogenic
response, vascular smooth muscle cells constrict as intra-
vascular pressure, and consequently the circumferential wall
tension, is elevated. Indeed, circumferential wall tension
depends on both the vessel radius R and transmural pressure
DP, defined as the difference between the intravascular and
extravascular pressures. According to Laplace’s law, as DP
rises, the vessel wall stretches, leading to increased wall
tension T:
T ¼ DP� R:
In the myogenic response, arterioles respond to the
increased wall tension by constricting to reduce the vessel
radius R and restore the wall tension to a normal level. The
myogenic response has been observed in the ONH. More
effective blood flow autoregulation was observed in the ONH
of healthy humans during an increase inMAP than IOP.24 Such
behavior could be compatible with a myogenic mechanism of
autoregulation.
The contractility of smooth muscle cells involved in the
myogenic response is regulated by ECs. In addition to chemical
agents, ECs respond to mechanical stimuli, such as fluid shear
stress and stretch.38 Externally applied forces with a clear di-
rection, such as shear stress from pulsatile flow or uniaxial
stretch, induce the release of vasoactive substances which then

Table 5 e Autoregulation studies in pathologic conditions
Study Methods Technique Main findings
Pournaras et al171 MAP elevation in NTG patients LDF NTG patients did not show abnormal ONH
blood flow regulation but only a greater
percent increase in vascular resistance
compared to healthy subjects
Galambos et al66 Investigation of autoregulatory control of
retrobulbar blood flow in response to postural
changes in NTG and POAG patients
CDI NTG and POAG patients exhibited an
insufficient compensatory response to
postural change, leading to accelerated flow
in the short PCAs
Shibata et al205 IOP elevation in hypercholesterolemic rabbits LSFG Hypercholesterolemia induced impairment in
the autoregulation of ONH blood flow and
deterioration in visual function and histology
Shibata et al204 IOP elevation in rabbits with induced diabetes
and induced gap junction uncoupling
LSFG Autoregulation was disrupted both in the
animals who were induced diabetes and in
those who received gap junction uncouplers
Wang et al234 Chronic unilateral IOP elevation induced in
monkeys by laser treatment to the trabecular
meshwork
Microspheres and LSFG Blood flow was compromised in the SNFL,
prelaminar, and laminar tissues, possibly due
to impaired autoregulation
Cull et al47 Chronic unilateral IOP elevation induced in
monkeys by laser treatment to the trabecular
meshwork
LSFG A 2-phase pattern of ONH blood flow
alteration was observed in treated animals.
ONH blood flow increased during the earliest
stage (while retinal nerve fiber layer thickness
was within 10% of baseline) followed by a
linear decline strongly correlated with loss of
RGCs
Wang et al237 Chronic unilateral IOP elevation induced in
monkeys by laser treatment to the trabecular
meshwork
LSFG Chronic IOP elevation caused no remarkable
change to the static autoregulation of
glaucomatous eyes
CDI, Color Doppler imaging; IOP, intraocular pressure; LDF, Laser Doppler flowmetry; LSFG, laser speckle flowgraphy; MAP, mean arterial
pressure; NTG, normal tension glaucoma; ONH, optic nerve head; PCA, posterior ciliary artery; POAG, primary open-angle glaucoma; RGC,
retinal ganglion cell; SNFL, superficial nerve fiber layer.
s u r v e y o f o p h t h a lm o l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6 175
determine ECs remodeling. This remodeling phenomenon in-
cludes changes in the orientation of ECs cytoskeletal fibers,38 the
morphology of cell surface49,152 and/or cell stiffness99,137,194,195 to
minimize the alterations in intracellular stress. Whereas ECs
remodeling results from molecular signaling due to mechano-
transduction, this adaptive behavior, in turn, modulates the
molecular signaling. Thus, a close coupling exists between
mechanics and vascular endothelium. In addition, experiments
have shown that myogenic tone of the PCAs is regulated by
prostaglandin formation and the release of endothelium-
derived relaxing factor by arteries.150 To our knowledge, regu-
lation of myogenic tone and sheer stress responses in the ONH
vasculature has not been studied. Further research is needed to
investigate the contribution of mechanical influences on ONH
blood flow regulation.
5.1.1. Nitric oxide and endothelin-1Two important vasoactive substances released by the ECs
after local stimulation by, for example, shear stress from
pulsatile flow or uniaxial stretch, are nitric oxide (NO) and
endothelin-1 (ET-1). A constant balance between the opposing
functions of NO and ET-1 is necessary for proper regulation of
the vascular system.156,157,232 NO is a potent vasodilator
secreted by smooth muscle cells that causes the dilation of
arterioles via activation of smooth muscle cells and the dila-
tion of capillaries via pericytes.93 Owing to the radical nature
of NO, it has a short half-life, which is made even shorter in
the presence of oxygen and superoxide and longer in the
absence or lower concentrations of oxygen.20,140 Because NO
cannot be stored in vivo, regulation of NO production is
controlled at the level of the biosynthesis.198 Hence, NO syn-
thesis largely depends on the amount of nitric oxide synthase
(NOS). Inhibiting NOS in cats resulted in a decrease in ONH
blood flow, both at baseline and with light flicker stimulation
of neuronal activity.27 Administration of a nonspecific NOS
inhibitor, NG-monomethyl-L-arginine, appeared to reduce
baseline ONH blood flow in humans.128,199 NO also exerts
antioxidative and neuroprotective effects by interacting with
reactive free radicals such as superoxide anions, hydroxyl,
and peroxyl lipid radicals.40
ET-1, a potent vasoconstrictor that is released from
ECs,8,156,179,180,200,232 acts on 3 types of ET receptors: ETA, ETB1,
and ETB2. More precisely, ETA receptors are present in vascular
smooth muscle and mediate vasoconstriction.156,179,200 The
stimulation of ETB1 receptors, which are located on ECs, results
in vasodilation via clearance of ET-1 and the release of NO and
prostacyclin.200 ETB2 receptors mediate vasoconstriction.167
Despite the opposing roles of the B1 and B2 receptors, a net
vasodilation is shown to result from the stimulation of the ETB
subtype receptors.238 Pharmacologically blocking ETA and ETB
receptors resulted in a significant increase in blood flow to the
retina, choroid, and ONH in patients with glaucoma as well as
healthy subjects, suggesting a potential role for endothelial
blockers to be used in the treatment of glaucoma.180

s u r v e y o f o p h t h a lmo l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6176
5.2. Metabolic response
Metabolic regulation results in the adjustment of blood flow
during inadequate or excessive nutrient supply, because of
either a change in the metabolic activity of the tissue or a
change in flow by virtue of cardiovascular factors such as
atherosclerosis or systemic vasospasm. No matter the cause,
when tissues are not receiving the appropriate amount of
blood flow, an adjustment is made by control mechanisms.
Overall, the vascular response to tissue demand is influenced
by the metabolic conditions in the tissue such as levels of
oxygen, carbon dioxide levels (mediated by pH), NO, ET-1, and
other chemical signals.157 For example, when the eye is
exposed to a flickering light, the number of action potentials
and the consequent need for (re)polarization of the RGC axon
membranes in the ONH is increased with each “on” and “off”
light switch. In response to this increased metabolic demand,
blood flow in the ONH appears to increase.184e186 Importantly,
metabolism in the various parts of RGC axons may be
controlled independently. In the prelaminar and lamina cri-
brosa regions, RGC axons are unmyelinated and acquire a
myelin sheath only after passing the posterior boundary of the
lamina cribrosa.11 The unmyelinated portions of the axons are
in need of energy to repolarize the axons along the entire
surface, whereas, in themyelinated portion, only the axons at
the nodes of Ranvier need to be repolarized. Thus, mito-
chondria are numerous andmetabolic activity is much higher
in the unmyelinated nerves, but less in the myelinated
portion.19 According to themetabolic hypothesis of blood flow
regulation, tissue perfusion and tissue metabolism are tightly
coupled in such a way that any reduction in arterial inflow
causes an increase of vasodilator metabolites in the tissue.157
5.2.1. OxygenAll living tissues require a steady supply of oxygen to meet,
but not exceed, their nutritional needs. Oxygen tension in a
tissue provides an important indication of its current meta-
bolic status. In hypoxic conditions, ONH blood flow is regu-
lated to establish a healthy partial pressure of oxygen in the
tissue. Under moderate hypoxic conditions, the intravascular
tissue’s partial pressure of oxygen at 200 mm depth within the
ONH was observed to be constant, reflecting, probably,
autoregulation of blood flow.25 Decreased partial pressure of
oxygen was found in the ONH when IOP was increased (OPP
was decreased) above 45mmHg.23 Both oxygenmetabolism in
the mitochondria of optic nerve cells and tissue oxygen ten-
sion were observed to decrease when hypoxia was induced.149
In contrast, under hyperoxic conditions, capillary blood flow
in the ONH decreases via vasoconstriction.115
5.2.2. Carbon dioxideCO2 is a vasodilator in all vascular beds at the posterior pole of
the eye.200 The mechanisms driving this vasodilation response
are not clearly understood. In human197 and animal studies,196
hypercapnia-induced vasodilation was shown to depend on
NO, whereas, in other animal studies, the vasodilation was
shown to be independent of NO.52,72 Hypercapnia triggers an
increase in flow to the short PCAs.189 Patients with untreated
primary open-angle glaucoma have shown a normal increase
in the blood velocity of the CRA with hypercapnia, but a
decrease in blood velocity in the OA.210 Although the prepon-
derance of data suggests that blood vessels dilate or constrict
in response to CO2 levels, it is possible that hypercapnia-
induced vasodilation is mainly because NO is brought into
play by lowered levels of oxygen, which would increase NO
half-life. Future research further defining the mechanisms of
this is needed.
5.2.3. Nitric oxideNOwas shown to mediate vasodilation in response to marked
hypoxia in the forearm resistance vessels in healthy
humans22 and in response to increased myocardial oxygen
demand.4 As mentioned in the previous sections, the role of
NO in hypercapnia-related vasodilation is not clearly
understood.
5.3. Neurovascular coupling
Experiments measuring vascular responses to light stimula-
tion have suggested that ONH blood flow and neuronal
activity of RGCs are coupled.68,185,186 Traditionally, in the ONH
and other regions like the retina and the brain, vascular
responses to changes in neural activity were assumed to be
controlled exclusively by a local metabolic feedback system in
which neural activity leads to energy demand and thus
vasodilation. This idea has been challenged, however,
following the discovery that feedforward mechanisms
mediate the vascular energy supply by neuronal activity.16
According to these mechanisms, active neurons either send
a signal directly to blood vessels or activate astrocytes to
release vasoactive agents onto the vessels to increase local
blood flow, a process known as “functional hyperemia.” Both
cases involve neurotransmitter signaling, particularly via
glutamate. In the brain, blocking glutamate receptors reduces
functional hyperemia but does not affect the energy use
associated with neuronal activity, providing evidence that
metabolic feedback and neurovascular feedforward mecha-
nisms can be distinguished and both contribute to blood flow
regulation in response to neuronal activity.151,216 The inter-
play between metabolic feedback and neuronal feedforward
mechanisms should then be taken into account whenever
considering clinical studies addressing ONH vascular
responses to light stimulation.
Astrocytes exhibit extremely complex and dynamic
behaviors. One of their functions is to control the extracellular
environment for neurons; for example, by clearing the space of
potassium after action potentials pass by and taking up excess
glutamate that leaks from synapses.100,101 Astrocytes also play
a fundamental role in regulating blood flow to the brain.16 As-
trocytes are the predominant glial cell type in the nonmyelin-
ated ONH inmostmammalian species.19 Structural similarities
suggest that retinal and ONH astrocytes may behave similarly
to cerebral astrocytes.10 Retinal and ONH astrocytes surround
blood vessels,8,233 and this close relationship further supports a
potential contribution of astrocytes to blood flow regulation in
the retina and ONH.
There is evidence of neurovascular coupling in the retina
and ONH occurring via glial signaling mediated by vasoactive

s u r v e y o f o p h t h a lm o l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6 177
agents released onto the vessels (not through nerve fibers and
synapsis with glutamate receptors on contractile cells). The
retinal vascular branches are devoid of such innervation once
they emerge onto the retinal surface, although the CRAwithin
the intraorbital optic nerve is innervated.243 Yet, retinal ves-
sels do retain receptors for various neurotransmitters on their
surface.58,104 To our knowledge, the innervation of the arte-
riolar branches of the ONH has not been studied yet. Future
research further elucidating the mechanisms of neuro-
vascular coupling in the retina and the ONH is essential.
Axon-glial signaling pathways have been observed in the
optic nerve.41,64,107 Following IOP elevation, autoregulation of
ONH blood flowwas not maintained in rabbit eyes where glial
cells were selectively impaired by a gliotoxic compound, L-2-
aminoadipic acid, indicating a possible involvement of astro-
cytes in ONH blood flow autoregulation.206 The cell processes
of astrocytes are connected to each other via gap junctions190
forming a functional syncytium that allows astrocytes to
communicate andmaintain control of the ionic andmetabolic
homeostasis in the ONH. Decreased gap junction communi-
cation between ONH astrocytes was reported under condi-
tions of elevated IOP.133 Closure of gap junctions interrupts
the continuity of astrocyte intercellular communication,
causing loss of cell-cell contact and homeostatic regulation.
Uncoupling gap junctions resulted in impaired ONH blood
flow autoregulation in healthy rabbits.204 Such conditions
may lead to RGC axonal loss and optic disk remodeling char-
acteristic of glaucomatous optic neuropathy.100
In studies on brain slice and isolated retina, evidence is
found that astrocytes contribute to blood flow regulation by
producing and releasingmetabolites of arachidonic acid. After
glutamate is released at synapses following neuronal activity,
some of the released neurotransmitter escapes the synaptic
cleft and activates metabotropic glutamate receptors on
astrocytes, increasing Ca2þ in astrocytes.170 The increased
Ca2þ activates phospholipase A2 and results in arachidonic
acid production. The build-up of arachidonic acid leads, in
turn, to the production of its metabolites, including prosta-
glandin E2 and epoxyeicosatrienoic acids (EETs), which dilate
nearby arterioles.74,138,163,164,219,245 In brain slices and in the
isolated retina, increased Ca2þ can also cause vessels to
constrict.44,138,145 This is made possible by the conversion of
arachidonic acid to 20-hydroxy-eicosatetraenoic acid (20-
HETE).138 When the synthetic enzymes for EETs and prosta-
glandin E2 are inhibited, glial-evoked vasodilation is reduced
by 88%.138,139 Under physiologic conditions, the effect of the
vasodilators prostaglandin E2 and EETs surpasses the effect of
20-HETE, and vasodilation occurs. However, under non-
physiologic conditions such as hyperoxia or elevated NO
levels, 20-HETEemediated vasoconstriction dominates.148
5.3.1. PotassiumGlial cells might also cause vasodilation (and hence increased
blood flow) by releasing potassium ions. Modest increases in
extracellular Kþ concentration cause the hyperpolarization of
smoothmuscle cells, a reduced influx of Ca2þ through voltage-
gated channels, and vasodilation. When glutamate released
from neurons activates astrocyte metabotropic glutamate
receptors, the resultant rise in astrocyte Ca2þ causes large-
conductance Ca2þ-activated Kþ channels in astrocyte
endfeet to open, releasing Kþ onto vessels.59 This potential
role of Kþ in neurovascular coupling has not been tested either
in the retina or in the ONH yet.
5.3.2. Nitric oxideNO is believed to mediate neurovascular coupling in the cer-
ebellum.242 Changes in neuronal activity induce Ca2þ
increases within neurons and activate neuronal NOS, leading
to the production of NO. NO is then able to permeate mem-
branes and diffuse to blood vessels, where it opens Kþ chan-
nels and relaxes vascular smooth muscle cells, leading to
vasodilation and increased blood flow.34 NO has also been
suggested to mediate neurovascular coupling in the ONH.
Increased NO levels were found in the ONH of humans during
changes in neuronal activity due to light flicker stimuli.26 In
addition, in the retina of humans54 and the retina and the
ONH of cats,114 systemic administration of nonselective NOS
inhibitors reduces flicker-evoked increases in blood flow.
These results would appear to support NO as an important
mediator of neurovascular coupling in the retina and theONH.
However, evidence has been found that NO could be pre-
dominantly a modulator of neurovascular coupling, but not a
mediator. In the whole-mount rat retina,138 at NO levels less
than 100 nmol/L, flickering lighteinduced vasodilation, but
not vasoconstriction. At increased NO levels, smaller vasodi-
lation was observed, while vasoconstriction became more
common. At 10 mmol/L NO, a flickering light evoked large
vasoconstriction, mediated by 20-HETE. It is still not clear how
NO inhibits flicked-induced vasodilation. The modulatory
mechanism of NO has been suggested to be due to its
inhibitions of P450 epoxygenase, which synthesizes the
vasodilator EETs. With less EETs being produced, vasodilation
will be smaller and vasoconstrictionmediated by 20-HETEwill
prevail.148
5.3.3. AdenosineAdenosine, which is produced when adenosine triphosphate
is hydrolyzed, is a vasodilator that contributes to functional
hyperemia in the human brain. Blocking adenosine receptors
has been shown to reduce the increase in blood flow evoked
by neuronal activity.113 Given its function in the brain, aden-
osine is also thought to be involved in the control of ocular
blood flow. Intravenous administration of adenosine was
shown to cause increased choroidal and ONH blood flow in
humans168 andwas established as an important participant in
mediating retinal blood flow autoregulation during hypoxia
and hypotension in piglets.71
5.3.4. LactateLactate, which is produced by anaerobic glycolysis and
released by both glial cells and neurons, is thought to
contribute to neurovascular coupling as a metabolic negative-
feedbackmediator. Blood flow in the human retina is sensitive
to changes in blood lactate levels,69 but lactate responses in
the ONH have not been established yet.
5.3.5. OxygenOxygen modulates neurovascular coupling in brain tissue by
altering the synthesis of glial and neuronalmessengers and by
altering the levels of lactate and adenosine. O2 is needed for

s u r v e y o f o p h t h a lmo l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6178
the synthesis of NO and the vasoactive messengers derived
from arachidonic acid. At in vivo levels of O2, the synthesis of
NO and 20-HETE is expected to be limited by the amount of O2
available.16 More specifically, 20-HETE formation is sup-
pressed in the presence of low O2 concentrations, leading to a
reduced vasoconstriction when arachidonic acid is generated
in astrocytes. A lower O2 also results in a reduced amount of
NO being present to inhibit the formation of vasodilatory EETs
in tissues. In addition, when O2 concentrations decrease, the
lack of energy for adenosine triphosphate synthesis causes an
increase in the level of extracellular adenosine, which binds to
adenosine A2A receptors on vascular smooth muscle cells to
depress vessel constriction.16 Moreover, low oxygen concen-
trations induce a decrease in the rate of oxidative phosphor-
ylation relative to the rate of glycolysis, resulting in lactate
production. Monocarboxylate transporters release the lactate
into the extracellular space, where it reduces the reuptake of
prostaglandin E2 by the prostaglandin transporter, promoting
vasodilation.74 Importantly, it is possible that oxygen modu-
lates neurovascular coupling by affecting NO half-life. Future
research to further elucidate the mechanisms involved is
needed.
5.4. The anatomic agents of blood flow regulation in theONH
Understanding which vessels in the ONH exhibit blood flow
regulation is still an open problem. In general, the structures
that control blood flow to a particular tissue are the resistance
arterioles (arterial branches smaller than 40 mm), which
change diameter actively to achieve autoregulation.172 Capil-
laries have smaller diameters than arterioles. Because resis-
tance to flow is inversely proportional to the fourth power of
the vessel radius (Poiseuille’s law),65 capillaries exhibit high
resistance individually; However, they are numerous and in
parallel array outweigh the reduction in vessel size, having a
large cross-sectional area that does not contribute much to
the net resistance between arteries and veins.246 In addition to
the arterial role in autoregulation, flow to a local capillary
network is controlled by a precapillary sphincter (where it
exists), a band of smooth muscle located where capillaries
originate from small arterioles.6 Finally, blood flow could also
be controlled by changes in the resistance of the capillaries
themselves because of the contractile properties of pericytes.6
The retinal and optic nerve capillaries lack precapillary
sphincters but have abundant pericytes.62,63,161 It has been
shown that pericytes respond to carbon dioxide concentra-
tions (mediated by pH), oxygen levels (affected by changes in
NO concentrations), and adenosine levels.161 Vascular smooth
muscle cells respond in a very similar manner.36,82,136 Peri-
cytes on isolated retinal capillaries were found to constrict or
dilate in response to neurotransmitters, following Ca2þ alter-
ations.173 In situ in brain slices, pericytes constricted in
response to noradrenaline and dilated in response to gluta-
mate,165 and in the isolated retina, blocking ionotropic
receptors of the neurotransmitter g-aminobutyric acid was
shown to constrict capillaries, suggesting that endogenous
transmitter release could regulate capillary diameter.83,165
Thus, capillaries are equipped to participate in the control of
ONH blood flow through metabolic feedback and neuronal
feedforward coupling mechanisms, but this still needs to be
further investigated.
In contrast to retinal capillaries, choroidal capillaries are
fenestrated and have sparse pericytes.161 The retinal tissue,
part of the central nervous system, has a preserved blood-
brain barrier due to the tight junctions between the retinal
pigment epithelial cells.161,179 Circulating vasoconstrictive
hormones, such as angiotensin, epinephrine, and so forth,
raise intracellular Ca2þ when they occupy receptors.157,161,200
In vascular smooth muscle, as well as in pericytes, an
increase of intracellular Ca2þ induces contraction of the cell.
Consequently, ECs produce more NO, which relax vascular
smooth muscle and pericytes. In this way, the circulating
hormones leak through fenestrated vessels, reach the
muscular coat of vessels in the region, causing vasoconstric-
tion in the skin and many visceral organs. In contrast, central
nervous vessels, where hormones can only affect ECs and are
prevented from reaching the contractile coat of the vessels to
the blood-tissue barrier, exhibit vasodilation.157,161,200 It is
unclear whether the branches of the PCAs that feed the
intrascleral portion of the optic nerve are innervated and/or
fenestrated. Such knowledge is fundamental to understand
how the intrascleral papillary tissue reacts to various insults,
including abnormally high IOP.
6. Pathologic effects of impaired blood flowregulation in the ONH
Impaired blood flow regulation has been suggested to render
the ONH more susceptible to damage by compromising blood
supply (leading to ischemia) and, consequently, oxygen
delivery (leading to hypoxia) in potentially dangerous situa-
tions of reduced BP, increased IOP, and/or increased local
metabolic demands.12,117,141 However, the mechanisms
through which the damage occurs are still not completely
understood.175,231 Evidence suggests that ischemia and
related hypoxia might influence the astrocytes in the ONH101
and/or the mitochondria of RGC axons,158 and that the ulti-
mate death of RGCs can occur via apoptosis158,175 and/or
autophagy.124
Astrocytes in the ONH are considered to be quiescent in
normal conditions,18 but they appear to become reactive in
response to pathologic conditions and contribute to changes
in the ONH environment, which do not favor the survival or
growth of the RGC axons.125 During ischemia, hydrogen
peroxide was observed to be abundantly produced and to
promote pathologic excitatory amino acid release and
swelling of reactive astrocytes.102 In glaucoma, ONH astro-
cytes were observed to have lower basal levels of antioxidant
glutathione, which plays an important role in protecting the
mitochondrial electron transport chain from damage by
oxidative stress in both astrocytes and neurons.132 Evidence
suggests that reactive astrocytes also produce NO in supra-
normal quantities, and this excessive production seems to
have neurotoxic effects on the RGC axons.126,143,147,244
Ischemia, hypoxia, and perfusion instability can also dam-
age the astrocyte-astrocyte gap junctions, and, as a conse-
quence, compromise the homeostasis of the RGC axons101 and

s u r v e y o f o p h t h a lm o l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6 179
reduce the autoregulation capabilities of the ONH vasculature,
thereby rendering the ONH even more susceptible to damage.
Impaired autoregulation might also cause perfusion
instability in the ONH,141 which, in turn, might compromise
the normal function of mitochondria in the RGCs and their
axons.158 Mitochondria play a critical role in the maintenance
of cellular homeostasis and they are abundantly present in
the RGCs, both inside and outside the eye globe.158 Mito-
chondria are involved in numerous metabolic functions,
including the metabolism of oxidative energy, the regulation
of intracellular calcium levels and pH, and the production of
reactive oxygen species, which promote and regulate
apoptosis.35,201
Apoptosis (“self-killing”) and autophagy (“self-eating”)
are 2 different mechanisms that regulate cell life. The rela-
tionship between apoptosis and autophagy is complex in the
sense that, under certain circumstances, autophagy consti-
tutes a stress adaptation that avoids cell death (and sup-
presses apoptosis), whereas in other cellular settings, it
constitutes an alternative cell-death pathway.131 Ischemia
and hypoxia increase the reactive oxygen species produc-
tion rate from mitochondria35,42,159 and favor the
mitochondria-mediated cellular apoptosis; however,
reactive oxygen species accumulation stimulates cellular
authophagy under conditions of nutrient deficiency,192
hypoxia, ischemia, and perfusion instability86 and other
pathologic conditions.57
7. Mathematical modeling of ONH blood flowregulation
In the previous sections, we have reviewed experimental and
clinical evidence of blood flow autoregulation in the ONH, the
mechanisms contributing to autoregulation and the patho-
logic consequences of vascular dysregulation. Despite sig-
nificant recent advances in the understanding of ONH blood
flow autoregulation, important questions remain unan-
swered. What are the relative contributions of various
mechanisms, including responses to mechanical, metabolic,
and neurovascular stimuli, in achieving ONH blood flow
autoregulation? Do these relative contributions change in
health and disease? Is the vascular energy supply by neural
activity in the ONH mediated by feedforward mechanisms, as
it happens in the brain? Are capillaries involved in ONH blood
flow autoregulation? To which extent do impairments in dAR
and sAR mechanisms contribute to glaucomatous damage?
Can dynamic and static mechanisms be independently
impaired?
The quest for answers to these questions is hindered by
limitations in both the technological and scientific tools
currently available to the community. Major limitations in
the current technologies for ONH measurements include the
fact that LDF, LSFG, and OCT angiography only provide ONH
blood flow estimates in arbitrary units and intereye
comparison is problematic. LDF appears to be mainly influ-
enced by blood flow changes in the more superficial layers of
the ONH than deeper ones, but to what extent remains un-
certain. OCT angiography provides measurements for the
ONH deeper layers which may contain spurious projections
from the superficial layers. In addition to the difficulties
related to structural and functional imaging of the ONH,
there are scientific challenges in designing experimental and
clinical studies capable of disentangling the complex inter-
play between chemical, mechanical, and hemodynamic
factors that contribute to the pathogenesis of optic neu-
ropathies. Given these challenges, mathematical modeling
provides a unique tool that can play a significant role in
advancing the understanding of ONH physiology in health
and disease. A mathematical model can serve as a virtual
laboratory where the influence of each factor acting on the
system can be singled out and investigated, from a theo-
retical viewpoint, in isolation or in relation with other fac-
tors. In the following, we review the main contributions to
the modeling of the biomechanics, perfusion, and autor-
egulation in the ONH and indicate some interesting future
directions of research.
7.1. Biomechanics of the ONH
Alterations in the ONH biomechanical response to changes in
IOP have been identified as a major factor in the pathogenesis
of glaucoma.28,174 Particular attention has been devoted to
the mathematical description of the mechanical stresses and
strains arising within the lamina cribrosa, which is thought
to be a primary site of axonal injury in glaucoma.176 A linear
model of elastic mechanics theory on the bending of thin
circular plate was developed for the lamina cribrosa.53 Such
an idealization allowed quantitative estimates to be obtained
of the extent to which the degree of fixity offered by the
connection with the sclera, the pretension caused by scleral
expansion, and the ratio between flexural and in-plane
stiffness influence the mechanical response of the lamina
cribrosa to IOP. An idealized, analytical microstructural
model of the ONH load bearing tissues based on an octagonal
cellular solid description of the porosity within the lamina
identified the material and geometrical properties of the
sclera as major determinants in the strain distributions
within the lamina.193 The analysis also showed that much
larger strains are developed perpendicular to the major axis
of an elliptical canal rather than in a circular canal. Eye-
specific finite element models based on experimentally
reconstructed geometries have been used recently.188,207,208
These models are used to study in depth influences of ge-
ometry and material properties of the ONH to changes in IOP
and to investigate growth and remodeling mechanisms in
glaucoma, including adaptation of tissue anisotropy, tissue
thickening/thinning, tissue elongation/shortening, and tissue
migration. Macro- and micro-scale strains are proposed as
potential control mechanisms governing mechanical home-
ostasis.76e80 Further development of these sophisticated
finite element models may benefit from the recent advances
in OCT imaging aimed at providing a more accurate charac-
terization of the architectural microstructure within the
lamina cribrosa.209
7.2. Perfusion of the ONH
Perfusion of the ONH results from the complex interplay
between BP, which provides the driving force for the blood

s u r v e y o f o p h t h a lmo l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6180
through the vasculature, “mechanical stress,” which acts as
external forces on the vessels, and “vascular regulation,”
which mediates vessel dilation/constriction to compensate
for changes in the system. To the best of our knowledge, only
one model combines biomechanics and hemodynamics in
the lamina cribrosa.33 In this model, the lamina cribrosa is
treated as a 2 dimensional poroelastic material, where blood
vessels are viewed as pores in a solid matrix. The presence of
blood vessels in the tissue defines a vascular porosity N
which changes with the local state of stress and strain. The
tissue permeability, which defines the ability of the porous
material to allow fluid passing through it, is assumed pro-
portional to the square of N; the solid matrix is assumed to
behave as nonlinear elastic material. Blood flow is driven by
the difference between the arterial pressure in the short PCAs
and the venous pressure in the CRV. The lamina cribrosa
deforms under the combined action of IOP, retrolaminar
tissue pressure and scleral tension. This exploratory 2-
dimensional analysis suggested that the degree of fixity at
the conjunction with the sclera has a strong influence on the
distributions of stresses and strains, as suggested in other
studies,53,193 but also on the blood flow within the lamina. In
particular, the inner surface of the lamina was found to be
more susceptible to experiencing reduced blood supply
following IOP elevation. Despite the many simplifying
assumptions adopted in the model, most importantly the
choice of a 2-dimensional geometry, the satisfactory quali-
tative agreement between experimental data and numerical
simulations encourages further investigation of poroelastic
models to describe the complex mechanisms governing ONH
perfusion.
7.3. Autoregulation of ONH blood flow
Mathematical models have been used to describe autor-
egulation of blood flow in various organs, including
brain,2,48,213,229 kidney,37,116,127,202 and skeletal muscle.13,31,227
To date, though, the theoretical modeling of autoregulation
in the ONH has not yet been addressed. Our group has
developed a vascular wall mechanics model to predict the
relative importance of regulatory mechanisms in achieving
blood flow regulation in the retina.14 Resistance vessels are
assumed to respond to changes in pressure, shear stress,
carbon dioxide (CO2), and the downstream metabolic state
communicated via conducted responses. The model quan-
tifies the relative contributions of different mechanisms to
retinal autoregulation and shows that the combined effects of
the metabolic and CO2 responses are critical for achieving
retinal autoregulation. It would be extremely interesting to
apply a similar modeling approach to the autoregulation in
the ONH, combine it with the poroelastic model mentioned
above,33 and explore the relative contributions of the various
mechanisms described in section 5. Most of the models for
blood flow autoregulation, including the one developed by our
group for the retinal circulation,14 are not time dependent.
Thus, they are suitable to study static, but not dynamic,
autoregulation. The dAR has been addressed by models for
brain154,228 and kidneys.135
7.4. Conclusions and future directions
The mathematical modeling of the interplay between
biomechanics, perfusion and autoregulation in the ONH is
still at its early stages, but is quickly attracting attention.
Elucidating the complex interactions of ONH perfusion and
tissue structure in health and disease using current imaging
methodologies is difficult, and mathematical modeling pro-
vides an approach to solving these limitations. One of the
main difficulties lies in the fact that the biophysical phe-
nomena governing the ONH physiology occur at different
scales in time and space. For example, BP, IOP and retro-
laminar tissue pressure oscillate within a cardiac cycle (1
second), but also during the course of a day (24 hours). The
characteristic time for the onset of autoregulation is
approximately 20 seconds,119 and the biomechanically
induced remodeling of the collagen network in the ONH takes
several months or years. The accurate modeling and simu-
lation of multiscale problems is still an open area of research,
and therefore the modeling of the ONH offers stimulating
opportunities for groundbreaking activities from both the
clinical and theoretical viewpoints. The application of dy-
namic modeling to reveal the mechanistic interplay of pre-
viously unseen physiologic relationships holds the potential
to advance medical care in ophthalmic disease and provide
patients and clinicians new hope for future diagnosis and
therapy.
8. Method of literature search
A literature search using the PubMed and Web of Science
search engines and available library databases was used with
reference cross-matching to obtain relevant peer reviewed
articles published on blood flow autoregulation and ocular
biomechanics and hemodynamics. The article search
included available published studies from 1900 to April 2015.
Separate searches were performed by the primary author
(D.P.), and 3 independent researchers (G.G., A.M.H., and J.A.)
until relevant articles were identified. A few selected articles
published before 1990 are included for their clinical relevance,
but the review ismainly based on articles published in the last
2 decades. The following were themajor search terminologies
used: “autoregulation in the optic nerve head,” “neurovascular
coupling,” “ocular biomechanics and hemodynamics,” “me-
chanical influences on autoregulation,” “metabolic autor-
egulation,” “effects of impaired blood flow regulation,” “optic
neuropathies,” “glaucoma,” “blood flow regulation modeling.”
Articles published in languages other than English were also
included during the literature search.
Acknowledgments
The authors acknowledge the valuable contribution of Ales-
sandra Cantagallo, who realized the anatomic drawings (Figs.
1e5) published in this article.

s u r v e y o f o p h t h a lm o l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6 181
r e f e r e n c e s
1. The Advanced Glaucoma Intervention Study (AGIS): 7. Therelationship between control of intraocular pressure andvisual field deterioration. The AGIS Investigators. Am JOphthalmol. 2000;130(4):429e40
2. Alastruey J, Moore SM, Parker KH, et al. Reduced modellingof blood flow in the cerebral circulation: coupling 1-D, 0-Dand cerebral auto-regulation models. Int J Numer MethodsFluids. 2008;56(8):1061e7
3. Alm A, Bill A. Ocular and optic nerve blood flow at normaland increased intraocular pressures in monkeys (Macacairus): a study with radioactively labelled microspheresincluding flow determinations in brain and some othertissues. Exp Eye Res. 1973;15(1):15e29
4. Altman JD, Kinn J, Duncker DJ, Bache RJ. Effect of inhibitionof nitric oxide formation on coronary blood flow duringexercise in the dog. Cardiovasc Res. 1994;28(1):119e24
5. Anderson DR. Introductory comments on blood flowautoregulation in the optic nerve head and vascular riskfactors in glaucoma. Surv Ophthalmol. 1999;43(Suppl 1):S5e9
6. Anderson DR. Glaucoma, capillaries and pericytes. 1. Bloodflow regulation. Ophthalmologica. 1996;210(5):257e62
7. Anderson DR. Ultrastructure of the optic nerve head. ArchOphthalmol. 1970;83(1):63e73
8. Anderson DR. Vascular supply to the optic nerve of primates.Am J Ophthalmol. 1970;70(3):341e51
9. Anderson DR. Ultrastructure of human and monkey laminacribrosa and optic nerve head. Arch Ophthalmol.1969;82(6):800e14
10. Anderson D, Hoyt W, Hogan M. The fine structure of theastroglia in the human optic nerve and optic nerve head.Trans Am Ophthalmol Soc. 1967;65:275e305
11. Andrews RM, Griffiths PG, Johnson MA, Turnbull DM.Histochemical localisation of mitochondrial enzyme activityin human optic nerve and retina. Br J Ophthalmol.1999;83(2):231e5
12. Araie M, Crowston J, Iwase A, et al. Clinical relevance ofocular blood flow (OBF) measurements including effects ofgeneral medications or specific glaucoma treatment, inWeinreb RN, Harris A (eds) Ocular Blood Flow in Glaucoma.Amsterdam, Kugler Publications; 2009, pp 59e128
13. Arciero JC, Carlson BE, Secomb TW. Theoretical model ofmetabolic blood flow regulation: roles of ATP release by redblood cells and conducted responses. Am J Physiol Heart CircPhysiol. 2008;295(4):H1562e71
14. Arciero J, Harris A, Siesky B, et al. Theoretical analysis ofvascular regulatory mechanisms contributing to retinalblood flow autoregulation. Invest Ophthalmol Vis Sci.2013;54(8):5584e93
15. Attariwala R, Giebs CP, Glucksberg MR. The influence ofelevated intraocular pressure on vascular pressures inthe cat retina. Invest Ophthalmol Vis Sci.1994;35(3):1019e25
16. Attwell D, Buchan AM, Charpak S, et al. Glial and neuronalcontrol of brain blood flow. Nature. 2010;468(7321):232e43
17. Awai T. Angioarchitecture of intraorbital part of humanoptic nerve. Jpn J Ophthalmol. 1985;29(1):79e98
18. Bachoo RM, Kim RS, Ligon KL, et al. Molecular diversity ofastrocytes with implications for neurological disorders. ProcNatl Acad Sci U S A. 2004;101(22):8384e9
19. Balaratnasingam C, Kang M, Yu P, et al. Comparativequantitative study of astrocytes and capillary distribution inoptic nerve laminar region. Exp Eye Res. 2014;121:11e22
20. Beckman JS, Beckman TW, Chen J, et al. Apparent hydroxylradical production by peroxynitrite: implications for
endothelial injury from nitric oxide and superoxide. ProcNatl Acad Sci U S A. 1990;87(4):1620e4
21. Berne RM, Knabb RM, Ely SW, Rubio R. Adenosine in the localregulation of blood flow: a brief overview. Fed Proc.1983;42(15):3136e42
22. Blitzer ML, Lee SD, Creager MA. Endothelium-derived nitricoxide mediates hypoxic vasodilation of resistance vessels inhumans. Am J Physiol. 1996;271(3 Pt 2):H1182e5
23. Blumenroder S, Augustin AJ, Koch FH. The influence ofintraocular pressure and systemic oxygen tension on theintravascular pO2 of the pig retina as measured withphosphorescence imaging. Surv Ophthalmol.1997;42(Suppl 1):S118e26
24. Boltz A, Schmidl D, Werkmeister R, et al. Regulation of opticnerve head blood flow during combined changes inintraocular pressure and arterial blood pressure. J CerebBlood Flow Metab. 2013;33(12):1850e6
25. Bouzas EA, Donati G, Pournaras CJ. Distribution andregulation of the optic nerve head tissue PO2. SurvOphthalmol. 1997;42(Suppl 1):S27e34
26. Buerk D, Riva C. Adenosine enhances functional activationof blood flow in cat optic nerve head during photicstimulation independently from nitric oxide. Miscovasc Res.2002;64(2):254e64
27. Buerk DG, Riva CE, Cranstoun SD. Nitric oxide has avasodilatory role in cat optic nerve head during flickerstimuli. Microvasc Res. 1996;52(1):13e26
28. Burgoyne CF. A biomechanical paradigm for axonal insultwithin the optic nerve head in aging and glaucoma. Exp EyeRes. 2011;93(2):120e32
29. Burgoyne CF, Downs JC, Bellezza AJ, et al. The optic nervehead as a biomechanical structure: a new paradigm forunderstanding the role of IOP-related stress and strain in thepathophysiology of glaucomatous optic nerve head damage.Prog Retin Eye Res. 2005;24(1):39e73
30. Caprioli J, Coleman AL. Blood Flow in Glaucoma D. Bloodpressure, perfusion pressure, and glaucoma. Am JOphthalmol. 2010;149(5):704e12
31. Carlson BE, Arciero JC, Secomb TW. Theoretical model ofblood flow autoregulation: roles of myogenic, shear-dependent, and metabolic responses. Am J Physiol HeartCirc Physiol. 2008;295(4):H1572e9
32. Carlstrom M, Wilcox CS, Arendshorst WJ. Renalautoregulation in health and disease. Physiol Rev.2015;95(2):405e511
33. Causin P, Guidoboni G, Harris A, et al. A poroelastic modelfor the perfusion of the lamina cribrosa in the optic nervehead. Math Biosci. 2014;257:33e41
34. Cavet M, Vittitow J, Impagnatiello F, et al. Nitric oxide (NO):an emerging target for the treatment of glaucoma. InvestOphthalmol Vis Sci. 2014;55(8):5005e15
35. Chan DC. Mitochondria: dynamic organelles in disease,aging, and development. Cell. 2006;125(7):1241e52
36. Chen Q, Anderson DR. Effect of CO2 on intracellular pH andcontraction of retinal capillary pericytes. Invest OphthalmolVis Sci. 1997;38(3):643e51
37. Chen J, Sgouralis I, Moore LC, et al. A mathematical model ofthe myogenic response to systolic pressure in the afferentarteriole. Am J Physiol Renal Physiol. 2011;300(3):F669e81
38. Chien S. Mechanotransduction and endothelial cellhomeostasis: the wisdom of the cell. Am J Physiol Heart CircPhysiol. 2007;292(3):H1209e24
39. Chiquet C, Lacharme T, Riva C, et al. Continuous response ofoptic nerve head blood flow to increase of arterial blood pressurein humans. Invest Ophthalmol Vis Sci. 2014;55(1):485e91
40. Chiueh CC. Neuroprotective properties of nitric oxide. Ann NY Acad Sci. 1999;890:301e11

s u r v e y o f o p h t h a lmo l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6182
41. Choi I, Chiu S. Expression of high-affinity neuronal and glialglutamate transporter in the rat optic nerve. Glia.1997;20(3):184e92
42. Christophe M, Nicolas S. Mitochondria: a target forneuroprotective interventions in cerebral ischemia-reperfusion. Curr Pharm Des. 2006;12(6):739e57
43. Chung H, Harris A, Evans D, et al. Vascular aspect in thepathophysiology of glaucomatous optic neuropathy. SurvOphthalmol. 1999;43(Supplement I):S43e50
44. Chuquet J, Hollender L, Nimchinsky EA. High-resolutionin vivo imaging of the neurovascular unit during spreadingdepression. J Neurosci. 2007;27(15):4036e44
45. Coleman AL, Caprioli J. The logic behind target intraocularpressure. Am J Ophthalmol. 2009;147(3):379e80
46. Costa V, Harris A, Anderson D, et al. Ocular perfusionpressure in glaucoma. Acta Ophthalmol.2014;92(4):e252e66
47. Cull G, Burgoyne C, Fortune B, Wang L. Longitudinalhemodynamic changes within the optic nerve head inexperimental glaucoma. Invest Ophthalmol Vis Sci.2013;54(6):4271e7
48. David T, Alzaidi S, Chatelin R, Farr H. Coupledautoregulation models. 13th International Conference onBiomedical Engineering, Vols 1-3. 2009;23(1-3):1896e1899.
49. Davies PF. Overview: temporal and spatial relationships inshear stress-mediated endothelial signalling. J Vasc Res.1997;34(3):208e11
50. Delles C, Michelson G, Harazny J, et al. Impaired endothelialfunction of the retinal vasculature in hypertensive patients.Stroke. 2004;35(6):1289e93
51. Delori FC, Pflibsen KP. Spectral reflectance of the humanocular fundus. Appl Opt. 1989;28(6):1061e77
52. Donati G, Pournaras CJ, Munoz JL, Tsacopoulos M. [The roleof nitric oxide in retinal vasomotor regulation]. Klin MonblAugenheilkd. 1994;204(5):424e6
53. Dongqi H, Zeqin R. A biomathematical model for pressure-dependent lamina cribrosa behavior. J Biomech.1999;32(6):579e84
54. Dorner GT, Garhofer G, Kiss B, et al. Nitric oxide regulatesretinal vascular tone in humans. Am J Physiol Heart CircPhysiol. 2003;285(2):H631e6
55. Downs JC, Roberts MD, Burgoyne CF, Hart RT. Multiscalefinite element modeling of the lamina cribrosamicroarchitecture in the eye. Conf Proc IEEE Eng Med BiolSoc. 2009;2009:4277e80
56. Drance SM, Douglas GR, Wijsman K, et al. Response of bloodflow to warm and cold in normal and low-tension glaucomapatients. Am J Ophthalmol. 1988;105(1):35e9
57. Essick EE, Sam F. Oxidative stress and autophagy in cardiacdisease, neurological disorders, aging and cancer. Oxid MedCell Longev. 2010;3(3):168e77
58. Ferrari-Dileo G. Beta 1 and beta 2 adrenergic binding sites inbovine retina and retinal blood vessels. Invest OphthalmolVis Sci. 1988;29(5):695e9
59. Filosa J, Bonev A, Straub S, et al. Local potassium signalingcouples neuronal activity to vasodilation in the brain. NatNeurosci. 2006;9:1397e403
60. Flammer J, Orgul S, Costa V, et al. The impact of ocular bloodflow in glaucoma. Prog Retin Eye Res. 2002;21(4):359e93
61. Folkow B, Sonnenschein RR, Wright DL. Loci of neurogenicand metabolic effects on precapillary vessels of skeletalmuscle. Acta Physiol Scand. 1971;81(4):459e71
62. Frank RN, Dutta S, Mancini MA. Pericyte coverage is greaterin the retinal than in the cerebral capillaries of the rat. InvestOphthalmol Vis Sci. 1987;28(7):1086e91
63. Frank RN, Turczyn TJ, Das A. Pericyte coverage of retinal andcerebral capillaries. Invest Ophthalmol Vis Sci.1990;31(6):999e1007
64. Fu C, Sretavan D. Ectopic vesicular glutamate release at theoptic nerve head and axon loss in mouse experimentalglaucoma. J Neurosci. 2012;32(45):15859e76
65. FungYC. Biomechanics: circulation. NewYork, Springer; 199766. Galambos P, Vafiadis J, Vilchez S, et al. Compromised
autoregulatory control of ocular hemodynamics in glaucomapatients after postural change. Ophthalmology.2006;113(10):1832e6
67. Galassi F, Giambene B, Varriale R. Systemic vasculardysregulation and retrobulbar hemodynamics in normal-tension glaucoma. Invest Ophthalmol Vis Sci.2011;52(7):4467e71
68. Garhofer G, Resch H, Weigert G, et al. Short-term increase ofintraocular pressure does not alter the response of retinaland optic nerve head blood flow to flicker stimulation. InvestOphthalmol Vis Sci. 2005;46(5):1721e5
69. Garhofer G, Zawinka C, Huemer K, et al. Flicker light-induced vasodilation in the human retina: effect of lactateand changes in mean arterial pressure. Invest OphthalmolVis Sci. 2003;44(12):2216e27
70. Geijer C, Bill A. Effects of raised intraocular pressure onretinal, prelaminar, laminar, and retrolaminar optic nerveblood flow in monkeys. Invest Ophthalmol Vis Sci.1979;18(10):1030e42
71. Gidday J, Park T. Adenosine-mediated autoregulation ofretinal arteriolar tone in the piglet. Invest Ophthalmol VisSci. 1993;34(9):2713e9
72. Gidday JM, Zhu Y. Nitric oxide does not mediateautoregulation of retinal blood flow in newborn pig. Am JPhysiol. 1995;269(3 Pt 2):H1065e72
73. Glucksberg MR, Dunn R. Direct measurement of retinalmicrovascular pressures in the live, anesthetized cat.Microvasc Res. 1993;45(2):158e65
74. Gordon GR, Choi HB, Rungta RL, et al. Brain metabolismdictates the polarity of astrocyte control over arterioles.Nature. 2008;456(7223):745e9
75. Grieshaber M, Mozaffarieh M, Flammer J. What is the linkbetween vascular dysregulation and glaucoma? SurvOphthalmol. 2007;52(Suppl 2):S144e54
76. Grytz R, Girkin CA, Libertiaux V, Downs JC. Perspectives onbiomechanical growth and remodeling mechanisms inglaucoma. Mech Res Commun. 2012;42:92e106
77. Grytz R, Meschke G. A computational remodeling approachto predict the physiological architecture of the collagen fibrilnetwork in corneo-scleral shells. Biomech ModelMechanobiol. 2010;9(2):225e35
78. Grytz R, Meschke G. Constitutive modeling of crimpedcollagen fibrils in soft tissues. J Mech Behav Biomed Mater.2009;2(5):522e33
79. Grytz R, Meschke G, Jonas JB. The collagen fibril architecturein the lamina cribrosa and peripapillary sclera predicted by acomputational remodeling approach. Biomech ModelMechanobiol. 2011;10(3):371e82
80. Grytz R, Sigal IA, Ruberti JW, et al. Lamina cribrosathickening in early glaucoma predicted by a microstructuremotivated growth and remodeling approach. Mech Mater.2012;44:99e109
81. Guidoboni G, Harris A, Cassani S, et al. Intraocularpressure, blood pressure, and retinal blood flowautoregulation: a mathematical model to clarify theirrelationship and clinical relevance. Invest Ophthalmol VisSci. 2014;55(7):4105e18
82. Haefliger IO, Chen Q, Anderson DR. Effect of oxygen onrelaxation of retinal pericytes by sodium nitroprusside.Graefes Arch Clin Exp Ophthalmol. 1997;235(6):388e92
83. Hall CN, Reynell C, Gesslein B, et al. Capillary pericytesregulate cerebral blood flow in health and disease. Nature.2014;508(7494):55e60

s u r v e y o f o p h t h a lm o l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6 183
84. Hamard P, Hamard H, Dufaux J. Blood flow rate in themicrovasculature of the optic nerve head in primary openangle glaucoma. A new approach. Surv Ophthalmol.1994;38(Suppl):S87e93, discussion S4.
85. Hamard P, Hamard H, Dufaux J, Quesnot S. Optic nerve headblood flow using a laser Doppler velocimeter andhaemorheology in primary open angle glaucoma and normalpressure glaucoma. Br J Ophthalmol. 1994;78(6):449e53
86. Hariharan N, Zhai P, Sadoshima J. Oxidative stressstimulates autophagic flux during ischemia/reperfusion.Antioxid Redox Signal. 2011;14(11):2179e90
87. Harris A, Januleviciene I, Siesky B, et al. Clinicalmeasurements of ocular blood flow, in Weinreb RN, Harris A(eds) Ocular Blood Flow in Glaucoma. Amsterdam, KuglerPublications; 2009, pp 19e56
88. Harris A, Jonescu-Cuypers C, Martin B, et al. Simultaneousmanagement of blood flow and IOP in glaucoma. ActaOphthalmol Scand. 2001;79(4):336e41
89. Harris A, Jonescu-Cuypers CP, Kagemann L, et al. Atlas ofocular blood flow. Oxford, UK, Butterworth-Heinemann; 2ed, 2010
90. Harris A, Rechtman E, Siesky B, et al. The role of optic nerveblood flow in the pathogenesis of glaucoma. OphthalmolClin North Am. 2005;18(3):345e53, v.
91. Harris A, Sergott R, Spaeth G, et al. Color Doppler analysis ofocular vessel blood velocity in normal-tension glaucoma.Am J Ophthalmol. 1994;118(5):642e9
92. Hayreh SS. The blood supply of the optic nerve head and theevaluation of itdmyth and reality. Prog Retin Eye Res.2001;20(5):563e93
93. Hayreh SS. Factors influencing blood flow in the optic nervehead. J Glaucoma. 1997;6(6):412e25
94. Hayreh SS. The 1994 Von Sallman Lecture. The optic nervehead circulation in health and disease. Exp Eye Res.1995;61(3):259e72
95. Hayreh SS. Inter-individual variation in blood supply of theoptic nerve head. Its importance in various ischemicdisorders of the optic nerve head, and glaucoma, low-tension glaucoma and allied disorders. Doc Ophthalmol.1985;59(3):217e46
96. Hayreh SS, Joos KM, Podhajsky PA, Long CR. Systemicdiseases associated with nonarteritic anterior ischemic opticneuropathy. Am J Ophthalmol. 1994;118(6):766e80
97. Hayreh SS, Servais GE, Virdi PS. Fundus lesions in malignanthypertension. V. Hypertensive optic neuropathy.Ophthalmology. 1986;93(1):74e87
98. Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL.Nocturnal arterial hypotension and its role in optic nervehead and ocular ischemic disorders. Am J Ophthalmol.1994;117(5):603e24
99. Helmke BP. Molecular control of cytoskeletal mechanics byhemodynamic forces. Physiology (Bethesda). 2005;20:43e53
100. Hernandez M. The optic nerve head in glaucoma: role ofastrocytes in tissue remodeling. Prog Retin Eye Res.2000;19(3):297e321
101. Hernandez MR, Miao H, Lukas T. Astrocytes in glaucomatousoptic neuropathy. Prog Brain Res. 2008;173:353e73
102. Hirrlinger J, Gutterer JM, Kussmaul L, et al. Microglial cells inculture express a prominent glutathione system for thedefense against reactive oxygen species. Dev Neurosci.2000;22(5-6):384e92
103. Hollows FC, Graham PA. Intra-ocular pressure, glaucoma,and glaucoma suspects in a defined population. Br JOphthalmol. 1966;50(10):570e86
104. Hoste AM, Boels PJ, Brutsaert DL, De Laey JJ. Effect of alpha-1 and beta agonists on contraction of bovine retinalresistance arteries in vitro. Invest Ophthalmol Vis Sci.1989;30(1):44e50
105. Huang D, Swanson EA, Lin CP, et al. Optical coherencetomography. Science. 1991;254(5035):1178e81
106. Inoue R, Hangai M, Kotera Y, et al. Three-dimensional high-speed optical coherence tomography imaging of laminacribrosa in glaucoma. Ophthalmology. 2009;116(2):214e22
107. Jeffery G, Sharp C, Malitschek B, et al. Cellular localisation ofmetabotropic glutamate receptors in the mammalian opticnerve: a mechanism for axon-glia communication. BrainRes. 1996;741(1-2):75e81
108. Jia Y, Morrison JC, Tokayer J, et al. Quantitative OCTangiography of optic nerve head blood flow. Biomed OptExpress. 2012;3(12):3127e37
109. Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherencetomography. Opt Express. 2012;20(4):4710e25
110. Kass MA, Heuer DK, Higginbotham EJ, et al. The OcularHypertension Treatment Study: a randomized trialdetermines that topical ocular hypotensive medicationdelays or prevents the onset of primary open-angleglaucoma. Arch Ophthalmol. 2002;120(6):701e13, discussion829e30.
111. Katsi V, Vlachopoulos C, Souretis G, et al. Associationbetween retinal microcirculation and aortic stiffness inhypertensive patients. Int J Cardiol. 2012;157(3):370e3
112. Klein R, Sharrett AR, Klein BE, et al. Are retinal arteriolarabnormalities related to atherosclerosis?: TheAtherosclerosis Risk in Communities Study. ArteriosclerThromb Vasc Biol. 2000;20(6):1644e50
113. Ko K, Ngai A, Winn H. Role of adenosine in regulation ofregional cerebral blood flow in sensory cortex. Am J Physiol.1990;259(6):H1703e8
114. Kondo M, Wang L, Bill A. The role of nitric oxide inhyperaemic response to flicker in the retina and optic nervein cats. Acta Ophthalmol Scand. 1997;75(3):232e5
115. Langhans M, Michelson G, Groh M. Effect of breathing 100%oxygen on retinal and optic nerve head capillary blood flowin smokers and non-smokers. Br J Ophthalmol.1997;81(5):365e9
116. Layton AT. Mathematical modeling of kidney transport.Wiley Interdiscip Reviews-Systems Biol Med.2013;5(5):557e73
117. Leske MC. Ocular perfusion pressure and glaucoma: clinicaltrial and epidemiologic findings. Curr Opin Ophthalmol.2009;20(2):73e8
118. Leske MC, Heijl A, Hussein M, et al. Factors for glaucomaprogression and the effect of treatment: the earlymanifest glaucoma trial. Arch Ophthalmol.2003;121(1):48e56
119. Liang Y, Downs J, Fortune B, et al. Impact of systemicblood pressure on the relationship between intraocularpressure and blood flow in the optic nerve head ofnonhuman primates. Invest Ophthalmol Vis Sci.2009;50(5):2154e60
120. Liang Y, Fortune B, Cull G, et al. Quantification of dynamicblood flow autoregulation in optic nerve head of rhesusmonkeys. Exp Eye Res. 2010;90(2):203e9
121. Lichter PR, Musch DC, Gillespie BW, et al. Interim clinicaloutcomes in the Collaborative Initial Glaucoma TreatmentStudy comparing initial treatment randomized tomedications or surgery. Ophthalmology.2001;108(11):1943e53
122. Lieberman MF, Maumenee AE, Green WR. Histologic studiesof the vasculature of the anterior optic nerve. Am JOphthalmol. 1976;82(3):405e23
123. Liew G, Campbell S, Klein R, et al. Ten-year longitudinalchanges in retinal microvascular lesions: the atherosclerosisrisk in communities study. Ophthalmology.2011;118(8):1612e8

s u r v e y o f o p h t h a lmo l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6184
124. Lin WJ, Kuang HY. Oxidative stress induces autophagy inresponse to multiple noxious stimuli in retinal ganglioncells. Autophagy. 2014;10(10):1692e701
125. Liu B, Chen H, Johns TG, Neufeld AH. Epidermal growthfactor receptor activation: an upstream signal for transitionof quiescent astrocytes into reactive astrocytes after neuralinjury. J Neurosci. 2006;26(28):7532e40
126. Liu B, Neufeld AH. Expression of nitric oxide synthase-2(NOS-2) in reactive astrocytes of the human glaucomatousoptic nerve head. Glia. 2000;30(2):178e86
127. Loutzenhiser R, Bidani A, Chilton L. Renal myogenicresponsedKinetic attributes and physiological role. Circ Res.2002;90(12):1316e24
128. Luksch A, Polak K, Beier C, et al. Effects of systemic NOsynthase inhibition on choroidal and optic nerve head bloodflow in healthy subjects. Invest Ophthalmol Vis Sci.2000;41(10):3080e4
129. Lundgren O. Autoregulation of intestinal blood flow:physiology and pathophysiology. J Hypertens Suppl.1989;7(4):S79e84
130. Mackenzie PJ, Cioffi GA. Vascular anatomy of the optic nervehead. Can J Ophthalmol. 2008;43(3):308e12
131. Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating andself-killing: crosstalk between autophagy and apoptosis. NatRev Mol Cell Biol. 2007;8(9):741e52
132. Malone PE, Hernandez MR. 4-Hydroxynonenal, a product ofoxidative stress, leads to an antioxidant response in opticnerve head astrocytes. Exp Eye Res. 2007;84(3):444e54
133. Malone P, Miao H, Parker A, et al. Pressure induces loss ofgap junction communication and redistribution ofconnexin-43 in astrocytes. Glia. 2007;55(10):1085e98
134. Marek B, Harris A, Kanakamedala P, et al. Cerebrospinal fluidpressure and glaucoma: regulation of trans-lamina cribrosapressure. Br J Ophthalmol. 2014;98(6):721e5
135. Marsh DJ, Sosnovtseva OV, Chon KH, Holstein-Rathlou NH.Nonlinear interactions in renal blood flow regulation. Am JPhysiol Regul Integr Comp Physiol. 2005;288(5):R1143e59
136. Matsugi T, Chen Q, Anderson DR. Adenosine-inducedrelaxation of cultured bovine retinal pericytes. InvestOphthalmol Vis Sci. 1997;38(13):2695e701
137. Matthews BD, Overby DR, Mannix R, Ingber DE. Cellularadaptation to mechanical stress: role of integrins, Rho,cytoskeletal tension and mechanosensitive ion channels.J Cell Sci. 2006;119(Pt 3):508e18
138. Metea M, Newman E. Glial cells dilate and constrict bloodvessels: a mechanism of neurovascular coupling. J Neurosci.2006;26(11):2862e70
139. Mishra A, Hamid A, Newman E. Oxygen modulation ofneurovascular coupling in the retina. Proc Natl Acad Sci U SA. 2011;108(43):17827e31
140. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology,pathophysiology, and pharmacology. Pharmacol Rev.1991;43(2):109e42
141. Moore D, Harris A, Wudunn D, et al. Dysfunctionalregulation of ocular blood flow: a risk factor for glaucoma?Clin Ophthalmol. 2008;2(4):849e61
142. Morgan WH, Yu DY, Alder VA, et al. The correlationbetween cerebrospinal fluid pressure and retrolaminartissue pressure. Invest Ophthalmol Vis Sci.1998;39(8):1419e28
143. Motallebipour M, Rada-Iglesias A, Jansson M, Wadelius C.The promoter of inducible nitric oxide synthase implicatedin glaucoma based on genetic analysis and nuclear factorbinding. Mol Vis. 2005;11:950e7
144. Movaffaghy A, Chamot S, Petrig B, Riva C. Blood flow in thehuman optic nerve head during isometric exercise. Exp EyeRes. 1998;67(5):561e8
145. Mulligan SJ, MacVicar BA. Calcium transients in astrocyteendfeet cause cerebrovascular constrictions. Nature.2004;431(7005):195e9
146. Nadler Z, Wang B, Wollstein G, et al. Repeatability of in vivo3D lamina cribrosa microarchitecture using adaptive opticsspectral domain optical coherence tomography. Biomed OptExpress. 2014;5(4):1114e23
147. Neufeld AH, Hernandez MR, Gonzalez M. Nitric oxidesynthase in the human glaucomatous optic nerve head.Arch Ophthalmol. 1997;115(4):497e503
148. Newman E. Functional hyperemia and mechanisms ofneurovascular coupling in the retinal vasculature. J CerebBlood Flow Metab. 2013;33(11):1685e95
149. Novack RL, Stefansson E, Hatchell DL. Intraocular pressureeffects on optic nerve-head oxidative metabolism measuredin vivo. Graefes Arch Clin Exp Ophthalmol.1990;228(2):128e33
150. Nyborg NC, Nielsen PJ. The level of spontaneous myogenictone in isolated human posterior ciliary arteries decreaseswith age. Exp Eye Res. 1990;51(6):711e5
151. Offenhauser N, Thomsen K, Caesar K, Lauritzen M. Activity-induced tissue oxygenation changes in rat cerebellar cortex:interplay of postsynaptic activation and blood flow.J Physiol. 2005;565(Pt 1):279e94
152. Ohashi T, Ishii Y, Ishikawa Y, et al. Experimental andnumerical analyses of local mechanical propertiesmeasured by atomic force microscopy for shearedendothelial cells. Biomed Mater Eng. 2002;12(3):319e27
153. Ohno-Matsui K, Futagami S, Yamashita S, Tokoro T. Zinn-Haller arterial ring observed by ICG angiography in highmyopia. Br J Ophthalmol. 1998;82(12):1357e62
154. Olufsen MS, Nadim A, Lipsitz LA. Dynamics of cerebralblood flow regulation explained using a lumped parametermodel. Am J Physiol Regul Integr Comp Physiol.2002;282(2):R611e22
155. Onda E, Cioffi GA, Bacon DR, Van Buskirk EM.Microvasculature of the human optic nerve. Am JOphthalmol. 1995;120(1):92e102
156. Orgul S, Gugleta K, Flammer J. Physiology of perfusion as itrelates to the optic nerve head. Surv Ophthalmol.1999;43:S17e26
157. Orgul S, Meyer P, Cioffi G. Physiology of blood flow regulationand mechanisms involved in optic nerve perfusion.J Glaucoma. 1995;4(6):427e43
158. Osborne NN. Mitochondria: their role in ganglion cell deathand survival in primary open angle glaucoma. Exp Eye Res.2010;90(6):750e7
159. Osborne NN, Wood JP, Chidlow G, et al. Ganglion cell deathin glaucoma: what do we really know? Br J Ophthalmol.1999;83(8):980e6
160. Panerai R. Assessment of cerebral pressure autoregulation inhumansda review of measurement methods. Physiol Meas.1998;19(3):305e38
161. Pasquale L, Jonas JB, Anderson DR. Anatomy and physiology,in Weinreb RN, Harris A (eds) Ocular Blood Flow inGlaucoma. Amsterdam, Kugler Publications; 2009, pp 3e13
162. Paulson OB, Strandgaard S, Edvinsson L. Cerebralautoregulation. Cerebrovasc Brain Metab Rev.1990;2(2):161e92
163. Peng X, Carhuapoma JR, Bhardwaj A, et al. Suppression ofcortical functional hyperemia to vibrissal stimulation in therat by epoxygenase inhibitors. Am J Physiol Heart CircPhysiol. 2002;283(5):H2029e37
164. Peng X, Zhang C, Alkayed NJ, et al. Dependency of corticalfunctional hyperemia to forepaw stimulation onepoxygenase and nitric oxide synthase activities in rats.J Cereb Blood Flow Metab. 2004;24(5):509e17

s u r v e y o f o p h t h a lm o l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6 185
165. Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectionalcontrol of CNS capillary diameter by pericytes. Nature.2006;443(7112):700e4
166. Pillunat L, Anderson D, Knighton R, et al. Autoregulation ofhuman optic nerve head circulation in response to increasedintraocular pressure. Exp Eye Res. 1997;64(5):737e44
167. Pollock DM, Keith TL, Highsmith RF. Endothelin receptorsand calcium signaling. FASEB J. 1995;9(12):1196e204
168. Polska E, Ehrlich P, Luksch A, Fuchsjager-Mayrl G. Effects ofadenosine on intraocular pressure, optic nerve head blood,and choroidal blood flow in healthy humans. InvestOphthalmol Vis Sci. 2003;44(7):3110e4
169. Porsaa K. Experimental studies on the vasomotorinnervation of the retinal arteries. Acta Ophthalmol.1941;19(S18):9e201
170. Porter JT, McCarthy KD. Hippocampal astrocytes in siturespond to glutamate released from synaptic terminals.J Neurosci. 1996;16(16):5073e81
171. Pournaras C, Riva C, Bresson-Dumont H, et al. Regulation ofoptic nerve head blood flow in normal tension glaucomapatients. Eur J Ophthalmol. 2004;14(3):226e35
172. Pries AR, Secomb TW, Gaehtgens P. Biophysical aspects ofblood flow in the microvasculature. Cardiovasc Res.1996;32(4):654e67
173. Puro DG. Physiology and pathobiology of the pericyte-containing retinal microvasculature: new developments.Microcirculation. 2007;14(1):1e10
174. Quigley HA. Glaucoma. Lancet. 2011;377(9774):1367e77175. Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res.
1999;18(1):39e57176. Quigley HA. Pathophysiology of the optic nerve head in
glaucoma, in McAllister JA, Wilson RP (eds) Glaucoma.London; Boston, Butterworths; 1986
177. Quigley HA, Broman AT. The number of people withglaucoma worldwide in 2010 and 2020. Br J Ophthalmol.2006;90(3):262e7
178. Rein H. Vasomotorische regulationen. Ergebnisse derPhysiologie. 1931;32(1):28e72
179. Resch H, Garhofer G, Fuchsjager-Mayrl G, et al. Endothelialdysfunction in glaucoma. Acta Ophthalmol.2009;87(1):4e12
180. Resch H, Karl K, Weigert G, et al. Effect of dual endothelinreceptor blockade on ocular blood flow in patients withglaucoma and healthy subjects. Invest Ophthalmol Vis Sci.2009;50(1):358e63
181. Riva CE, Cranstoun SD, Petrig BL. Effect of decreased ocularperfusion pressure on blood flow and the flicker-inducedflow response in the cat optic nerve head. Microvasc Res.1996;52(3):258e69
182. Riva C, Falsini B, Logean E. Flicker-evoked responses ofhuman optic nerve head blood flow: luminance versuschromatic modulation. Invest Ophthalmol Vis Sci.2001;42(3):756e62
183. Riva CE, Geiser M, Petrig BL, Beijing PRCOBFRA. Ocular bloodflow assessment using continuous laser Doppler flowmetry.Acta Ophthalmol. 2010;88(6):622e9
184. Riva CE, Grunwald JE, Petrig BL. Autoregulation of humanretinal blood flow. An investigation with laser Dopplervelocimetry. Invest Ophthalmol Vis Sci. 1986;27(12):1706e12
185. Riva CE, Harino S, Petrig BL, Shonat RD. Laser Dopplerflowmetry in the optic nerve. Exp Eye Res.1992;55(3):499e506
186. Riva C, Hero M, Titze P, Petrig B. Autoregulation of humanoptic nerve head blood flow in response to acute changes inocular perfusion pressure. Graefe’s Arch Clin ExpOphthalmol. 1997;235(10):618e26
187. Riva C, Logean E, Falsini B. Visually evoked hemodynamicalresponse and assessment of neurovascular coupling in the
optic nerve and retina. Prog Retin Eye Res.2005;24(2):183e215
188. Roberts MD, Grau V, Grimm J, et al. Remodeling of theconnective tissue microarchitecture of the lamina cribrosain early experimental glaucoma. Invest Ophthalmol Vis Sci.2009;50(2):681e90
189. Roff EJ, Harris A, Chung HS, et al. Comprehensiveassessment of retinal, choroidal and retrobulbarhaemodynamics during blood gas perturbation. GraefesArch Clin Exp Ophthalmol. 1999;237(12):984e90
190. Rose C, Ransom B. Gap junctions equalize intracellular Naþconcentration in astrocytes. Glia. 1997;20(4):299e307
191. Rosengarten B, Hecht M, Kaps M. Brain activity affectsdynamic but not static autoregulation. Exp Neurol.2007;205(1):201e6
192. Russell FD, Hamilton KD. Nutrient deprivation increasesvulnerability of endothelial cells to proinflammatory insults.Free Radic Biol Med. 2014;67:408e15
193. Sander EA, Downs JC, Hart RT, et al. A cellular solid model ofthe lamina cribrosa: mechanical dependence onmorphology. J Biomech Eng. 2006;128(6):879e89
194. Satcher R, Dewey CF Jr, Hartwig JH. Mechanical remodelingof the endothelial surface and actin cytoskeleton induced byfluid flow. Microcirculation. 1997;4(4):439e53
195. Sato M, Ohashi T. Biorheological views of endothelial cellresponses to mechanical stimuli. Biorheology.2005;42(6):421e41
196. Sato E, Sakamoto T, Nagaoka T, et al. Role of nitric oxide inregulation of retinal blood flow during hypercapnia in cats.Invest Ophthalmol Vis Sci. 2003;44(11):4947e53
197. Schmetterer L, Findl O, Strenn K, et al. Role of NO in the O2and CO2 responsiveness of cerebral and ocular circulation inhumans. Am J Physiol. 1997;273(6 Pt 2):R2005e12
198. Schmetterer L, Polak K. Role of nitric oxide in the control ofocular blood flow. Prog Retin Eye Res. 2001;20(6):823e47
199. Schmidl D, Boltz A, Kaya S, et al. Role of nitric oxide in opticnerve head blood flow regulation during an experimentalincrease in intraocular pressure in healthy humans. Exp EyeRes. 2013;116:247e53
200. SchmidlD,GarhoferG,SchmettererL.Thecomplex interactionbetween ocular perfusion pressure and ocular bloodflowdrelevance for glaucoma. Exp Eye Res. 2011;93(2):141e55
201. Schon EA, Manfredi G. Neuronal degeneration andmitochondrial dysfunction. J Clin Invest. 2003;111(3):303e12
202. Sgouralis I, Layton AT. Theoretical assessment of renalautoregulatory mechanisms. Am J Physiol Renal Physiol.2014;306(11):F1357e71
203. Shah R, Wormald RP. Glaucoma. BMJ Clin Evid. 2011;2011204. Shibata M, Oku H, Sugiyama T, et al. Disruption of gap
junctions may be involved in impairment of autoregulationin optic nerve head blood flow of diabetic rabbits. InvestOphthalmol Vis Sci. 2011;52(5):2153e9
205. Shibata M, Sugiyama T, Hoshiga M, et al. Changes in opticnerve head blood flow, visual function, and retinal histologyin hypercholesterolemic rabbits. Exp Eye Res.2011;93(6):818e24
206. Shibata M, Sugiyama T, Kurimoto T, et al. Involvement ofglial cells in the autoregulation of optic nerve head bloodflow in rabbits. Invest Ophthalmol Vis Sci.2012;53(7):3726e32
207. Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Modelingindividual-specific human optic nerve head biomechanics.Part I: IOP-induced deformations and influence of geometry.Biomech Model Mechanobiol. 2009;8(2):85e98
208. Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Modelingindividual-specific human optic nerve head biomechanics.Part II: influence of material properties. Biomech ModelMechanobiol. 2009;8(2):99e109

s u r v e y o f o p h t h a lmo l o g y 6 1 ( 2 0 1 6 ) 1 6 4e1 8 6186
209. Sigal IA, Wang B, Strouthidis NG, et al. Recent advances inOCT imaging of the lamina cribrosa. Br J Ophthalmol.2014;98(Suppl 2):ii34e9
210. Sines D, Harris A, Siesky B, et al. The response of retrobulbarvasculature to hypercapnia in primary open-angle glaucomaand ocular hypertension. Ophthalmic Res. 2007;39(2):76e80
211. Singh S, Dass R. The central artery of the retina. II. A study of itsdistribution and anastomoses. Br J Ophthalmol. 1960;44:280e99
212. Sossi N, Anderson D. Effect of elevated intraocular pressureon blood flow. Occurrence in cat optic nerve head studiedwith iodoantipyrine I 125. Arch Ophthalmol.1983;101(1):98e101
213. Spronck B, Martens EG, Gommer ED, van de Vosse FN.A lumped parameter model of cerebral blood flow controlcombining cerebral autoregulation and neurovascularcoupling. Am J Physiol Heart Circ Physiol.2012;303(9):H1143e53
214. Srikanth K, Kumar MA, Selvasundari S, Prakash ML. ColourDoppler imaging of ophthalmic artery and central retinalartery in glaucoma patients with and without diabetesmellitus. J Clin Diagn Res. 2014;8(4):VC01e2
215. Srinivasan VJ, Adler DC, Chen Y, et al. Ultrahigh-speedoptical coherence tomography for three-dimensional and enface imaging of the retina and optic nerve head. InvestOphthalmol Vis Sci. 2008;49(11):5103e10
216. St Lawrence KS, Ye FQ, Lewis BK, et al. Measuring the effectsof indomethacin on changes in cerebral oxidativemetabolism and cerebral blood flow during sensorimotoractivation. Magn Reson Med. 2003;50(1):99e106
217. Sugiyama T, Araie M, Riva CE, et al. Use of laser speckleflowgraphy in ocular blood flow research. Acta Ophthalmol.2010;88(7):723e9
218. Sugiyama T, Utsumi T, Azuma I, Fujii H. Measurement ofoptic nerve head circulation: comparison of laser speckleand hydrogen clearance methods. Jpn J Ophthalmol.1996;40(3):339e43
219. Takano T, Tian GF, PengW, et al. Astrocyte-mediated controlof cerebral blood flow. Nat Neurosci. 2006;9(2):260e7
220. Takayama J, Tomidokoro A, Ishii K, et al. Time course of thechange in optic nerve head circulation after an acuteincrease in intraocular pressure. Invest Ophthalmol Vis Sci.2003;44(9):3977e85
221. Tamaki Y, Araie M, Fukaya Y, et al. Effects of lomerizine, acalcium channel antagonist, on retinal and optic nerve headcirculation in rabbits and humans. Invest Ophthalmol VisSci. 2003;44(11):4864e71
222. Tamaki Y, Araie M, Kawamoto E, et al. Non-contact, two-dimensional measurement of tissue circulation in choroidand optic nerve head using laser speckle phenomenon. ExpEye Res. 1995;60(4):373e83
223. Tomita K, Araie M, Tamaki Y, et al. Effects of nilvadipine, acalcium antagonist, on rabbit ocular circulation and opticnerve head circulation in NTG subjects. Invest OphthalmolVis Sci. 1999;40(6):1144e51
224. Tamaki Y, Araie M, Tomita K, Tomidokoro A. Time-course ofchanges in nicardipine effects on microcirculation in retinaand optic nerve head in living rabbit eyes. Jpn J Ophthalmol.1996;40(2):202e11
225. Tamaki Y, AraieM, Tomita K, UrashimaH. Effects of pranidipine,a new calcium antagonist, on circulation in the choroid, retinaand optic nerve head. Curr Eye Res. 1999;19(3):241e7
226. Tomita K, Tomidokoro A, Tamaki Y, et al. Effects ofsemotiadil, a novel calcium antagonist, on the retina andoptic nerve head circulation. J Ocul Pharmacol Ther.2000;16(3):231e9
227. Ursino M, Colantuoni A, Bertuglia S. Vasomotion and bloodflow regulation in hamster skeletal muscle microcirculation:
a theoretical and experimental study. Microvasc Res.1998;56(3):233e52
228. Ursino M, Giannessi M. A model of cerebrovascularreactivity including the circle of willis and corticalanastomoses. Ann Biomed Eng. 2010;38(3):955e74
229. Ursino M, Lodi CA. A simple mathematical model of theinteraction between intracranial pressure and cerebralhemodynamics. J Appl Physiol (1985). 1997;82(4):1256e69
230. van Beek A, Claassen J, Rikkert M. Cerebral autoregulation:an overview of current concepts and methodology withspecial focus on the elderly. J Cereb Blood Flow Metab.2008;28(6):1071e85
231. Vasudevan SK, Gupta V, Crowston JG. Neuroprotectionin glaucoma. Indian J Ophthalmol. 2011;59(Suppl):S102e13
232. Venkataraman S, Flanagan J, Hudson C. Vascular reactivityof optic nerve head and retinal blood vessels in glaucomadareview. Microcirculation. 2010;17(7):568e81
233. Wang L, Burgoyne C, Cull G, et al. Static blood flowautoregulation in the optic nerve head in normal andexperimental glaucoma. Invest Ophthalmol Vis Sci.2014;55(2):873e80
234. Wang L, Cioffi GA, Cull G, et al. Immunohistologic evidencefor retinal glial cell changes in human glaucoma. InvestOphthalmol Vis Sci. 2002;43(4):1088e94
235. Wang L, Cull G, Piper C, et al. Anterior and posterior opticnerve head blood flow in nonhuman primate experimentalglaucoma model measured by laser speckle imagingtechnique and microsphere method. Invest Ophthalmol VisSci. 2012;53(13):8303e9
236. Wang B, Nevins JE, Nadler Z, et al. Reproducibility of in-vivoOCT measured three-dimensional human lamina cribrosamicroarchitecture. PLoS One. 2014;9(4):e95526
237. Wang B, Nevins JE, Nadler Z, et al. In vivo lamina cribrosamicro-architecture in healthy and glaucomatous eyes asassessed by optical coherence tomography. InvestOphthalmol Vis Sci. 2013;54(13):8270e4
238. White LR, Bakken IJ, Sjaavaag I, et al. Vasoactivitymediated by endothelin ETA and ETB receptors in isolatedporcine ophthalmic artery. Acta Physiol Scand.1996;157(2):245e52
239. Williamson TH, Harris A. Color Doppler ultrasoundimaging of the eye and orbit. Surv Ophthalmol.1996;40(4):255e67
240. Winkler M, Jester B, Nien-Shy C, et al. High resolutionthree-dimensional reconstruction of the collagenousmatrix of the human optic nerve head. Brain Res Bull.2010;81(2-3):339e48
241. Wong TY, Mitchell P. The eye in hypertension. Lancet.2007;369(9559):425e35
242. Yang G, Zhang Y, Ross M. Attenuation of activity-inducedincreases in cerebellar blood flow in mice lacking neuronalnitric oxide synthase. Am J Physiol Heart Circ Physiol.2003;285(1):H298e304
243. Ye XD, Laties AM, Stone RA. Peptidergic innervation of theretinal vasculature and optic nerve head. Invest OphthalmolVis Sci. 1990;31(9):1731e7
244. Yuan L, Neufeld AH. Activated microglia in the humanglaucomatous optic nerve head. J Neurosci Res.2001;64(5):523e32
245. Zonta M, Angulo MC, Gobbo S, et al. Neuron-to-astrocytesignaling is central to the dynamic control of brainmicrocirculation. Nat Neurosci. 2003;6(1):43e50
246. Zweifach B, Lipowski H. Pressure-flow relations in blood andlymph microcirculation, in Renkin EM, Michel CC, Geiger SR(eds) Microcirculation. 4. Bethesda, Md, AmericanPhysiological Society; 1984, pp 251e307