Anemia Aplastic
Transcript of Anemia Aplastic

Anemia aplastic
The term aplastic anemia describes a clinical syndrome in which there is a deficiency of red cells, neutrophils,
and monocytes, and platelets without morphologic evidence of another marrow disorder. Marrow examination
shows a near absence of hematopoietic precursor cells and fatty replacement. The disorder can be induced by
toxic chemicals (e.g., benzene), specific viruses (e.g., Epstein-Barr virus), or can be inherited (e.g., Fanconi’s
anemia). Most cases occur without an evident incitant and are the result of autoreactive T lymphocytes that
suppress or destroy hematopoietic cells. The disease may be ameliorated or sometimes cured by
immunosuppressive therapy, especially antithymocyte globulin. For those with a suitable donor, allogeneic
stem cell therapy is often curative. The disease, even after successful treatment with immunosuppressive
agents, has a propensity to evolve into a clonal hematopoietic disorder such as paroxysmal nocturnal
hemoglobinuria, oligoblastic or acute myelogenous leukemia.
Acronyms and abbreviations that appear in this chapter include: ALG, antilymphocyte globulin; ALS,
antilymphocyte serum; ATG, antithymocyte globulin; BFU–E, erythroid burst-forming units; CFU-GM,
granulocyte-macrophage colony-forming units; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV,
human herpes virus; HIV, human immunodeficiency virus; IL, interleukin; NMRI, nuclear magnetic resonance
imaging; PNH, paroxysmal nocturnal hemoglobinuria; SCF, stem cell factor.
DEFINITION AND HISTORY
Aplastic anemia results from a failure of blood cell production in the marrow. This results in a markedly
hypocellular marrow and varying degrees of anemia, granulocytopenia, and thrombocytopenia. Most cases of
aplastic anemia are acquired. The decrease in hematopoiesis may be secondary to toxic effects of offending
agents on marrow stem cells; in many cases, however, the pathogenesis is the suppression of blood cell
progenitor proliferation and maturation by autoreactive lymphocytes. The disease also may occur as the result
of an inherited disorder, especially Fanconi anemia. A close association also exists between the clonal
hemopathy, PNH, and aplastic anemia.
Aplastic anemia was first recognized by Ehrlich in 1888.1 He described a young pregnant woman who died of
severe anemia and neutropenia; autopsy examination revealed a fatty marrow with essentially no
hematopoiesis. The name aplastic anemia was subsequently applied to this disease in 1904.2 Over the next 30
years many conditions that caused pancytopenia were confused with aplastic anemia based on incomplete or
inadequate histologic study of the patient’s marrow. Marrow biopsies are required to document that a
hypocellular marrow aspirate represents hypoplasia or aplasia and to exclude other conditions that may
infiltrate, replace, or suppress normal marrow cells.3
ETIOLOGY AND PATHOGENESIS

It is unclear why some individuals who are exposed repeatedly to potential marrow toxins sustain severe and
often irreversible marrow injury, whereas most have no untoward effects. It has been postulated that there is a
genetic predisposition based on a high incidence of HLA class II antigens DR24 and DPw35 in patients with
marrow aplasia. The disease may result from the accumulated effects of multiple noxious exposures on
pluripotential stem cells. Ultimately, all stem cells sustain sufficient damage to hinder their ability to replicate.
Alternatively, such exposures may induce a single abnormal cell to proliferate in a clonal fashion and to
somehow hinder the growth of normal stem cells. The result in the first case would be aplastic anemia from a
polyclonal injury (classical aplastic anemia) to stem cells and/or very early multipotential progenitor cells and
in the latter injury to a single stem cell resulting in a monoclonal disorder (e.g., paroxysmal nocturnal
hemoglobinuria—aplastic anemia syndrome). Although classic aplastic anemia and paroxysmal nocturnal
hemoglobinuria are thought to be distinct clinical entities, studies suggest an overlap in these conditions. As
many as 40 percent of patients with otherwise typical aplastic anemia have evidence of glycosyl-
phosphatidylinositol molecule defects in leukocytes and red cells as judged by flow cytometry, analogous to
that seen in PNH.6,7 and 8 Aplastic anemia, hypoplastic myelodysplastic syndrome, and paroxysmal nocturnal
hemoglobinuria each may eventually evolve into acute myelogenous leukemia.9,10,11,12 and 13
STEM CELLS, MICROENVIRONMENT, CYTOKINES
Potential mechanisms responsible for acquired marrow cell failure include induced defects in hematopoietic
stem cells, failure of the stromal microenvironment of the marrow, impaired production or release of
hemopoietic growth factors, and cellular or humoral immune suppression of the marrow. Several pathogenic
abnormalities have been found in patients with aplastic anemia14,15,16,17 and 18; however, it is unclear
whether these are inciting events or represent epiphenomena secondary to the disease. Although it is not
possible to assess human totipotential stem cells in culture, their progeny are easily identified by clonal growth
in vitro. The number of CFU-GM and BFU–E are reduced markedly in patients with
aplastic anemia.19,20,21,22,23,24 and 25 The more immature long-term culture initiating cells are also
reduced to about 1 percent of normal values.26 CD34-positive hematopoietic cells, in which fraction the
hematopoietic stem cells reside, are also correspondingly low.27,28 These findings suggest that reduced
hematopoietic stem cells are the primary defect in the abnormal hematopoiesis. Stem cell inhibition may,
however, occur by a T-cell–mediated suppressor effect. Early studies showed inhibition of colony growth by
autologous marrow lymphocytes or by blood or marrow mononuclear cells from patients with aplastic anemia
when cocultured with normal marrow.20,23,29,30 and 31 In many cases the inhibition was thought to result
from transfusion sensitization rather than autoimmunity.32,33 However, culture studies in patients with
aplastic anemia prior to transfusion34 or before and after successful treatment35,36 are highly suggestive of a
T-cell–mediated suppressor cell phenomenon. Alternatively, injured hemopoietic stem cells may present
neoantigens that serve as a stimulus for secondary T-cell–mediated suppression, which serves to worsen the
aplasia.
Based on studies in rats, a theory of “seed” (stem cells) versus “soil” (microenvironment) abnormality

was established.37 Modest doses of irradiation to a limb lead to transient aplasia, with recovery secondary to
ingress of circulating stem cells. With doses of 20 Gy (2000 rad) and greater, there is similar aplasia and
recovery; however, permanent aplasia develops in this limb several months later. This appears to result from
late damage to the marrow microenvironment, which may also occur in certain cases of aplastic anemia.
Pathogenetic studies have been conducted of the residual marrow damage in mice that
receive busulfan.38,39 Although blood counts are usually normal, both marrow progenitor cells and stem cells
are decreased substantially. Some animals progress to chronic hypoplastic marrow failure, whereas others
retain relatively normal bloodcounts.38 When treated with chloramphenicol, animals exposed to busulfan have
a marked decrease in progenitor cells, whereas controls are unaffected.39 Perhaps similar repeated exposures
with different agents may be necessary to induce aplastic anemia in humans.
The hematopoietic defects, macrocytic anemia, and mild hypoplasia occur in two strains of mice (WWv and
Sl/Sld). WWv anemia is cured by lethal irradiation and infusion of stem cells from nonaffected litter mates,
whereas the Steel (Sl) defect is not corrected by stem cell transplantation because it is the result of an
abnormal microenvironment.40 WWv cells are deficient in the proto-oncogene kit,41 which encodes a tyrosine
kinase receptor for the hemopoietic growth factor kit ligand42 (see Chap. 14). Sl/Sld mice do not produce kit
ligand, also called stem cell factor (SCF). Similar defects have not been observed in patients with aplastic
anemia. Serum levels of SCF have been either moderately low or normal in several studies of
aplastic anemia.43,44 and 45 Although SCF augments the growth of hemopoietic colonies from aplastic
marrows,46 its use in patients has not led to clinical remissions. Another early acting growth factor, Flt-3
ligand, is 30- to 100-fold elevated in the serum of patients with aplastic anemia.47 Short-term clonal assays for
marrow stromal cells have shown variable defects in stromal cell function. Fibroblasts grown from patients
with severe aplastic anemia have subnormal cytokine production. However, serum levels of granulocyte
colony-stimulating factor,48 erythropoietin,49 and thrombopoietin50 are usually high. Synthesis of IL-1, an
early stimulator of hemopoiesis, is decreased in mononuclear cells from patients with aplastic anemia, but it is
produced normally by cells from patients with myelodysplastic syndrome.51 Levels of cytokines with
inhibitory effects on hemopoiesis are increased in most patients with severe aplastic anemia. Increased
mononuclear cell production of interferon-g,52,53 IL-2,54 and tumor necrosis factor-a55,56 has been noted
spontaneously or in response to certain mitogens. Elevated serum levels of interferon-g have been found in 30
percent of patients with aplastic anemia, and interferon-g expression has been detected in the marrow of most
patients with acquired aplastic anemia.57 Moreover, addition of antibodies to interferon enhances in vitro
colony growth of marrow cells from affectedpatients.52 This suggests a potential role for interferon-g in either
the initiation or propagation of the defect in aplastic anemia.
Studies of the microenvironment have shown relatively normal stromal cell proliferation and growth
factor production.58,59 and 60 These findings, coupled with the limited response of patients with aplastic
anemia to the known growth factors, suggest that cytokine deficiency is not the etiologic problem in most
cases.

Potential causes for aplastic anemia are shown in Table 31-1. The disorder may follow exposure to various
chemicals, drugs, radiation, or viruses. In addition, connective tissue disorders and pregnancy may be
associated with marrow aplasia. Constitutional forms, particularly Fanconi anemia, develop in the setting of an
underlying genetic defect. As many as 65 percent of cases of aplastic anemia, however, are idiopathic.
TABLE 31-1 ETIOLOGIC CLASSIFICATION OF APLASTIC ANEMIA
CHEMICALS
Benzene was the first chemical linked to aplastic anemia, based on studies in factory workers before the
twentieth century.61 Despite its known properties as a marrow toxin, benzene is still used widely as a solvent
and is employed in the manufacture of chemicals, drugs, dyes, and explosives. It has been a vital chemical in
the manufacture of rubber and leather goods and has been used widely in the shoe industry, leading to an
increased risk for aplastic anemia and leukemia in workers in all theseindustries.62,63,64,65 and 66 In China,
where benzene is still used widely in industry, benzene poisoning was found in 0.5 percent of the workers;
aplastic anemia among workers was sixfold higher than in the general population.67
The U.S. Occupational Safety and Health Administration has lowered the permissible exposure limit to
benzene to 1 ppm,68 after it was shown that exposure to 100 ppm was associated with leukopenia in about
one-third of workers.69 Other hematologic abnormalities have been observed in patients exposed to benzene,
including hemolytic anemia, marrow hyperplasia, myeloid metaplasia, and acute
myelogenousleukemia.62,63,64,65,66 and 67,70
There appears to be a relationship between the use of insecticides related to benzene and aplastic anemia.
Chlorinated hydrocarbons and organophosphate compounds have been implicated in 280 cases reported in
the literature.71 DDT (chlorophenothane), lindane, and chlordane appear to be the most common insecticides
involved. Aplastic anemia was reported following the use of lindane in home vaporizers for disinfection. This
practice continued until the 1970s, when over 30 case reports of aplastic anemia led to
its curtailment.72 Occasional cases still occur following heavy exposure at industrial plants or after its use as
a pesticide.73 Lindane is metabolized in part to pentachlorophenol (PCP), another toxic chlorinated
hydrocarbon that is manufactured for use as a wood preservative. Many cases of aplastic anemia and related
blood disorders have been attributed to PCP over the past 25 years.72,73,74 and 75 Prolonged exposures to
petroleum distillates in the form of Stoddard solvent76 and acute exposure of toluene through the practice of
glue sniffing77 have also been reported to cause aplasia. Trinitrotoluene (TNT), an explosive used extensively
during World Wars I and II, is absorbed readily by inhalation and through the skin. Many fatal cases of aplastic
anemia were observed in munitions workers exposed to TNT in Great Britain from 1940 to 1946.78
DRUGS
Chloramphenicol is a nitrobenzene compound with broad-spectrum antibiotic activity. It was introduced in
1948 and used widely during the 1950s and 1960s by various routes of administration, often for trivial

indications. Reports of an association with aplastic anemia began early after its introduction.79,80 and 81
Indiscriminant use of the drug continued, however, despite warnings to limit its use to infections caused by
organisms that were resistant to other agents or for which no other drugs were available.82 The risk of
developing aplastic anemia in patients treated with chloramphenicol is about 1 in 20,000, or 10 to 50 times that
of the general population.83,84 and 85 Unfortunately, reports of fatal aplastic anemia continue to appear with
topical or systemic use of thedrug.86,87
Two adverse hematopoietic responses to the drug occur. Suppression of marrow function occurs in many
individuals receiving the drug, with reduced erythroid cell production characterized by a rise in serum iron,
accumulation of iron in the mitochondria, and vacuolization of marrow erythroid precursor cells.88,89,90 and
91 Reticulocytes decrease and the hematocrit declines as inhibition of red cell production continues. Mild
decreases in circulating neutrophils and platelets are also observed in some patients. These effects are probably
the result of inhibition of mitochondrial protein synthesis and are reversible after discontinuation of the drug.
There appears to be no relationship between this common, temporary inhibition of hematopoiesis and the rare,
but devastating, severe aplastic anemia that ensues in a small proportion of patients. This latter reaction may
occur weeks to months after treatment and does not appear related to the total drug exposure nor to the route of
administration. Based on the nearly simultaneous development of aplastic anemia in identical twins given this
agent,92 it was postulated that there is a genetic defect that predisposes to this severe reaction to the drug.
With chloramphenicol, the many cases of aplastic anemia strongly established the potential for toxic effects
with this drug. Owing to a lower incidence of aplastic anemia with many other drugs, however, it has often
been difficult to isolate the single offending agent. Perhaps the only drug for which there is good statistical
information is quinacrine (Atabrine).93 This drug was administered to all U.S. troops in the South Pacific and
Asiatic theaters of operations as prophylaxis for malaria during 1943 and 1944. The incidence of aplastic
anemia was compared to army personnel in the United States and Europe who did not receive quinacrine. The
incidence of aplastic anemia was 7 to 28 cases per 1,000,000 personnel per year in the prophylaxis zones,
whereas nontreated soldiers had 1 to 2 cases per 1,000,000 personnel per year. In contrast to other drugs, the
aplasia occurred during administration of the offending agent and was preceded by a characteristic rash in
nearly half the cases.
Many other drugs have been implicated in sporadic cases of aplastic anemia, but owing to limited reporting of
information, it is possible that the spectrum of drug-induced aplastic anemia is not fully appreciated. A list of
drugs that have been implicated is shown in Table 31-2. Antineoplastic drugs such as alkylating agents,
antimetabolites, and certain cytotoxic antibiotics all have the potential for producing marrow aplasia. In
general, this is transient, is an extension of their pharmacologic action, and resolves within several weeks of
completing chemotherapy. Although unusual, severe hypoplasia can follow use of the alkylating agent
busulfan and may persist for extended intervals. Patients may develop marrow aplasia 2 to 5 years after
discontinuation of alkylating agent therapy. These cases often evolve into hypoplastic myelodysplastic
syndromes.

TABLE 31-2 DRUGS ASSOCIATED WITH APLASTIC ANEMIA
Other types of drugs may be associated with occasional cases of aplastic anemia. These include analgesics as
well as antiarthritic and anti-inflammatory agents. The most serious offenders in the latter group include gold
salts, penicillamine, and the butazone compounds. Many nonsteroidal analgesics have been associated with
sporadic cases of marrow aplasia. Anticonvulsant medications, particularly carbamazepine, are marrow
suppressants. The sulfonamides and their derivatives, which include diuretics and oral hypoglycemic agents,
have been associated with cases of aplastic anemia. Many of these drugs are known to induce selective
cytopenias, such as agranulocytosis, which are usually reversible after discontinuation of the offending agent.
These reversible reactions are not correlated with the risk of aplastic anemia, casting doubt on the effectiveness
of routine monitoring of blood counts as a strategy to avoid aplastic anemia.
Since aplastic anemia remains a rare event, it may occur because of an underlying metabolic predisposition in
susceptible individuals. In the case of phenylbutazone-associated aplasia, there is delayed oxidation and
clearance of a related compound, acetanilide, as compared to either normal controls or those with aplastic
anemia due to other causes.94 This suggests excess accumulation of the drug as a potential mechanism for the
aplasia. In some cases drug interactions or synergy may be required to induce aplasia. Cimetidine, a histamine
H2-receptor antagonist, is occasionally implicated in cytopenias and in causing aplastic anemia, perhaps owing
to a direct effect on hematopoietic stem cells.95,96 This drug accentuates the marrow-suppressive effects of
the chemotherapy drug carmustine97 and in several instances has been reported as a possible cause of aplasia
when given with chloramphenicol.87
RADIATION
Chronic exposure to low doses of radiation or use of localized radiation for ankylosing spondylitis is
associated with an increased, but delayed, risk of developing aplastic anemia and
acute leukemia.98,99 Patients who were given thorium dioxide (Thorotrast) as an intravenous contrast medium
suffered numerous late complications, including malignant liver tumors, acute leukemia, and chronic
aplastic anemia.100 Chronic radium poisoning with osteitis of the jaw, osteogenic sarcoma, and aplastic
anemia was seen in workers who painted watch dials with luminous paint when they moistened the
brushesorally.101
Acute exposures to large doses of radiation are associated with the development of marrow aplasia and a
gastrointestinal syndrome.102,103 Total body exposure to between 1 and 2.5 Gy (100 to 250 rad) leads to
gastrointestinal symptoms and depression of leukocyte counts, but most patients recover. A dose of 4.5 Gy
leads to death in half the individuals (LD50) owing to marrow failure. Higher doses in the range of 10 Gy are
universally fatal unless the patient receives extensive supportive care followed by marrow transplantation.
Aplastic anemia associated with nuclear accidents was seen after the disaster that occurred at the Chernobyl
nuclear power station in the Ukraine in 1986.104,105,106 and 107
VIRUSES

A striking relationship between hepatitis and the subsequent development of aplastic anemia has been the
subject of a number of case reports; this association was emphasized by two major reviews in the
1970s.108,109 In the aggregate, these reports summarized findings in over 200 cases. In many instances, the
hepatitis was improving or had resolved when the aplastic anemia was noted 4 to 12 weeks later.
Approximately 10 percent of cases occurred more than 1 year after the initial diagnosis of hepatitis. Most
patients were young (18 to 20 years), two-thirds were male, and their survival was short (10 weeks). Although
hepatitis A and B have been implicated in aplastic anemia in a small number of cases, most cases are related to
non-A, non-B hepatitis.110,111 Severe aplastic anemia developed in 9 of 31 patients who underwent liver
transplantation for non-A, non-B hepatitis but in none of 1463 patients transplanted for
otherindications.112 Several lines of evidence indicate there is no association with hepatitis C virus,
suggesting that a hitherto unknown viral agent is involved.113,114 and 115
EBV has been implicated in the pathogenesis of aplastic anemia.116,117 The onset usually occurs within 4 to
6 weeks of infection. In some cases infectious mononucleosis is subclinical, with a finding of atypical
lymphocytes in the blood film and serological results consistent with a recent infection. EBV has been detected
in marrow cells,118 but it is uncertain whether aplasia results from a direct effect or an immunologic response
by the host. Some patients have recovered following therapy with antithymocyteglobulin.113,116,118
A number of other viruses have been implicated in the pathogenesis of marrow failure. B-19 parvovirus, the
cause of fifth disease, leads to transient erythroid aplasia but is not known to induce
aplastic anemia.113,119 HHV-6 has caused severe marrow aplasia subsequent to bone marrow transplantation
for other disorders.120 HIV infection is frequently associated with varying degrees of cytopenia. The marrow
is often cellular, but occasional cases of aplastic anemia have been noted.121,122 and 123 In these patients,
marrow hypoplasia may result both from viral suppression and from the many drugs used to control viral
replication in this disorder.
MISCELLANEOUS CAUSES
Rheumatoid arthritis is not ordinarily associated with severe aplastic anemia, but an epidemiologic study in
France revealed a sevenfold increase in the incidence of aplastic anemia in patients with this disorder.124 It is
uncertain whether the aplastic anemia is related directly to rheumatoid arthritis or to the various drugs used to
treat the condition (gold salts, D-penicillamine, and nonsteroidal agents). Occasional cases of aplastic anemia
are seen in conjunction with systemic lupus erythematosus.125 In vitro studies have suggested the presence of
an antibody126,127 or suppressor cell128,129 directed against hematopoietic progenitor cells. Patients have
recovered after plasmapheresis,126,127 glucocorticoids,129 or cyclophosphamide therapy,128,130 suggesting
a possible immune etiology.
Eosinophilic fasciitis, an uncommon connective tissue disorder with painful swelling and induration of the skin
and subcutaneous tissue, has been associated with aplastic anemia on at least 14 occasions.131,132,133 and
134 Although it may be antibody-mediated in some cases, it has been largely unresponsive to therapy. One
patient improved with immunosuppressive therapy using ATG and cyclosporine.133 In another case, a partial

remission was achieved following therapy with ATG.
There are a number of reports of pregnancy-associated aplastic anemia, but the relationship between the two
conditions is not clear.135,136,137,138,139,140 and 141 In some patients, preexisting aplastic anemia is
exacerbated with pregnancy, only to improve following termination of the pregnancy.136,139,140 In other
cases, the aplasia develops during pregnancy with recurrences during
subsequentpregnancies.136,140,141 Termination of pregnancy or delivery may improve the marrow function,
but the disease may progress to a fatal outcome even afterdelivery.135,136,137,138,139,140 and 141 Therapy
may include elective termination of early pregnancy, supportive care, immunosuppressive therapy, or marrow
transplantation after delivery.
HEREDITARY APLASTIC ANEMIA
FANCONI ANEMIA
The most common form of constitutional aplastic anemia was described in three brothers by Fanconi in
1927.142 Since that time nearly 800 cases have been recorded, either by reports in the literature or through an
International Fanconi AnemiaRegistry.143,144,145 and 146
Fanconi anemia is inherited as an autosomal recessive condition. It is estimated to be present in one in a
million individuals, although it is more frequent in Afrikaners of European descent and in southern Italy.
At least eight gene mutations have been associated with the development of Fanconi anemia. The genes have
been designated FAA through FAH. The great majority of patients have mutations of FAA or FAC. It has been
proposed that the A and C gene products, which are cytoplasmic proteins, form a complex with the products of
genes B, E, F, and G, which are adaptors or phosphorylators; the complex translocates to the nucleus, where it
subserves its normal function. In the presence of a mutant gene product, normal function is disturbed leading to
effects in sensitive tissues, including hematopoietic cells. Mutation of the D product appears to effect tissue
cells through a different mechanism, perhaps downstream from the complex.147,148
Blood counts and marrow cellularity are often normal until 5 to 10 years of age, when pancytopenia develops
gradually over an extended interval. Thrombocytopenia may precede the development of granulocytopenia and
anemia. The marrow becomes hypocellular, and in vitro colony assays reveal a decrease in CFU-GM and
BFU–E.145 It is often associated with abnormal skin pigmentation typical of café-au-lait lesions. Growth
retardation results in short stature. Skeletal anomalies, especially dysplastic radii and thumbs, occur in half the
patients. Heart, kidney, and eye defects may be present. Microcephaly and mental retardation may be present.
Hypogonadism occurs. Hematologic and visceral manifestations are combined in more than a third of patients,
but some may have anemia and inconspicuous somatic changes whereas others may have the anomalies with
little hematopoietic disorder. A few may be virtuallyunaffected.145,146 and 147 In the past, children with a
similar onset of aplastic anemia without congenital abnormalities were thought to have a different disorder
termed Estren-Dameshek syndrome.149 These children, whose lymphocytes show sensitivity to
diepoxybutane, have the same inherited disorder without skeletal abnormalities.144
Random chromatid breaks are present in myeloid cells, lymphocytes, and chorionic villus preparations. This

chromosome damage is intensified after exposure to DNA cross-linking agents such as mitomycin C or
diepoxybutane. The hypersensitivity of the chromosomes of marrow cells or lymphocytes to the latter agent is
used as a diagnostic test for this condition. Cell-cycle progression is prolonged at the G2 to M transition phase
of the cell mitotic cycle, and the cells are more susceptible to oxygen toxicity when cultured in vitro.147 It is
important to test the lymphocytes from pediatric patients with aplastic anemia for sensitivity to diepoxybutane,
since therapy for Fanconi anemia differs from that used for idiopathic aplastic anemia.
Most patients with Fanconi anemia do not respond to ATG or cyclosporine but do improve with androgen
preparations, often for as long as several years. Relapses occur gradually, with eventual death by age 10 to 20
years from progressive marrow failure or from conversion to acute myelogenous leukemia (approximately 10
percent of patients). Allogeneic stem cell transplantation is curative for this disorder.150,277 A marked
reduction in dosage of the marrow-conditioning regimen of cyclophosphamide and radiation is necessary
owing to the undue sensitivity of the tissues to alkylatingagents.150
DYSKERATOSIS CONGENITA AND SCHWACHMAN-DIAMOND SYNDROME
Dyskeratosis congenita and Schwachman-Diamond syndrome (pancreatic insufficiency with neutropenia) are
two rare disorders that may also evolve into aplastic anemia. Dyskeratosis is usually inherited as a recessive X-
chromosome–linked disorder although rare cases are autosomal. The disease is reflected in reticulate skin
pigmentation, leukoplakia, and dystrophic nails. A variety of noncutaneous anomalies have also been
observed. The skin and mucosal lesions appear in adolescence, and aplastic anemia usually develops in
early adulthood.151 Schwachman-Diamond syndrome is manifest by pancreatic insufficiency and steatorrhea.
Neutropenia is present in virtually all patients and granulocytopenia and thrombocytopenia in about one-third
to one-half. Thus a substantial plurality of patients have bi- or tricytopenia with hypoplastic marrows. There is
a significant risk of progression to myelogenous leukemia.152,278 Severe hematopoietic dysfunction can be
treated successfully with allogeneic stem cell transplantation.
CLINICAL FEATURES
EPIDEMIOLOGY
The incidence of aplastic anemia is estimated to be 2 to 5 cases per 1,000,000 population per year based on
retrospective studies.83 The incidence rates in Sweden (13 cases per 1,000,000 per year),153 Israel (8 cases
per 1,000,000 per year),84 and the United States (5 to 12 new cases per 1,000,000 per year)154,155 suggest
that the rate in industrialized countries is about 5 to 10 cases per 1,000,000 per year.
SIGNS AND SYMPTOMS
The onset of aplastic anemia may be insidious, with a gradual fall in red cells leading to pallor, weakness, and
fatigue, or it may be more dramatic with fever, chills, and pharyngitis or other infections resulting from
neutropenia. Dependent petechiae, bruising, and bleeding secondary to thrombocytopenia are seen often and
may be the initial clue to the underlying marrow disorder.
Physical examination is generally unrevealing except for evidence of infection or bleeding. Oral purpuric
lesions (wet purpura) suggest a platelet count of less than 10,000/µl (10 × 109/liter), which portends a higher

risk for cerebral hemorrhage. Retinal hemorrhages may be seen with severe anemia or thrombocytopenia.
Lymphadenopathy or splenomegaly are not ordinarily found in aplastic anemia; such findings suggest recent
infection or an alternative diagnosis such as leukemia or lymphoma.
LABORATORY FEATURES
BLOOD FINDINGS
Patients with aplastic anemia have varying degrees of pancytopenia. Anemia is associated with a low
reticulocyte index.156 The reticulocyte count is usually less than 1.0 percent and may be zero. Macrocytosis
may result from the high levels of erythropoietin,157 stimulating the few residual erythroblasts to mature more
rapidly,158 or from an abnormal clone of erythroid cells, such as is seen in myelodysplasia or paroxysmal
nocturnal hemoglobinuria.10,11,12 and 13 The total leukocyte count is low; the differential cell count reveals a
marked decrease in neutrophils. The absolute neutrophil count is the most important prognostic feature, with a
count of less than 500/µl (0.5 × 109/liter) associated with increased risk of infections and less than 200/µl (0.2
× 109/liter) associated with a dire prognosis. Lymphocyte production is thought to be normal, but patients may
have mild lymphopenia. Platelets are reduced, but they function normally. On occasion, only one cell line is
depressed initially, which may lead to an early diagnosis of red cell aplasia or amegakaryocytic
thrombocytopenia. In such patients, other cell lines will fail shortly thereafter (days to weeks) and permit a
definitive diagnosis.
MARROW FINDINGS
MORPHOLOGY
The marrow aspirate typically contains numerous spicules with empty fatty spaces and relatively few
hematopoietic cells. Lymphocytes, plasma cells, macrophages, and mast cells may be prominent, but this
probably reflects a lack of other cells rather than an increase in these elements. On occasion, some spicules are
cellular or even hypercellular, but megakaryocytes are usually reduced. These areas of residual hematopoiesis
do not appear to be of prognostic significance. Residual granulocytic cells generally appear normal, but it is
not unusual to see mild dysplastic or megaloblastoid features in the erythroid cells. At times they are
macrocytic or macronormoblastic, presumably due to the high levels of erythropoietin. Marrow biopsy is
essential to confirm the overall hypocellularity (Fig. 31-1), since a poor yield of cells is seen on marrow
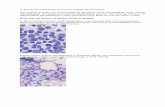
aspirates in a number of other hematologic disorders.
FIGURE 31-1 Marrow biopsy in aplastic anemia. The marrow is devoid of hematopoietic cells and contains
only scattered lymphocytes and stromal cells.
Severe aplastic anemia has been defined by the International Aplastic Anemia Study Group159 as a marrow of
less than 25 percent cellularity, or less than 50 percent cellularity with less than 30 percent hematopoietic cells,
with at least two of the following: neutrophil count less than 500/µl (0.5 × 109/liter), platelet count less than
20,000/µl (20 × 109/liter), and anemia with a corrected reticulocyte index of less than 1 percent. Those patients

with a neutrophil count less than 200/µl (0.2 × 109/liter) have an extremely poor prognosis and are
characterized as having very severe aplastic anemia.160
If a lymphocytosis is noted in the marrow or blood, a tartrate-resistant acid phosphatase determination as well
as immunophenotyping should be done to exclude early cases of hairy-cell leukemia161 or acute
lymphocytic leukemia.162
PROGENITOR CELL GROWTH
In vitro granulocytic-monocytic (CFU-GM) and erythroid (BFU–E) colony assays reveal a marked reduction
in progenitor cells.19,20,21,22,23,24 and 25,29,30,31,32,33,34 and 35 In some instances, improvement in
colony growth after incubation with anti-T-cell monoclonal antibodies may predict improvement after
immunosuppressive therapy163; this has not, however, been a universal finding.
CYTOGENETICS
Cytogenetic analysis may be difficult to perform owing to low cellularity; thus, multiple aspirates may be
required to provide sufficient cells for study. The results are often normal in aplastic anemia and abnormal in
myelodysplastic syndromes; one study, however, suggested that 4 percent of patients with otherwise typical
aplastic anemia had abnormalities usually seen in myelodysplasia.164 Two of three patients with aplastic
anemia and cytogenetic abnormalities who did not have marrow transplants progressed to a myelodysplastic
syndrome, suggesting that the cytogenetic abnormalities of such patients represent or will soon evolve into a
clonal (neoplastic) disorder.
RADIOLOGIC FINDINGS
NMRI can be used to distinguish between marrow fat and hematopoietic cells.165 This may provide a better
overall estimate of marrow aplasia than morphologic techniques and may differentiate hypoplastic
myelodysplastic syndrome from aplasticanemia.166,167
PLASMA AND URINE FINDINGS
Studies should include assessment of antibodies to hepatitis A, B, and C; hepatitis B antigen; heterophile
determination; and antibodies to EBV. A sucrose hemolysis test and leukocyte and red cell
immunophenotyping6,7 and 8 should be done prior to transfusions to exclude paroxysmal nocturnal
hemoglobinuria (see Chap. 36).
The serum has high levels of hematopoietic growth factors, including erythropoietin, thrombopoietin, and
myeloid colony-stimulating factors.48,49 and 50
Serum iron values are usually high, and 59Fe clearance is prolonged, with decreased incorporation into
circulating red cells.168 Ferrokinetic studies or marrow scanning following injection of technetium sulfur
colloid or indium chloride may be required to assess overall marrow cellularity and function.
DIFFERENTIAL DIAGNOSIS
Both marrow aspirate and biopsy are essential to show the overall marked hypocellularity and to exclude other
causes of pancytopenia. Several disorders may be confused with severe aplastic anemia at the initial
presentation. Approximately 10 percent of patients with myelodysplastic syndromes present with hypoplasia

rather than a hypercellular marrow. This possibility is suspected if there is abnormal blood film morphology,
as seen with myelodysplasia (see Chap. 92). Marrow erythroid precursors in myelodysplasia have both
megaloblastoid and dysplastic features, with dumbbell and cloverleaf nuclei, Howell-Jolly bodies, and
increased siderotic granules. Granulocyte precursors have reduced granulation, with abnormal blue cytoplasm
in the promyelocytes and acquired Pelger-Huüet anomalies in the mature cells. Megakaryocytes may have
hyperlobulation or may have a single nucleus in a small cell (unilobular micromegakaryocytes). Cytogenetic
abnormalities are often found but are not essential for the diagnosis of myelodysplastic syndromes. MRI
studies may be useful in differentiating severe aplastic anemia from myelodysplastic syndromes by showing a
diffuse cellular pattern in the latterdisorders.165,166 and 167 Paroxysmal nocturnal hemoglobinuria is usually
identified by a positive sucrose hemolysis test, or abnormal CD59 red cell membrane proteins as detected by
flow cytometry.6,7 and 8
Acute lymphocytic leukemia may initially have a hypoplastic phase, but even then clumps of lymphocytic cells
are often seen.162 Less often, hairy-cell leukemia may be preceded by a hypoplastic phase.161 Both
conditions can be differentiated from severe aplastic anemia by use of special stains such as tartrate-resistant
acid phosphatase for hairy-cell leukemia and by immunophenotyping using flow cytometry for acute
lymphocytic leukemia.
TREATMENT
The median survival of untreated patients with aplastic anemia [neutrophil count <500/µl (0.5 × 109/liter)] is 3
to 6 months, with only 20 percent surviving beyond a year.169 Very severe aplastic anemia [neutrophils
<200/µl (0.2 × 109/liter)] has an extremely poorprognosis.160 Those patients with less severe disease [i.e.,
neutrophils >1000/µl (1.0 × 109/liter)], who do not require red cell or platelet transfusions, may be treated
conservatively with supportive care, as there are occasional cases of spontaneous recovery.
MARROW TRANSPLANTATION
Prompt and aggressive therapy is usually indicated for most patients with severe disease. The major curative
approach is allogeneic marrow transplantation.170 and 171 This treatment modality is described in Chap. 18.
Only one-third of all patients have compatible donors. Many transplants have been performed using partially
matched siblings or unrelated histocompatible donors recruited through the National Marrow Donor Program
or similar organizations in other countries.172 Umbilical cord blood may evolve as an alternative source of
unrelated donors for transplantation.173
IMMUNOSUPPRESSIVE THERAPY
ANTILYMPHOCYTE AND ANTITHYMOCYTE GLOBULIN
Many cases of aplastic anemia may be mediated by a cellular immune reaction directed against hematopoietic
stem or progenitor cells. The recovery of their own cells was observed in some recipients of mismatched
allogeneic transplants after treatment withALG.174 Improvement occurred in benzene- or 32P-induced
aplastic anemia in rabbits after ALG treatment and transplantation with mismatched marrow cells.175,176 The
requirement for high-dose immunosuppressive treatment to allow engraftment in half the syngeneic

transplants170,177 also supports the concept of a cellular immune reaction. Subsequently, many investigators
found evidence of T-cell–mediated suppressor mechanisms by in vitro marrow
culture studies.20,23,29,31,32,33,34,35 and 36 Some of these observations were undoubtedly due to
transfusion sensitization, but a number of studies supported the notion of an active immune (T-lymphocyte–
mediated) suppressor mechanism being responsible for the aplasia. Although ALS and ATG appear to act by
reducing cytotoxic T cells, these preparations also release hematopoietic growth factors from certain
T cells.178,179
There is about a 50 percent response rate with ALS or ATG.180,181,182,183 and 184 Therapy is given for 4 to
10 days with doses of 15 to 40 mg/kg daily. Since the antisera are prepared in horses, it is important to perform
skin tests against horse serum prior toadministration.185 If positive, pretreatment with glucocorticoids for 24 h
lessens reactions. Fever and chills are common during the first day of treatment; these can be reduced with oral
prednisone, 20 mg, daily. During treatment, accelerated platelet destruction is often seen. This leads to an
increase in transfusion requirements over the 10-day treatment interval. Serum sickness occurs commonly 7 to
10 days from the first dose. This is characterized by spiking fevers, skin rashes, and arthralgias. The clinical
manifestations of serum sickness can be prevented by increasing prednisone to 60 to 80 mg daily, from day 10
to day 17 of the treatment course.
GLUCOCORTICOIDS
Marrow recovery can occur after very high doses of glucocorticoids.186,187Methylprednisolone in the range
of 500 to 1000 mg daily for 3 to 14 days has been successful, but the side effects can be severe. These include
marked glycosuria, electrolyte disturbances, gastric distress, psychosis, increased infections, and aseptic
necrosis of the hips.
Repeated responses to immunosuppressive therapy have been observed. As shown in Fig. 31-2, a 45-year-old
woman with severe aplastic anemia responded completely to ATG and remained well for nearly 9 months. At
relapse, she received a second course of ATG and recovered for another 6 months. She developed an
anaphylactic reaction with a third exposure to ATG. There were several subsequent responses to 14-day
infusions of methylprednisolone (1 g/day), but no appreciable responses to nandrolone decanoate,
cyclosporine, or goat antilymphocyte globulin; she became refractory to therapy and died of fungal sepsis.
FIGURE 31-2 Responses to repeated cycles of immunosuppressive therapy. A 45-year-old woman with severe
aplastic anemia had two complete responses following treatment with horse antithymocyte globulin (ATG).
After an anaphylactic reaction on the third exposure, she received 1 g of methylprednisolone (MP),
intravenously daily for 14 days. This led to a partial response on three occasions. She was essentially
unresponsive to further treatment with nandrolone decanoate (DECA), cyclosporine (CSA), or goat
antilymphocyte globulin (ALG).

CYCLOSPORINE
Another approach to immunosuppressive therapy uses cyclosporine, a cyclic polypeptide that inhibits IL-2
production by T lymphocytes and prevents expansion of cytotoxic T cells in response to IL-2. After the initial
report in 1984 of its ability to induce remission,188 many groups have utilized cyclosporine as primary
treatment,189,190,191 and 192 in patients refractory to ATG or glucocorticoids,190,191,192,193,194 and 195
in combination with granulocyte colony-stimulating factors,196,197 or in varying combinations with other
modes of therapy.198 Cyclosporine is administered orally at 3 to 7 mg/kg per day for at least 4 to 6 months.
Dosage adjustments may be required to maintain trough blood levels of 300 to 500 ng/ml. Renal impairment is
common and may require increased hydration or dose adjustments to keep creatinine values below 2 mg/dl.
Responses are usually seen by 3 months and may range from achieving transfusion independence to complete
remission. Approximately 25 percent of patients respond to this agent, but the response rate has ranged from 0
to 80 percent in variousreports.198
Although immunosuppression with ALG or ATG has the longest experience and a seemingly better response
rate, there are certain advantages to cyclosporine. This drug does not require hospitalization or use of central
venous catheters. Fewer platelet transfusions are required during the first few weeks of therapy, where
accelerated platelet needs are seen in patients receiving ALG or ATG. It is unclear whether cyclosporine
should be considered primary therapy for most patients, with views ranging from cautious optimism to marked
reservation concerning its effectiveness. A French cooperative trial showed equal effectiveness of ATG plus
prednisone compared tocyclosporine.199 In this crossover study of newly diagnosed patients, survivals of
about 65 percent were observed 12 months after diagnosis. Improved in vitro tests to predict responsiveness
may help tailor specific therapy for each patient in the future.194
COMBINATION THERAPY
Both low-dose and high-dose methylprednisolone have been used in combination with ATG
and oxymethylone.200,201 Responses and survival were similar in both groups of patients. Androgens have
been used in conjunction with immunosuppressive agents in randomized trials with both negative202 and
positive results.201,203 Nonrandomized trials suggest that coadministration of androgens with ATG or ALG
improves the response rate.204 At this time, it is unclear whether androgen preparations should be added to
primary therapy; their use should be avoided in patients with posthepatitis aplastic anemia or in those with
abnormal liver function tests.
Several trials have examined the effect of adding cyclosporine to basic regimens of ALG and glucocorticoids.
Overall response rates increased from 47 percent to 70 percent in one study205 and from 39 percent to 65
percent in another.206 With combined therapy a 67 percent response rate was noted at 3 months, which
improved to 78 percent at 1year.207 Although granulocyte colony-stimulating factor alone may be
detrimental,208 its addition to ALG, glucocorticoids, and cyclosporine appeared to improve
responserates.209 Eighty-three percent of 40 patients with very severe aplastic anemia had trilineage
reconstitution and became transfusion independent within 4 months of therapy.

These encouraging results with immunosuppressive therapy are in some instances equal to the results after
marrow transplantation.210,211 and 212 The results from UCLA indicated a 49 percent survival for 146
patients treated with ATG alone.210 This did not differ from the survival of age-matched patients that
underwent bone marrow transplantation during the same time interval. However, the survival after transplants
improved after 1984, from 43 percent to 72 percent, indicating better results with improved transplant
protocols. Similar results were seen in Seattle,211 where 227 patients were treated with ATG alone, and 168
received bone marrow transplants. The actuarial survival at 15 years was 38 percent following ATG therapy
and 69 percent after bone marrow transplant. However, later results with combination immunosuppressive
therapy showed better results. Forty-eight children treated at Memorial Sloan-Kettering between 1983 and
1992 had a 10-year survival of 76 percent for bone marrow transplantation and 74 percent for combined
immunosuppressive therapy.212 Thus, immunosuppression may be preferable for patients who are greater than
30 to 40 years of age and who may experience delay in finding a suitable donor. Marrow transplants are,
however, curative for aplastic anemia, whereas many long-term sequelae have been found after
immunosuppressive therapy.213,214 and 215 Several surveys have shown a substantial rate of relapse or
conversion to other stem cell disorders. Of 358 patients responding to immunosuppressive therapy, 74 relapsed
after a mean of 2.1 years. The actuarial incidence was 35 percent at 10 years.216 In the Swiss experience,217
29 of 129 patients treated with ALG developed myelodysplasia, leukemia, paroxysmal nocturnal
hemoglobinuria, or combined disorders. This tendency to relapse and to develop clonal hematologic disorders
has been reviewed by the European Cooperative Group for Bone Marrow Transplantation in 468 patients, most
of whom receivedATG.218 The risk of a hematologic complication increased continuously and reached 57
percent at 8 years after immunosuppressive therapy. A further survey indicated 42 malignancies in 860 patients
treated with immunosuppression, whereas only 9 malignancies were seen in 748 patients who received
marrow transplants.219 Careful morphologic review of the blood and marrow before and after therapy may
distinguish those patients at risk for late hematologic complications.220
HIGH-DOSE CYCLOPHOSHAMIDE
High-dose cyclophosphamide has been used as a unique form ofimmunosuppression.221 Although it would
seem inappropriate to administer high doses of chemotherapy to patients with severe marrow aplasia, this
approach was based on observations of autologous recovery after preparative therapy for
allogeneictransplants.221 Ten patients received cyclophosphamide at 45 mg/kg per day intravenously for 4
days with or without cyclosporine for an additional 100 days. Gradual neutrophil and platelet recovery ensued
over 3 months. Seven patients responded completely and remain in remission 11 years after treatment.
ANDROGENS
A variety of androgenic and anabolic steroids have been used for aplastic anemia. Testosterone,
methyltestosterone, testosterone enanthate, fluoxymesterone, norethandrolone, oxymetholone, nandrolone
decanoate, danazol, and etiocholanolone have all been employed. These agents stimulate the production of
erythropoietin, and their metabolites stimulate erythropoiesis when added to marrow cultures in vitro.222

Randomized trials have not shown efficacy when androgens were used as primary therapy for severe or
moderately severe aplastic anemia.159,202 However, a French study showed that high doses of androgens
were superior to low doses in patients with only moderately severe aplasia.223 Large series of patients were
reported in which survival seemed improved as compared with historical controls,224 but this could equally be
attributed to better supportive care. Many physicians use androgens in those patients with moderate degrees of
marrow aplasia who have failed initial treatment with immunosuppressive agents. The response of one patient
who was resistant to fluoxymesterone, ATG, and cyclosporine is shown in Fig. 31-3. There was progressive
marrow recovery after 5 months of treatment with etiocholanolone and nandrolonedecanoate.225
FIGURE 31-3 Response to etiocholanolone and nandrolone decanoate. A 62-year-old woman with severe
aplastic anemia failed to improve after treatment with fluoxymesterone (Halotestin)(1¯, ×5 months),
methylprednisolone (M Pred)(2
, 14 days), antithymocyte globulin (ATG) (3¯, 10 days), or cyclosporine (CYS)(4
, ×1 month). Marrow recovery occurred during therapy with 3-b-etiocholanolone and nandrolone decanoate.
(Reproduced from Seewald et al,225 with permission.)
Androgens should be continued for at least 3 to 6 months, since responses may require prolonged treatment.
Nandrolone decanoate, 400 mg intramuscularly per week, is a good initial drug. Concerns about local
hematomas are usually obviated by firm local pressure for 30 min following injection. Long-term survivors
after androgen therapy have essentially the same progression to clonal hematologic disorders as patients
treated with immunosuppressive agents.224
CYTOKINES
Despite their effectiveness in accelerating recovery from chemotherapy, these agents have been far less
effective in achieving long-term benefits in patients with severe aplastic anemia. Daily treatment with
granulocyte-macrophage colony-stimulating factor226,227 and 228 or granulocyte colony-stimulating
factor229 has improved marrow cellularity and increased neutrophil counts approximately 1.5- to 10-fold.
Unfortunately, in nearly all patients, the blood counts return to baseline within several days of cessation of
therapy. Although occasional patients show evidence of trilineage marrow recovery with long-term
therapy,229,230,231 and 231 the vast majority do not respond. In fact, physicians have been cautioned not to
use hemopoietic growth factors as primarytherapy.208 Repeated transfusions of blood and platelets while
awaiting definitive therapy by bone marrow transplant or immunosuppression can reduce the chances of
responding to such therapy. Therapy with myeloid growth factors is probably best reserved for instances of
proved infections or as a preventive measure prior to dental work or other procedures that would compromise
mucosal barriers. Doses of 250 to 300 µg daily by subcutaneous injection are easiest to administer and seem to
be associated with the fewest side effects.
IL-1, a potent stimulator of marrow stromal cell production of other cytokines, and IL-3 have been ineffective

in small numbers of patients with severe aplastic anemia.233,234These disappointing results with cytokines
are not unexpected, as previous work has suggested high serum levels of growth factors in patients with
aplastic anemia. Moreover, the majority of patients appear to have a stem cell defect, which may be
unresponsive to factors that act on more mature progenitor cells.
OTHER THERAPY
High doses of intravenous gamma globulin have been given to small numbers of patients with severe aplastic
anemia235,236 and 237 because of its success in treating certain cases of antibody-mediated pure red cell
aplasia. Some improvement was noted in four of six patients treated. Other treatments that are occasionally
successful include lymphocytapheresis238,239 and acyclovir240 (perhaps owing to a viral etiology in some
cases).
SPLENECTOMY
Removal of the spleen does not increase hematopoiesis but may increase neutrophil and platelet counts two- to
threefold and improve survival of transfused red cells or platelets in highly sensitized individuals.241 The
surgical morbidity and mortality in patients with virtually no platelets makes this a questionable therapeutic
procedure.
SUPPORTIVE CARE
All patients should have immediate HLA typing performed.242 Where marrow transplant is a consideration,
HLA typing of all siblings should be performed as soon as possible. Blood and platelet transfusions should be
used sparingly, if at all, in potential transplant recipients to minimize sensitization. Most young adults can
tolerate hemoglobin levels of 7 to 8 g/dl; platelets need to be given only for active hemorrhage or severe
thrombocytopenia with platelet counts less than 10,000/µl (10 × 109/liter). It is important not to transfuse
patients with red cells or platelets from family members, since this may sensitize patients to minor
histocompatibility antigens, increasing the risk of graft rejection after marrow transplantation. Following a
marrow transplant, or in those individuals in whom transplantation is not a consideration, family members may
be ideal donors for platelet apheresis products.
It is important to assess the risk of bleeding in each individual patient and not to transfuse solely on the basis of
platelet counts. Some patients tolerate platelet counts of 8000 to 10,000/µl (8 to 10 × 109/liter) without undue
bruising or bleeding.243 In some cases, administration of 4 to 12 g daily of e-aminocaproic acid seems to
reduce the bleeding tendency and to avoid transfusion requirements without influencing the
plateletcounts.244 Pooled random-donor platelets may be used until sensitization ensues, although it is
preferable to use single-donor platelets from the onset to minimize sensitization to HLA or platelet antigens.
Subsequently, single-donor apheresis products or HLA-matched platelets may be required.
Platelet refractoriness has been a major problem with long-term transfusion support. This may occur
transiently, with fever or infection, or as a lasting problem secondary to HLA sensitization. In the past, this
occurred in approximately 50 percent of patients after 8 to 10 weeks of transfusion support. Filtration of blood
and platelet concentrates to remove leukocytes reduces this problem to approximately 15 to 20 percent of

patients receiving chronic transfusions.245 In some patients who are refractory to platelets, this problem can be
overcome by administering high-dose intravenous gamma globulin246,247 or by immunoabsorbent pheresis,
using a column to remove circulating IgG complexes.248
Packed red cells are usually given to alleviate symptoms of anemia; transfusions are often indicated at
hemoglobin values below 7 to 8 g/dl. These products should also be given through leukocyte-removal filters to
lessen leukocyte and platelet sensitization and to reduce subsequent transfusion reactions. Since each unit of
red cells provides approximately 200 to 250 mg of iron, there are long-term consequences of transfusion-
induced hemosiderosis. This is not usually a major problem in patients who respond to transplantation or
immunosuppressive therapy but is an issue in nonresponders who require continued support. Consideration
should be given to chelation therapy with deferoxamine to avoid or reduce severe iron overload.249
CMV antibody titers should be obtained on admission; until these results are available, only CMV-negative
blood products should be given to potential transplant recipients to minimize problems with CMV infections
after transplantation. Once a patient is shown to be CMV-positive, this restriction is no longer necessary.
Leukocyte-depletion filters will also decrease the risk of transmitting CMV.
Neutropenic precautions should be applied to hospitalized patients with neutrophil counts less than 1000/µl (1
× 109/liter). Precautions vary widely from institution to institution. One approach is to use private rooms, with
requirements for face masks and hand-washing with antiseptic soap. Fresh fruits and vegetables should be
avoided to prevent bacterial colonization or infection. When patients with aplastic anemia become febrile,
cultures should be obtained from the blood, urine, and any suspicious lesions. Broad-spectrum antibiotics
should be initiated promptly, without awaiting culture results (see Chap. 17). The choice of antibiotics depends
on local practices; major organisms of concern include Staphylococcus aureus, S. epidermidis (in patients with
venous access devices), and gram-negative organisms. Patients with persistent culture-negative fevers should
receive antifungal treatment with fluconazole, itraconazole, or amphotericin.
In the past, leukocyte transfusions were used on a daily basis to reduce the short-term mortality from
infections. Approximately 1010 leukocytes were obtained from normal donors by apheresis. However, there
was generally a high proportion of mononuclear cells rather than neutrophils in these products. With a 1- to 2-h
half-life in febrile patients, it was unusual to detect more than 100 to 200 neutrophils per microliter for more
than a few hours after transfusion. The yield of neutrophils can be increased by administering GM-CSF or G-
CSF to the donor,250 but most physicians avoid using white cell products since present-day antibiotics are
usually sufficient to carry a patient through an episode of sepsis. Notable exceptions include documented
invasive aspergillosis unresponsive to amphotericin (particularly in the post-transplant setting) and infections
with organisms resistant to all known antibiotics.
COURSE AND PROGNOSIS
Before marrow transplantation and immunosuppressive therapy, more than 25 percent of the patients died
within 4 months of diagnosis; half succumbed within 1 year.169,251,252Marrow transplantation is highly
successful and curative for 75 to 85 percent of untransfused patients and for 55 to 60 percent of those with

multiple previous transfusions. Unfortunately, as many as 20 to 30 percent of transplant survivors suffer the
deleterious consequences of severe graft-versus-host disease. Immunosuppressive therapy leads to a marked
improvement in about 50 to 70 percent of the patients; some are hematologically normal, but many continue
with moderate anemia or thrombocytopenia. Relapse occurs in about 15 percent of patients, and indeed the
underlying stem cell defect may progress over 10 years to paroxysmal nocturnal hemoglobinuria, a
myelodysplastic syndrome, or acute myelogenous leukemia in as many as 40 percent of initial responders to
immunosuppressivetherapy.213,214,215,216,217,218 and 219
At diagnosis, the prognosis is largely related to the absolute neutrophil count and platelet count. In the past, the
prognosis appeared worse when the disease followedhepatitis.108,109,253 Recent results with
immunosuppression254 or bone marrow transplant255 are equivalent to that seen with idiopathic or drug-
induced cases. Children appear to respond better than adults, both with transplantation and with androgen
therapy, especially those with mild or moderate disease. Constitutional aplastic anemia responds temporarily to
androgens and glucocorticoids but is invariably fatal unless treated by transplantation.145,150
CHAPTER REFERENCES
1.
Ehrlich P: Uber einen Fall von Anamie mit Bemerkungen uüber regenerative Veranderungen des
Knochenmarks. Charite Ann 13:300, 1888.
2.
Chauffard M: Un cas d’aneémie pernicieuse aplastique. Bull Soc Med Hop Paris 21:313, 1904.
3.
Scott JL, Cartwright GE, Wintrobe MM: Acquired aplastic anemia: an analysis of thirty-nine cases and review
of the pertinent literature. Medicine 38:119, 1959.
4.
Chapuis B, Von Fliedner VE, Jeannet M, et al: Increased frequency of DR2 in patients with aplastic anemia
and increased DR sharing in their parents. Br J Haematol 63:51, 1986.
5.
Odum N, Platz P, Morling N, et al: Increased frequency of HLA-DPw3 in severe aplastic anemia (AA). Tissue
Antigens 20:184, 1987.
6.
Scubert J, Vogt HG, Skowronek M, et al: Development of the glycosylphosphatidylinositol-anchoring defect
characteristic for paroxysmal nocturnal hemoglobinuria in patients with aplastic anemia. Blood 83:2313, 1994.

7.
Griscelli-Bennaceur A, Gluckman E, Scrobohaci ML, et al: Aplastic anemia and paroxysmal nocturnal
hemoglobinuria: search for a pathogenetic link. Blood 85:1354, 1995.
8.
Schrezenmeier H, Hertenstein B, Wagner B, Ragavashar A, Heimpel H: A pathogenetic link between aplastic
anemia and paroxysmal nocturnal hemoglobinuria is suggested by a high frequency of aplastic anemia patients
with a deficiency of phosphatidylinositol glycan anchored proteins. Exp Hematol 23:81, 1995.
9.
Damashek W: What do aplastic anemia, paroxysmal nocturnal hemoglobinuria and hypoplastic leukemia have
in common? (editorial). Blood 30:251, 1967.
10.
Editorial: Aplasia leukaemia syndrome. Lancet 1:1425, 1989.
11.
Marsh JCW, Geary CG: Is aplastic anaemia a preleukaemic disorder? (annotation). Br J Haematol 77:447,
1991.
12.
Socie G: Could aplastic anaemia be considered a pre-pre-leukaemic disorder? Eur J Haematol 57(suppl):60,
1996.
13.
Young NS: The problem of clonality in aplastic anemia: Dr. Damashek’s riddle, restated (perspective). Blood
79:1385, 1992.
14.
Camitta BM, Storb R, Thomas ED: Aplastic anemia: pathogenesis, diagnosis, treatment and prognosis. N Engl
J Med 306:645, 712, 1982.
15.
Marmont AM, Bacigalupo A: Aplastic anaemia: pathogenesis and treatment. Haematologica 73:133, 1988.
16.
Nissen C: The pathophysiology of aplastic anemia. Semin Hematol 28:313, 1991.

17.
Young NS: Immune pathophysiology of acquired aplastic anaemia. Eur J Haematol 57(suppl):55, 1996.
18.
Young NS, Maciejewski J: The pathophysiology of acquired aplastic anemia. N Engl J Med 336:1365, 1997.
19.
Kurnick JE, Robinson WA, Dickey CA: In vitro granulocytic colony-forming potential of bone marrow from
patients with granulocytopenia and aplastic anemia. Proc Soc Exp Biol Med 137:917, 1971.
20.
Kagan WA, Ascensao J, Pahwa R, et al: Aplastic anemia: presence in human bone marrow of cells that
suppress myelopoiesis. Proc Natl Acad Sci USA 73:2890, 1976.
21.
Kern P, Heimpel H, Heit W, et al: Granulocytic progenitor cells in aplastic anaemia. Br J Haematol 35:613,
1977.
22.
Hoak HL, Goselink HM, Veenkof, et al: Acquired aplastic anaemia in adults. IV. Histological and CFU studies
in transplanted and nontransplanted patients. Scand J Haematol 19:159, 1977.
23.
Hoffman R, Zanjani ED, Lutton JD, et al: Suppression of erythroid-colony formation by lymphocytes from
patients with aplastic anemia. N Engl J Med 296:10, 1977.
24.
Hansi W, Rich I, Heimpel H, et al: Erythroid colony forming cells in aplastic anaemia. Br J Haematol 37:483,
1977.
25.
Moriyama Y, Sato M, Kinoshita Y: Studies of hematopoietic stem cells. XI. Lack of erythroid burst-forming
units (BFU-E) in patients with aplastic anemia. Am J Hematol 6:11, 1979.
26.
Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS: A severe and consistent deficit in marrow and

circulating primitive hematopoietic cells (long-term culture-initiating cells) in acquired aplastic anemia. Blood
88:1983, 1996.
27.
Maciejewski JP, Anderson S, Katevas P, Young NS: Phenotypic and functional analysis of bone marrow
progenitor cell compartment in bone marrow failure. Br J Haematol 87:227, 1994.
28.
Scopes J, Bagnara M, Gordon-Smith EC, Ball SE, Gibson FM: Haemopoietic progenitor cells are reduced in
aplastic anaemia. Br J Haematol 86:427, 1994.
29.
Ascensao J, Kagan W, Moore M, et al: Aplastic anemia: evidence for an immunological mechanism. Lancet
1:669, 1976.
30.
Nissen C, Cornu P, Gratwohl A, Speck B: Peripheral blood cells from patients with aplastic anaemia in partial
remission suppress growth of their own bone marrow precursors in culture. Br J Haematol 45:233, 1980.
31.
Bacigalupo A, Podesta M, VanLint MT, et al: Severe aplastic anaemia: correlation of in vitro tests with clinical
response to immunosuppression in twenty patients. Br J Haematol 47:423, 1981.
32.
Singer JW, Brown JE, James MC, et al: Effect of peripheral blood lymphocytes from patients with aplastic
anemia on granulocyte colony growth from HLA-matched and mismatched marrows: effect of transfusion
sensitization. Blood 52:37, 1978.
33.
Torok-Storb B, Sieff C, Storb R, et al: In vitro tests for distinguishing possible immune-mediated aplastic
anemia from transfusion-induced sensitization. Blood 55:211, 1980.
34.
Singer JW, Doney KC, Thomas ED: Co-culture studies of 16 untransfused patients with aplastic anemia.
Blood 54:180, 1979.

35.
Mangan KF, Mullaney MT, Rosenfeld CS, Shadduck RK: In vitro evidence for disappearance of erythroid
progenitor T suppressor cells following allogeneic bone marrow transplantation for severe aplastic anemia.
Blood 71:144, 1988.
36.
Teramura M, Kobayashi S, Iwabe K, Yoshinaga K, Mizoguchi H: Mechanism of action of antithymocyte
globulin in the treatment of aplastic anaemia: in vitro evidence for the presence of immunosuppressive
mechanism. Br J Haematol 96:80, 1997.
37.
Knospe WH, Crosby WH: Aplastic anaemia: a disorder of the bone-marrow sinusoidal microcirculation rather
than stem cell failure? Lancet 1:20, 1971.
38.
Morley, A, Blake J: An animal model of chronic aplastic marrow failure. I. Late marrow failure after busulfan.
Blood 44:49, 1974.
39.
Morley A, Trainor K, Remes J: Residual marrow damage: possible explanation for idiosyncrasy to
chloramphenicol. Br J Haematol 31:525, 1976.
40.
Harrison DE: Use of genetic anaemia in mice as tools for haematological research. Clin Haematol 8:239, 1979.
41.
Geissler EN, Ryan MA, Housman DE: The dominant-white spotting (W) locus of the mouse encodes the c-kit
proto-oncogene. Cell 55:185, 1988.
42.
Huang E, Nocka K, Beier Dr, et al: The hematopoietic growth factor KL is encoded by the Sl locus and is the
ligand of the c-kit receptor, the gene product of the W locus. Cell 63:225, 1990.
43.
Wodnar-Filipowicz A, Yancik S, Moser Y, et al: Levels of soluble stem cell factor in serum of patients with
aplastic anemia. Blood 81:3159, 1993.

44.
Nimer SD, Leung DHY, Wolin MJ, Golde DW: Serum stem cell factor levels in patients with aplastic anemia.
Int J Hematol 60:185, 1994.
45.
Kojima S, Matsuyama T, Kodera Y: Plasma levels and production of soluble stem cell factor by marrow
stromal cells in patients with aplastic anaemia. Br J Haematol 99:440, 1997.
46.
Wodnar-Filipowicz A, Tichelli A, Zsebo KM, Speck B, Nissen C: Stem cell factor stimulates the in vitro
growth of bone marrow cells from aplastic anemia patients. Blood 79:3196, 1992.
47.
Lyman SD, Seaberg M, Hanna R, et al: Plasma/serum levels of flt3 ligand are low in normal individuals and
highly elevated in patients with Fanconi anemia and acquired aplastic anemia. Blood 86:4091, 1995.
48.
Kojima S, Matsuyama T, Kodera Y, et al: Measurement of endogenous plasma granulocyte colony-stimulating
factor in patients with acquired aplastic anemia by a sensitive chemiluminescent immunoassay. Blood 87:1303,
1996.
49.
Kojima S, Matsuyama T, Kodera Y: Circulating erythropoietin in patients with acquired aplastic anaemia. Acta
Haematol 94:117, 1995.
50.
Emmons RVD, Reid DM, Cohen RL, et al: Human thrombopoietin levels are high when thrombocytopenia is
due to megakaryocyte deficiency and low when due to increased platelet destruction. Blood 87:4068, 1996.
51.
Nakao S, Matsushima K, Young N: Deficient interleukin I production by aplastic anaemia monocytes. Br J
Haematol 71:431, 1989.
52.
Zoumbos N, Gascon P, Djeu J, Young NS: Interferon is a mediator of hematopoietic suppression in aplastic
anemia in vitro and possibly in vivo. Proc Natl Acad Sci USA 82:188, 1985.

53.
Laver J, Castro-Malaspina H, Kernan NA, et al: In vitro interferon-gamma production by cultured T-cells in
severe aplastic anaemia: correlation with granulomonopoietic inhibition in patients who respond to anti-
thymocyte globulin. Br J Haematol 69:545, 1988.
54.
Gascon P, Zoumbos NC, Scala G, et al: Lymphokine abnormalities in aplastic anemia: implications for the
mechanism of action of antithymocyte globulin. Blood 65:407, 1985.
55.
Hinterberger W, Adolf G, Bettelheim P, et al: Lymphokine overproduction in severe aplastic anemia is not
related to blood transfusions. Blood 74:2713, 1989.
56.
Shinohara K, Ayame H, Tanaka M, et al: Increased production of tumor necrosis factor alpha by peripheral
blood mononuclear cells in the patients with aplastic anemia. Am J Hematol 37:75, 1991.
57.
Nistico, A, Young, NS: Gamma-interferon gene expression in the bone marrow of patients with aplastic
anemia. Ann Intern Med 120:463, 1994.
58.
Marsh JC, Chang J, Testa NG, et al: In vitro assessment of marrow “stem cell” and stromal cell function in
aplastic anaemia. Br J Haematol 78:258, 1991.
59.
Kojima S, Matsuyama T, Kodera Y: Hematopoietic growth factors released by marrow stromal cells from
patients with aplastic anemia. Blood 79:2256, 1992.
60.
Holmberg LA, Seidel K, Leisenring W, Torok-Storb B: Aplastic anemia: analysis of stromal cell function in
long-term marrow cultures. Blood 84:3685, 1994.
61.
Santesson GG: Uüber chronische Vergiftungen mit Steinkohlenteerbenzin. vier Todesfaülle. Arch Hyg Berl
31:336, 1897.

62.
Rangau U, Snyder R: Scientific update on benzene. Ann N Y Acad Sci 837:105, 1997.
63.
Goldstein BD: Benzene toxicity. Occup Med 3:541, 1988.
64.
Aksoy B: Hematotoxicity and carcinogenicity of benzene. Environ Health Perspect 82:193, 1989.
65.
Smith MT: Overview of benzene-induced aplastic anaemia. Eur J Haematol 60(suppl):107, 1996.
66.
Paci E, Buiatti E, Costatini AS, et al: Aplastic anemia, leukemia and other cancer mortality in a cohort of shoe
workers exposed to benzene. Scand J Work Environ Health 15:313, 1989.
67.
Yin SN, Li GL, Tain FD, et al: Leukaemia in benzene workers: a retrospective cohort study. Br J Indust Med
44:124, 1987.
68.
Yardley-Jones A, Anderson D, Parke DV: The toxicity of benzene and its metabolism and molecular pathology
in human risk assessment. Br J Indust Med 48:437, 1991.
69.
Hamilton A: The lessening menace of benzol poisoning in American industry. J Indust Hyg 10:227, 1928.
70.
Aksoy M, Dincol K, Akgun T, et al: Hematological effects of chronic benzene poisoning in 217 workers. Br J
Indust Med 28:296, 1971.
71.
Fleming LE, Timmeny MA: Aplastic anemia and pesticides. JOM 35:1106, 1993.
72.
Rugman FP, Cosstick R: Aplastic anaemia associated with organochlorine pesticide: case reports and review of
evidence. J Clin Pathol 43:98, 1990.

73.
Rauch AE, Kowalsky SF, Lesar TS, et al: Lindane (Kwell)-induced aplastic anemia. Arch Intern Med
150:2393, 1990.
74.
Sanchez-Medal L, Castanedo JP, Garcia-Rojas F: Insecticides and aplastic anemia. N Engl J Med 269:1365,
1963.
75.
Roberts HJ: Pentachlorophenol-associated aplastic anemia, red cell aplasia, leukemia and other blood
disorders. J Fla Med Assoc 77:86, 1990.
76.
Prager D, Peters C: Development of aplastic anemia and the exposure to Stoddard solvent. Blood 35:286,
1970.
77.
Powers D: Aplastic anemia secondary to glue sniffing. N Engl J Med 273:700, 1965.
78.
Crawford MAD: Aplastic anaemia due to trinitrotoluene intoxication. Br Med J 2:430, 1954.
79.
Claudon DB, Holbrook AA: Fatal aplastic anemia associated with chloramphenicol (Chloromycetin) therapy.
JAMA 149:912, 1952.
80.
Smiley RK, Cartwright GE, Wintrobe MM: Fatal aplastic anemia following chloramphenicol (Chloromycetin)
administration. JAMA 149:914, 1952.
81.
Sturgeon P: Fatal aplastic anemia in children following chloramphenicol (Chloromycetin) therapy. JAMA
149:918, 1952.
82.
Dameshek W: Chloramphenicol (Chloromycetin) and the bone marrow (editorial). Blood 7:755, 1952.

83.
Wallerstein RO, Condit PK, Kasper PK, et al: Statewide study of chloramphenicol therapy and fatal aplastic
anemia. JAMA 208:2045, 1969.
84.
Modan B, Segal S, Shani M, Sheba C: Aplastic anemia in Israel: evaluation of the etiological role of
chloramphenicol on a community-wide basis. Am J Med Sci 270:441, 1975.
85.
Smick K, Condit PK, Proctor RL, Sutcher V: Fatal aplastic anemia: an epidemiological study of its
relationship to the drug chloramphenicol. J Chronic Dis 17:899, 1964.
86.
Brodsky E, Zeidan Z, Biger Y, Schneider M: Topical application of chloramphenicol eye ointment followed by
fatal bone marrow aplasia. Isr J Med Sci 25:54, 1989.
87.
West BC, DeVault GA Jr, Clement JC, Williams DM: Aplastic anemia associated with parenteral
chloramphenicol: review of 10 cases, including the second case of possible increased risk with cimetidine. Rev
Infect Dis 10:1048, 1988.
88.
Rubin D, Weisberger A, Botti RE, Storaasli JP: Changes in iron metabolism in early chloramphenicol toxicity.
J Clin Invest 37:1286, 1958.
89.
Saidi P, Wallerstein RO, Aggeler P: Effect of chloramphenicol on erythropoiesis. J Lab Clin Med 57:247,
1961.
90.
Scott JL, Finegold SM, Belkin GA, Lawrence JS: A controlled double-blind study of the hematologic toxicity
of chloramphenicol. N Engl J Med 272:1137, 1965.
91.
Weisberger AS: Mechanisms of action of chloramphenicol. JAMA 209:97, 1969.

92.
Nagao T, Mauer AM: Concordance for drug-induced aplastic anemia in identical twins. N Engl J Med 281:7,
1969.
93.
Custer RP: Aplastic anemia in soldiers treated with Atabrine (quinacrine). Am J Med Sci 212:211, 1946.
94.
Cunningham JL, Leyland MJ, Delamore IW, Price-Evans DA: Acetanilide oxidation in phenylbutazone-
associated hypoplastic anaemia. Br Med J 3:313, 1974.
95.
Chang HK, Morrison SL: Bone marrow suppression associated with cimetidine. Ann Intern Med 91:580, 1979.
96.
Tonkonow B, Hoffman R: Aplastic anemia and cimetidine. Arch Intern Med 140:1123, 1980.
97.
Volkin RL, Shadduck RK, Winkelstein A, et al: Potentiation of carmustine-cranial-irradiation-induced
myelosuppression by cimetidine. Arch Intern Med 142:243, 1982.
98.
Court-Brown WM, Doll R: Mortality from cancer and other causes after radiotherapy for ankylosing
spondylitis. Br Med J 2:1317, 1965.
99.
Darby SC, Doll R, Gill SK, Smith PG: Long term mortality after a single treatment course with x-rays in
patients treated with ankylosing spondylitis. Br J Cancer 55:179, 1987.
100.
Johnson SAN, Bateman CJT, Beard MEJ, et al: Long-term haematological complications of Thorotrast. Q J
Med 182:259, 1977.
101.
Martland HS: The occurrence of malignancy in radioactive persons: a general review of data gathered in the
study of the radium dial painters, with special reference to the occurrence of osteogeneic sarcoma and the
inter-relationship of certain blood diseases. Am J Cancer 15:2435, 1931.

102.
Cronkite EP, Haley TJ: Clinical aspects of acute radiation injury, in Manual on Radiation Haematology, pp
169–173. International Atomic Energy Agency, Vienna, 1971.
103.
Mettler FA Jr, Moseley RD Jr: Medical Effects of Ionizing Irradiation, pp. 1–185. Grune and Stratton, New
York, 1985.
104.
Gale RP: Immediate medical consequences of nuclear accidents: lessons from Chernobyl. JAMA 258:625,
1987.
105.
Champlin RE, Kastenberg WE, Gale RP: Radiation accidents and nuclear energy: medical consequences and
therapy. Ann Intern Med 109:730, 1988.
106.
Baranov A, Gale RP, Guskova A, et al: Bone marrow transplantation after the Chernobyl nuclear accident. N
Engl J Med 311:205, 1989.
107.
Gale RP: USSR: Follow-up after Chernobyl. Lancet 1:401, 1990.
108.
Ajlouni K, Doeblin TD: The syndrome of hepatitis and aplastic anaemia. Br J Haematol 27:345, 1974.
109.
Hagler L, Pastore RA, Bergin JJ: Aplastic anemia following viral hepatitis: report of 2 fatal cases and literature
review. Medicine (Baltimore) 54:139, 1975.
110.
Pol S, Driss F, Devergie A, et al: Is hepatitis C virus involved in hepatitis-associated aplastic anemia? Ann
Intern Med 113:435, 1990.
111.
Hibbs JR, Frickhofen N, Rosenfeld SJ, et al: Aplastic anemia and viral hepatitis: non-A, non-B, non-C? JAMA
267:2051, 1992.

112.
Tzakis AG, Arditi M, Whitington PF, et al: Aplastic anemia complicating orthotopic liver transplantation for
non-A, non-B hepatitis. N Engl J Med 319:393, 1988.
113.
Kurtzman G, Young N: Viruses and bone marrow failure. Baillieres Clin Haematol 2:51, 1989.
114.
Hibbs JR, Issaragrisl S, Young NS: High prevalence of hepatitis C viremia among aplastic anemia patients and
controls from Thailand. Am J Trop Med Hyg 46:564, 1992.
115.
Brown KE, Tisdale J, Barrett AJ, Dunbar CE, Young NS: Hepatitis-associated aplastic anemia. N Engl J Med
336:1059, 1997.
116.
Shadduck RK, Winkelstein A, Zeigler Z, et al: Aplastic anemia following infectious mononucleosis: possible
immune etiology. Exp Hematol 7:264, 1979.
117.
Lazarus KH, Baehner RL: Aplastic anemia complicating infectious mononucleosis: a case report and review of
the literature. Pediatrics 67:907, 1981.
118.
Baranski B, Armstrong G, Truman JT, et al: Epstein-Barr virus in the bone marrow of patients with aplastic
anemia. Ann Intern Med 109:695, 1988.
119.
Young N: Hematologic and hematopoietic consequences of B-19 parvovirus infection. Semin Hematol 25:159,
1988.
120.
Rosenfeld CS, Rybka WB, Weinbaum D, et al: Late graft failure due to dual bone marrow infection with
variants A and B of human Herpesvirus-6. Exp Hematol 23:626, 1995.

121.
Vinters HV, Mah V, Mohrmann R, Wiley CA: Evidence for human immunodeficiency virus (HIV) infection
of the brain in a patient with aplastic anemia. Acta Neuropathol 76:311, 1988.
122.
Samuel D, Castaing D, Adam R, et al: Fatal acute HIV infection with aplastic anaemia, transmitted by liver
graft. Lancet 1:1221, 1988.
123.
Morales CE, Sriram I, Baumann MA: Myelodysplastic syndrome occurring as possible first manifestation of
human immunodeficiency virus infection with subsequent progression to aplastic anaemia. Int J STD AIDS
1:55, 1990.
124.
Baumelou E, Guiguet M, Mary JY, et al: Epidemiology of aplastic anemia in France: a case control study. I.
Medical history and medication use. Blood 81:1471, 1993.
125.
Stricke RB, Shuman MA: Aplastic anemia complicating systemic lupus erythematosus: response to androgens
in two patients. Am J Hematol 17:193, 1984.
126.
Fitchen JJ, Cline MJ, Saxon A, Golde DW: Serum inhibitors of hematopoiesis in a patient with aplastic anemia
and systemic lupus erythematosus: recovery after exchange plasmapheresis. Am J Med 66:537, 1979.
127.
Bailey FA, Lilly M, Bertoli LF, Ball GV: An antibody that inhibits in vitro bone marrow proliferation in a
patient with systemic lupus erythematosus and aplastic anemia. Arthritis Rheum 31:901, 1989.
128.
Roffe C, Cahill MR, Samanta A, et al: Aplastic anaemia in systemic lupus erythematosus: a cellular immune
mechanism? Br J Rheumatol 30:301, 1991.
129.
Sumimoto S, Kawai M, Kasajima Y, Hamamoto T: Aplastic anemia associated with systemic lupus
erythematosus. Am J Hematol 38:329, 1991.

130.
Winkler A, Jackson RW, Kay DS, et al: High-dose intravenous cyclophosphamide treatment of systemic lupus
erythematosus-associated aplastic anemia (letter). Arthritis Rheum 31:693, 1988.
131.
Hoffman R, Dainiak N, Sibrack L, et al: Antibody-mediated aplastic anemia and diffuse fasciitis. N Engl J
Med 300:718, 1979.
132.
Narayanan MN, Liu Yin JA, Love EM, et al: Eosinophilic fasciitis and aplastic anaemia. Clin Lab Haematol
10:471, 1988.
133.
Debusscher L, Bitar N, DeMaubeuge J, et al: Eosinophilic fasciitis and severe aplastic anemia: favorable
response to either antithymocyte globulin or cyclosporin A in blood and skin disorders. Transplant Proc
20:310, 1988.
134.
Blaser KU, Steiger U, Wursch A, Speck B: Eosinophilic fasciitis with aplastic anemia and Hashimoto’s
thyroiditis. Review of the literature and report of a typical example. Schweiz Med Wochenschr 119:1899,
1989.
135.
Evans IL: Aplastic anaemia in pregnancy remitting after abortion. Br Med J 3:166, 1968.
136.
Fleming AF: Hypoplastic anaemia in pregnancy. J Obstet Gynaecol Br Commonw 75:138, 1968.
137.
Goldstein JM, Coller BS: Aplastic anemia in pregnancy: recovery after normal spontaneous delivery (letter).
Ann Intern Med 82:537, 1975.
138.
Suda T, Omine M, Tsuchiya J, Maekawa T: Prognostic aspects of aplastic anaemia in pregnancy. Experience
on 6 cases and review of the literature. Blut 36:285, 1978.

139.
Aitchison RGM, Marsh JCW, Hows JM, et al: Pregnancy associated aplastic anaemia: a report of 5 cases and
review of current management. Br J Haematol 73:541, 1989.
140.
Pajor A, Kelemen E, Szak’acs Z, Lehoczky D: Pregnancy in idiopathic aplastic anemia (report of 10 patients).
Eur J Obstet Gynecol Reprod Biol 45:19, 1992.
141.
Bourantas K, Makrydimas G, Georgiou I, Repousis P, Lolis D: Aplastic anemia: report of a case with recurrent
episodes in consecutive pregnancies. J Reprod Med 42:672, 1997.
142.
Fanconi G: Familiaüre infantile perniziosaartige anaümie (pernizioüses blutbild und konstitution). Jahrbuch
Kinderheil 117:257, 1927.
143.
Gordon-Smith EC, Rutherford TR: Fanconi anaemia—constitutional, familial aplastic anaemia. Baillieres Clin
Haematol 2:139, 1989.
144.
Gordon-Smith EC, Rutherford TR: Fanconi anemia: constitutional aplastic anemia. Semin Hematol 28:104,
1991.
145.
Alter BP: Fanconi’s anemia: current concepts: Am J Pediatr Hematol Oncol 14:170, 1992.
146.
Auerbach AD, Rogatko A, Schroeder-Kurth TM: International Fanconi Anemia Registry: first report, in
Fanconi Anemia: Clinical, Cytogenetic and Experimental Aspects, edited by TM Schroeder-Kurth, AD
Auerbach, G Obe, pp 3–17. Springer-Verlag, Berlin, 1989.
147.
D’Apolito M, Zelante L, Savoia A: Molecular basis of Fanconi anemia. Hematologica 83:533, 1998.

148.
Garcia-Higuera I, Kuang Y, D’Andrea AD: The molecular and cellular biology of Fanconi anemia. Curr Opin
Hematol 6:83, 1999.
149.
Estren S, Damshek W: Familial hypoplastic anemia of childhood: report of 8 cases in 2 families with
beneficial effects of splenectomy in 1 case. Am J Dis Child 73:671, 1947.
150.
Gluckman E, Auerbach A, Horowitz MM, et al: Bone marrow transplantation for Fanconi anemia. Blood
86:2856, 1995.
151.
Dokal I: Dyskeratosis congenita: an inherited bone marrow failure syndrome. Br J Haematol 92:775, 1996.
152.
Ginzberg H, Shin J, Ellis L, et al: Schwachman syndrome: phenotypic manifestations of sibling sets and
isolated cases in a large patient cohort are similar. J Pediatr 135:81, 1999.
153.
Bottiger LE, Westerholm B: Aplastic anemia. I. Incidence and aetiology. Acta Med Scand 192:315, 1972.
154.
Szklo M, Sensenbrenner L, Markowitz J, et al: Incidence of aplastic anemia in metropolitan Baltimore: a
population based study. Blood 66:115, 1985.
155.
Linet MS, McCaffrey LD, Morgan WF, et al: Incidence of aplastic anemia in a three county area in South
Carolina. Cancer Res 46:426, 1986.
156.
Marsh JCW, Hows JM, Bryett KA, et al: Survival after antilymphocyte globulin for aplastic anemia depends
on disease severity. Blood 70:1046, 1987.
157.
Alexanian R: Erythropoietin excretion in bone marrow failure and hemolytic anemia. J Lab Clin Med 82:438,
1973.

158.
Stohlman F Jr: Erythropoiesis. N Engl J Med 267:342, 392, 1962.
159.
Camitta BM, Thomas ED, Nathan DG, et al: A prospective study of androgens and bone marrow
transplantation for treatment of severe aplastic anemia. Blood 53:504, 1979.
160.
Bacigalupo A, Hows J, Gluckman E, et al: Bone marrow transplantation (BMT) versus immunosuppression for
the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol
70:177, 1988.
161.
Krause JR: Aplastic anemia terminating in hairy cell leukemia. A report of 2 cases. Cancer 53:1533, 1984.
162.
Reid MM, Summerfield GP: Distinction between a leukaemic prodrome of childhood acute lymphoblastic
leukaemia and aplastic anaemia. J Clin Pathol 45:697, 1992.
163.
Torok-Storb B, Doney K, Brown SL, Prentice RL: Correlation of two in vitro tests with clinical response to
immunosuppressive therapy in 54 patients with severe aplastic anemia. Blood 63:349, 1984.
164.
Applebaum FR, Barrall J, Storb R, et al: Clonal cytogenetic abnormalities in patients with otherwise typical
aplastic anemia. Exp Hematol 15:1134, 1987.
165.
Olson DO, Shields AF, Scheurich CJ, et al: Magnetic resonance imaging of the bone marrow in patients with
leukemia, aplastic anemia, and lymphoma. Invest Radiol 21:540, 1986.
166.
Steiner RM, Mitchell DG, Rao VM, et al: Magnetic resonance imaging of bone marrow: diagnostic value in
diffuse hematologic disorders. Magn Reson Q 6:17, 1990.

167.
Negendank W, Weissman D, Bey TM, et al: Evidence for clonal disease by magnetic resonance imaging in
patients with hypoplastic marrow disorders. Blood 78:2872, 1991.
168.
Finch CA, Duebelbeiss K, Cook JD, et al: Ferrokinetics in man. Medicine (Baltimore) 49:17, 1970.
169.
Camitta BM, Thomas ED, Nathan DG, et al: Severe aplastic anemia: a prospective study of the effect of early
marrow transplantation on acute mortality. Blood 48:63, 1976.
170.
Storb R, Longton G, Anasetti C, et al: Changing trends in marrow transplantation for aplastic anemia. Bone
Marrow Transplant 10(suppl 1):45, 1992.
171.
Margolis DA, Cammita BM: Hematopoietic stem cell transplantation for severe aplastic anemia. Curr Opin
Hematol 5:441, 1998.
172.
Kernan NA, Bartsch G, Ash RC, et al: Analysis of 462 transplantations from unrelated donors facilitated by
the National Marrow Donor Program. N Engl J Med 318:593, 1993.
173.
Wagner JE, Rosenthal J, Sweetman R, et al: Successful transplantation of HLA-matched and HLA-
mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host
disease. Blood 88:795, 1996.
174.
Matheé G, Amiel JL, Schwarzenberg L, et al: Bone marrow graft in man after conditioning by antilymphocytic
serum. Br Med J 2:131, 1970.
175.
Speck B, Kissling M: Successful bone marrow grafts in experimental aplastic anemia using antilymphocytic
serum for conditioning. Eur J Clin Biol Res 16:1047, 1971.

176.
Speck B, Kissling M: Studies on bone marrow transplantation and experimental 31P-induced aplastic anaemia
after conditioning with antilymphocyte serum. Acta Haematol 50:193, 1973.
177.
Champlin RE, Feig SA, Sparkes RS, Gale RP: Bone marrow transplantation from identical twins in the
treatment of aplastic anaemia: implication for the pathogenesis of the disease. Br J Haematol 56:455, 1984.
178.
Mangan KF, D’Alessandro L, Mullaney MT: Action of antithymocyte globulin on normal human erythroid
progenitor cell proliferation in vitro: erythropoietic growth-enhancing factors are released from marrow
accessory cells. J Lab Clin Med 107:353, 1986.
179.
Kawano Y, Nissen C, Gratwohl A, Speck B: Immunostimulatory effects of different antilymphocyte globulin
preparations: a possible clue to their clinical effect. Br J Haematol 68:115, 1988.
180.
Speck B, Gluckman E, Haak HL, et al: Treatment of aplastic anaemia by antilymphocyte globulin with and
without allogeneic bone-marrow infusions. Lancet 2:1145, 1977.
181.
Champlin RE, Ho W, Gale RP: Antilymphocyte globulin treatment in patients with aplastic anemia. N Engl J
Med 308:113, 1983.
182.
Camitta B, O’Reilly RJ, Sensenbrenner L, et al: Antithoracic duct lymphocyte globulin therapy of severe
aplastic anemia. Blood 62:883, 1983.
183.
Young N, Griffith P, Brittain E, et al: A multi-center trial of antithymocyte globulin in aplastic anemia and
related diseases. Blood 72:1861, 1989.
184.
Camitta BM, Doney K: Immunosuppressive therapy for aplastic anemia: indications, agents, mechanisms, and
results. Am J Pediatr Hematol Oncol 12:411, 1990.

185.
Bielory L, Wright R, Nienhuis AW, et al: Antithymocyte globulin hypersensitivity in bone marrow failure
patients. JAMA 260:3164, 1988.
186.
Bacigalupo A, Van Lint MT, Cerri R, et al: Treatment of severe aplastic anemia with bolus 6-
methylprednisolone and antilymphocyte globulin. Blut 41:168, 1980.
187.
Issaragrisil S, Tangnai-Trisorana Y, Siriseriwan T, et al: Methylprednisolone therapy in aplastic anaemia:
correlation of in vitro tests and lymphocyte subsets with clinical response. Eur J Haematol 40:343, 1988.
188.
Stryckmans PA, Dumont JP, Velu T, Debusscher L: Cyclosporine in refractory severe aplastic anemia (letter).
N Engl J Med 310:655, 1984.
189.
Lazzarino M, Morra E, Canevari A, et al: Cyclosporine in the treatment of aplastic anaemia and pure red-cell
aplasia. Bone Marrow Transplant 4(suppl 4):165, 1989.
190.
Hinterberger-Fischer M, Hoücker P, Lechner K, et al: Oral cyclosporin-A is effective treatment for untreated
and also for previously immunosuppressed patients with severe bone marrow failure. Eur J Haematol 43:136,
1989.
191.
Toütterman TH, Hoüglund M, Bengtsson M, et al: Treatment of pure red-cell aplasia and aplastic anaemia with
cyclosporin: long-term clinical effects. Eur J Haematol 42:126, 1989.
192.
Leeksma OC, Thomas LLM, van der Lelie J, van Oers MHJ, Kr.von dem Borne AEG, Goudsmit R:
Effectiveness of low dose cyclosporine in acquired aplastic anaemia with severe neutropenia. Neth J Med
41:143, 1992.
193.
Leonard EM, Raefsky E, Griffith P, et al: Cyclosporine therapy of aplastic anaemia, congenital and acquired
red-cell aplasia. Br J Haematol 72:278, 1989.

194.
Tong J, Bacigalupo A, Piaggio G, et al: Severe aplastic anemia (SAA): response to cyclosporin A (CyA) in
vivo and in vitro. Eur J Haematol 46:212, 1991.
195.
Nakao S, Yamaguchi M, Shiobara S, et al: Interferon-g gene expression in unstimulated bone marrow
mononuclear cells predicts a good response to cyclosporine therapy in aplastic anemia. Blood 79:2531, 1992.
196.
Kojima S, Fukada M, Miyajima Y, Matsuyama T: Cyclosporine and recombinant granulocyte colony-
stimulating factor in severe aplastic anemia (letter). N Engl J Med 313:920, 1990.
197.
Bertrand Y, Amri F, Capdeville R, et al: The successful treatment of two cases of severe aplastic anaemia with
granulocyte colony-stimulating factor and cyclosporine A (case report). Br J Haematol 79:648, 1991.
198.
Schrezenmeier H, Schlander M, Raghavachar A: Cyclosporin A in aplastic anemia—report of a workshop.
Ann Hematol 65:33, 1992.
199.
Gluckman E, Esperou-Bourdeau H, Baruchel A, et al: Multicenter randomized study comparing cyclosporine-
A alone and antithymocyte globulin with prednisone for treatment of severe aplastic anemia. Blood 79:2540,
1992.
200.
Doney K, Pepe M, Storb R, et al: Immunosuppressive therapy of aplastic anemia: results of a prospective,
randomized trial of antithymocyte globulin (ATG), methylprednisolone and oxymetholone to ATG, very high-
dose methylprednisolone, and oxymetholone. Blood 79:2566, 1992.
201.
Bacigalupo A, Chaple M, Hows J, et al: Treatment of aplastic anaemia (AA) with antilymphocyte globulin
(ALG) and methylprednisolone (MPred) with or without androgens: a randomized trial from the EBMT SAA
working party. Br J Haematol 83:145, 1993.

202.
Champlin RE, Ho WG, Feig SA, et al: Do androgens enhance the response to antithymocyte globulin in
patients with aplastic anemia? A prospective randomized trial. Blood 66:184, 1985.
203.
Kaltwasser JP, Dix U, Schalk KP, Vogt H: Effect of androgens on the response to antithymocyte globulin in
patients with aplastic anaemia. Eur J Haematol 40:111, 1988.
204.
Gluckman E, Devergie P, Poros A, et al: Results of immunosuppression in 170 cases of severe aplastic
anaemia. Br J Haematol 51:541, 1982.
205.
Park CW, Han CH, Kim CC, et al: Immunomodulation therapy for severe aplastic anemia: ALG versus ALG
plus cyclosporin-A. Korean J Intern Med 4:28, 1989.
206.
Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al: Treatment of aplastic anemia with antilymphocyte
globulin and methylprednisolone with or without cyclosporine. N Engl J Med 314:1299, 1991.
207.
Rosenfeld SJ, Kimball J, Vining D, Young NS: Intensive immunosuppression with antithymocyte globulin and
cyclosporine as treatment for severe acquired aplastic anemia. Blood 85:3058, 1995.
208.
Marsh JCW, Socie G, Schrezenmeier H, et al: Haemopoietic growth factors in aplastic anaemia: a cautionary
note. Lancet 344:172, 1994.
209.
Bacigalupo A, Broccia G, Corda G, et al: Antilymphocyte globulin, cyclosporine, and granulocyte colony-
stimulating factor in patients with acquired severe aplastic anemia (SAA): a pilot study of the EBMT SAA
working party. Blood 85:1348, 1995.
210.
Paquette RL, Tebyani N, Frane M, et al: Long-term outcome of aplastic anemia in adults treated with
antithymocyte globulin: comparison with bone marrow transplantation. Blood 85:283, 1995.

211.
Doney K, Leisenring W, Storb R, Appelbaum FR: Primary treatment of acquired aplastic anemia: outcomes
with bone marrow transplantation and immunosuppressive therapy. Ann Intern Med 126:107, 1997.
212.
Gillio AP, Boulad F, Small TN, et al: Comparison of long-term outcome of children with severe aplastic
anemia treated with immunosuppression versus bone marrow transplantation. Biol Blood Marrow Transplant
3:18, 1997.
213.
De Planque MM, Kluin-Nelemans HC, Van Krieken HJM, et al: Evolution of acquired severe aplastic anaemia
to myelodysplasia and subsequent leukaemia in adults. Br J Haematol 70:55, 1988.
214.
Tichelli A, Gratwohl A, Wuürsch A, et al: Late haematological complications in severe aplastic anaemia. Br J
Haematol 69:413, 1988.
215.
Moore MAS, Castro-Malaspina H: Immunosuppression in aplastic anemia—postponing the inevitable? N Engl
J Med 314:1358, 1991.
216.
Schrezenmeier H, Marin P, Raghavachar A, et al: Relapse of aplastic anaemia after immunosuppressive
treatment: a report from the European Bone Marrow Transplantation Group SAA Working Party. Br J
Haematol 85:371, 1993.
217.
Tichelli A, Gratwohl A, Nissen C, Speck B: Late clonal complications in severe aplastic anemia. Leuk
Lymphoma 12:167, 1994.
218.
De Planque MM, Bacigalupo A, Wuürsch A, et al: Long-term follow-up of severe aplastic anaemia patients
treated with antithymocyte globulin. Br J Haematol 73:121, 1989.
219.
Socieé G, Henry-Amar M, Bacigalupo A, et al: Malignant tumors occurring after treatment of aplastic anemia.
N Engl J Med 319:1152, 1993.

220.
Tichelli A, Gratwohl A, Nissen C, et al: Morphology in patients with severe aplastic anemia treated with
antilymphocyte globulin. Blood 80:337, 1992.
221.
Brodsky RA, Sensenbrenner LL, Jones RJ: Complete remission in severe aplastic anemia after high-dose
cyclophosphamide without bone marrow transplantation. Blood 87:491, 1996.
222.
Shahidi NT: Androgens and erythropoiesis. N Engl J Med 289:72, 1973.
223.
French Cooperative Group for the Study of Aplastic and Refractory Anemias: Androgen therapy in aplastic
anemia: a comparative study of high and low doses of 4 different androgens. Scand J Haematol 36:346, 1986.
224.
Najean Y, Haguenauer O (for the Cooperative Group for the Study of Aplastic and Refractory Anemias):
Long-term (5–20 years) evolution of non-grafted aplastic anemias. Blood 76:2222, 1990.
225.
Seewald TR, Zeigler ZR, Gardner FH: Successful treatment of severe refractory aplastic anemia with 3-b
etiocholanolone and nandrolone decanoate. Am J Hematol 31:216, 1989.
226.
Antin JH, Smith BR, Holmes W, Rosenthal DS: Phase I/II study of recombinant human granulocyte-
macrophage colony-stimulating factor in aplastic anemia and myelodysplastic syndrome. Blood 72:705, 1988.
227.
Vadhan-Raj S, Buescher S, Broxmeyer HE, et al: Stimulation of myelopoiesis in patients with aplastic anemia
by recombinant human granulocyte-macrophage colony-stimulating factor. N Engl J Med 319:1628, 1988.
228.
Guinan EC, Sieff CA, Oette DH, Nathan DG: A phase I/II trial of recombinant granulocyte-macrophage
colony-stimulating factor for children with aplastic anemia. Blood 76:1077, 1990.
229.
Sonoda Y, Ohno Y, Fujii H, et al: Multilineage response in aplastic anemia patients following long-term

administration of filgrastim (recombinant human granulocyte colony stimulating factor). Stem Cells 11:543,
1993.
230.
Bessho M, Jinnai I, Hirashima K, et al: Trilineage recovery by combination therapy with recombinant human
granulocyte colony-stimulating factor and erythropoietin in patients with aplastic anemia and refractory
anemia. Stem Cells 12:604, 1994.
231.
Imamura M, Kobayashi M, Kobayashi S, et al: Combination therapy with recombinant human granulocyte
colony-stimulating factor and erythropoietin in aplastic anemia. Am J Hematol 48:29, 1995.
232.
Kojima S: Use of hematopoietic growth factors for treatment of aplastic anemia. Bone Marrow Transplant
18(suppl 3):S36, 1996.
233.
Ganser A, Lindemann A, Siepelt G, et al: Effects of recombinant human interleukin-3 in aplastic anemia.
Blood 76:1287, 1990.
234.
Walsh CE, Liu JM, Anderson SM, et al: A trial of recombinant human interleukin-1 in patients with severe
refractory aplastic anaemia. Br J Haematol 80:106, 1992.
235.
Kapoor N, Hvizdala E, Good RA: High-dose intravenous gamma globulin as an approach to treatment of
antibody mediated pancytopenia. Br J Haematol 59:98, 1988.
236.
Sadowitz PD, Dubowy RL: Intravenous immunoglobulin in the treatment of aplastic anemia. Am J Pediatr
Hematol Oncol 12:198, 1990.
237.
Bodenstein H: Successful treatment of aplastic anemia with high-dose immunoglobulin (letter). N Engl J Med
314:1368, 1991.

238.
Ito T, Haraiwa M, Ishikawa Y, et al: Lymphocytapheresis in a patient with severe aplastic anaemia. Acta
Haematol 80:167, 1988.
239.
Morales-Polanco MR, Sanchez-Valle E, Guerrero-Rivera S, Gutierrez-Alamillo L, Delgado-Marquez B:
Treatment results of 23 cases of severe aplastic anemia with lymphocytapheresis. Arch Med Res 28:85, 1997.
240.
Goémez-Almaguer D, Marfil-Rivera J, Kudish-Wersh A: Acyclovir in the treatment of aplastic anemia. Am J
Hematol 29:172, 1988.
241.
Speck B, Tichelli A, Widmer E, et al: Splenectomy as an adjuvant measure in the treatment of severe aplastic
anaemia. Br J Haematol 92:818, 1996.
242.
Nissen C, Gratwohl A, Speck B: Management of aplastic anaemia. Eur J Haematol 46:193, 1991.
243.
Beutler E: Platelet transfusions: the 20,000/µl trigger. Blood 81:1411, 1993.
244.
Zeigler ZR: Effects of epsilon aminocaproic acid on primary haemostasis. Haemostasis 21:313, 1991.
245.
Sniecinski I, O’Donnell MR, Nowicki B, Hill LR: Prevention of refractoriness and HLA-alloimmunization
using filtered blood products. Blood 71:1402, 1988.
246.
Zeigler ZR, Shadduck RK, Rosenfeld CS, et al: High-dose intravenous gamma globulin improves responses to
single-donor platelets in patients refractory to platelet transfusion. Blood 70:1433, 1987.
247.
Zeigler ZR, Shadduck RK, Rosenfeld CS, et al: Intravenous gamma globulin decreases platelet associated IgG
and improves transfusion responses in platelet refractory states. Am J Hematol 38:15, 1991.

248.
Christie DJ, Howe RB, Lennon SS, Sauro SC: Treatment of refractoriness to platelet transfusion by protein A
column therapy. Transfusion 33:234, 1993.
249.
Wolfe LC, Olivieri NF, Sallan D, et al: Prevention of cardiac disease by subcutaneous deferoxamine in patients
with thalassemia major. N Engl J Med 312:1600, 1985.
250.
Caspar CB, Seger RA, Burger J, Gmuür J: Effective stimulation of donors for granulocyte transfusions with
recombinant methionyl granulocyte colony-stimulating factor. Blood 81:2866, 1993.
251.
Lewis SM: Course and prognosis in aplastic anemia. Br Med J 1:1027, 1965.
252.
Lynch RE, Williams DM, Reading JC, Cartwright GE: The prognosis in aplastic anemia. Blood 45:517, 1975.
253.
Najean Y, Pecking A: Prognostic factors in acquired aplastic anemia. A study of 352 cases. Am J Med 67:564,
1979.
254.
Bacigalupo A: Aetiology of severe aplastic anaemia and outcome after allogeneic bone marrow transplantation
or immunosuppression therapy. Eur J Haematol 57(suppl):16, 1996.
255.
Kiem HP, McDonald GB, Myerson D, et al: Marrow transplantation for hepatitis-associated aplastic anemia: a
follow-up of long-term survivors. Biol Blood Marrow Transplant 2:93, 1996.
256.
Best WR: Drug-associated blood dyscrasias. JAMA 185:286, 1963.
257.
The International Agranulocytosis and Aplastic Anemia Study: Risks of agranulocytosis and aplastic anemia: a
first report of their relation to drug use with special reference to analgesics. JAMA 256:1749, 1986.

258.
Retsagi G, Kelly JP, Kaufman DW: Risk of agranulocytosis and aplastic anaemia in relation to use of
antithyroid drugs: International Agranulocytosis and Aplastic Anaemia Study. Br Med J 297:262, 1988.
259.
International Agranulocytosis and Aplastic Anemia Study: Anti-infective drug use in relation to the risk of
agranulocytosis and aplastic anemia. Arch Intern Med 149:1036, 1989.
260.
Kelly JP, Kaufman DW, Shapiro S: Risks of agranulocytosis and aplastic anemia in relation to the use of
cardiovascular drugs: the International Agranulocytosis and Aplastic Anemia Study. Clin Pharmacol Ther
49:330, 1991.
261.
Kaufmann DW, Kelly JP, Jurgelon JM, et al: Drugs in the aetiology of agranulocytosis and aplastic anaemia.
Eur J Haematolo 57(suppl):23, 1996.
262.
Bithell TC, Wintrobe MM: Drug-induced aplastic anemia. Semin Hematol 4:194, 1967.
263.
Williams DM, Lynch RE, Cartwright GE: Drug-induced aplastic anemia. Semin Hematol 10:195, 1973.
264.
Heimpel H, Heit W: Drug-induced aplastic anaemia. Clin Haematol 9:641, 1980.
265.
Williams DM: Pancytopenia, aplastic anemia and pure red cell aplasia, in Wintrobe’s Clinical Hematology,
10th ed, edited by GR Lee, J Foerster, J Lukens, et al, pp 1452–1459. Williams & Wilkins, Baltimore, 1999.
266.
Adamson JW, Erslev AJ: Aplastic anemia, in Hematology, 4th ed, edited by WJ Williams, E Beutler, AJ
Erslev, MA Lichtman, pp 158–174. McGraw-Hill, New York, 1990.
267.
Fernandez deSevilla T, Alegre J, Ocana I, Pahissa A: Aplastic anemia secondary to ticlopidine. Med Clin
(Barc) 90:308, 1988.

268.
Mataix R, Ojeda E, Carmen-Perez M, Jimenez S: Ticlopidine and severe aplastic anaemia. Br J Haematol
80:125, 1992.
269.
Garnier G, Taillan B, Pesce A, et al: Ticlopidine and severe aplastic anaemia. Br J Haematol 81:459, 1992.
270.
Doney K, Storb R, Buckner CD, Thomas ED: Treatment of gold-induced aplastic anaemia with
immunosuppressive therapy. Br J Haematol 68:469, 1988.
271.
Yan A, Davis P: Gold induced marrow suppression: a review of 10 cases. J Rheumatol 17:47, 1990.
272.
Biswas N, Ahn WH, Goldman JM, Schwartz JM: Aplastic anemia associated with antithyroid drugs (case
report). Am J Med Sci 301:190, 1991.
273.
Keisu M, Wiholm BE, Ost A, Mortimer O: Acetazolamide-associated aplastic anaemia. J Intern Med 228:627,
1990.
274.
Mangan KF, Zidar B, Shadduck RK, et al: Interferon-induced aplasia: evidence for T-cell mediated
suppression of hematopoiesis and recovery after treatment with horse anti-human thymocyte globulin. Am J
Hematol 19:401, 1985.
275.
Harousseau JL, Milpied N, Bourhis JS, et al: Lethal aplasia after treatment with alpha-interferon of recurrent
chronic myeloid leukemia following allogeneic bone marrow graft (letter). Presse Med 17:80, 1988.
276.
Kaufman DW, Kelly JP, Anderson T, Harmon DC, Shapiro S: Evaluation of case reports of aplastic anemia
among patients treated with felbamate. Epilepsia 38:1265, 1997.

277.
Guardiola Ph, Pasquini R, Dokal I, et al: Outcome of 69 allogeneic stem cell transplantations for Fanconi
anemia using HLA-matched unrelated donors. Blood 95:422, 2000.
278.
Dror Y, Freedman MH: Schwachman-Diamond Syndrome. Blood 94:3048, 1999.
Books@Ovid
Copyright © 2001 McGraw-Hill
Ernest Beutler, Marshall A. Lichtman, Barry S. Coller, Thomas J. Kipps, and Uri Seligsohn
Williams Hematology