Analysis of Plant Genomes Using Flow Cytometry
-
Upload
international-institute-of-tropical-agriculture -
Category
Technology
-
view
1.750 -
download
4
description
Transcript of Analysis of Plant Genomes Using Flow Cytometry

Laboratory of Molecular Cytogenetics and Cytometry,Institute of Experimental Botany, Olomouc, Czech Republic
Analysis of Plant Genomes UsingFlow Cytometry
Jaroslav Doležel

IEB, Olomouc
Czech Republic
Our location
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

Our weather


Our research
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
• Analysis of plant genome structure and
evolution
- Constitution and evolution of hybrid genomes of
Festuca x Lolium hybrids (Festuloliums)
- Development and application of Chromosome
Genomics to analyze complex genomes of
wheat, barley and rye
- Evolution of Musa genome at chromosomal and
molecular level

http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
New facility for plant genomics

Musa Genome Resources Centre
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

Principles of flow cytometry
Application in plants
Flow cytometry of plant genomes
Analysis and sorting cell nuclei
Outline
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

DetectorExcitation
light source
Flow cytometry involves the
analysis of fluorescence and light
scatter properties of particles in
flow, moving with respect to the
point of measurement
The sample for flow cytometry
should be a suspension of single
particles (no clumps allowed)
How does a flow cytometry work?
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

1934: a proposal for a „flow
cytometer“ (Moldavan)
1947: the first working flow
cytometer (Gucker)
1953: hydrodynamic focusing
(Crossland-Taylor)
1965: fluidic switch sorter
(Kamentsky)
1965: electrostatic cell sorter
(Fulwyler)
1969: fluorescence measurement
Hydrodynamic
focusing
zone
Sample Sheath fluid
Light beam
Hydrodynamic focusing
A brief history
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

Sheath Fluid Sample
Laser
Fluorescencedetectors
BeamSplitter
Drop-Charging Signal
Filter
Filter
Collecting Lens forFluorescent Light
LightDetector
Collecting Lens forForward-ScatteredLight
Vibration Transducer
Flow Chamber
Focusing Lens
Positively ChargedDeflection Plate
Negatively ChargedDeflection Plate
Waste
Right CollectorLeft Collector
ObscurationBar
ElectronicsConsole
Flow cytometer and sorter
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

BD FACSVantage (two lasers, 8 parameters) Close-up
And the real thing …
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

Measurements of:
– Light scatter
• Particle size
• Surface, internal cell structure
– Fluorescence detection: in multiple wavelength bands
• Total intensity (integral)
• Maximum intensity
• Polarization
• Lifetime
– With labeling reagents, provides information about:
• Amount of: DNA, RNA, protein, surface molecules,…
• Environment within a cell or membrane
What can a flow cytometry do?
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

• System pressure: up to 100
psi
• Drop drive: up to 200 kHz
• Sort rate: up to 70,000 cells /
sec
Sorting rare cells
(hematopoietic stem cells,
fetal cells, circulating
dendritic cells)
Large-scale sorting
(chromosome purification,
separation of X- and Y-
chromosome bearing sperm)
Some cytometers are very sophisticated
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
High-speed flow cytometer and sorter (MoFlo, Cytomation)

OptoFlow MICROCYTE Partec CYFLOW
And some specialized and compact
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

Analysis and
sorting of:
• Microspores
• Protoplasts
• Cell nuclei
• Chromosomes
• Chloroplasts
Flow cytometry in plants
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

Applications:
• Relative DNA content
• DNA content in absolute units (genome size)
• Nuclear DNA base content (AT/GC ratio)
• Gene expression (nuclear-targeted GFP)
• Nuclei purification (proteins, DNA, RNA)
Flow cytometry of plant cell nuclei
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

Estimation of DNA content – the stone age
DNS-Bestimmung an Keimwurzeln von Vicia faba L.
mit Hilfe der Impulscytophotometrie
Friedrich Otto Heller
Institut für Landwirtschaftliche Botanik der Universität Bonn
Vorgetragen auf der Botaniker-Tagung in Hannover an
21. September 1972
Ber. Deutsch. Bot. Ges. 86:437-441, 1973
• Suspension of intact nuclei prepared by lysis of protoplasts
obtained after enzymatic digestion of root tips
• DNA stained with ethidium bromide
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

Breakthrough in 1983
Rapid Flow Cytometric Analysis of the Cell Cycle in Intact
Plant Tissues
David W. Galbraith, Kristi R. Harkins,
Joyce M. Maddox, Nicola M. Ayres,
Dharam P. Sharma, Ebrahim Firoozabady
Science 220: 1049-1051, 1983
• Suspensions of intact nuclei prepared by chopping small amounts
of fresh plant tissues with a sharp razor blade
• Nuclei stained in the crude homogenate with mithramycin
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

Flow cytometric analysis of
relative fluorescence intensity of
nuclei in suspension
Peak representing
G1 nuclei with 2C
DNA content
Peak representing
G2 nuclei with 4C
DNA content
Relative DNA content
Nuclei isolation
buffer + DNA
stain
20 mg of fresh
leaf tissue
Isolation of nuclei
by chopping
Removal of large
debris by filtration
Estimation of relative nuclear DNA content
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html Galbraith et al., Science 220: 1049, 1983

Fluorochrome Primary Binding Mode Wavelength (nm)*
Excitation Emission
Ethidium bromide** Intercalation 530 605
Propidium iodide** Intercalation 540 615
Hoechst 33258 AT-binding 365 465
Hoechst 33342 AT-binding 360 460
DAPI AT-binding 365 450
DIPI AT-binding 365 450
Chromomycin A3 GC-binding 445 570
Mihtramycin GC-binding 445 575
Olivomycin GC-binding 440 560
* Dye-DNA complex
**Binds also to double stranded RNA!
Fluorescent dyes for DNA
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

G1
S
G2M
DNA content
DNA content and cell cycle
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
2C 4C
DNA content
2C
4C
2C -
4C
4C

The distribution as it is actually
measured, broadened
because of imperfections in
the staining and measurement
procedure.
G1 (2C)
G2 (4C)
S
Nuclear DNA content
Num
ber
of
nucle
i
Nuclear DNA contentN
um
ber
of
nucle
i
Analysis of nuclear DNA content
Distribution of nuclear DNA content in a population of
asynchronously growing cells:
Ideal distribution as it would
be measured in a perfect
system.
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

5 μm
Metaphase spreads
are difficult to prepare
Chromosomes are
very small
An easy method for ploidy screening?
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

0 50 100 150 200 250
Known ploidy
(diploid, 2x)2C
0
100
200
300
400
500
4C0
100
200
300
400
500
0 50 100 150 200 250
Tetraploid (4x)
4C
8C0
100
200
300
400
500
0 50 100 150 200 250
3C
6C
Triploid (3x)
Relative nuclear DNA content (channel number)
Nu
mb
er
of n
ucle
i
Advantages:
Convenient and rapid (>100 samples per working day)
Does not require dividing (mitotic) cells
Non-destructive (only milligram amounts of plant
tissues are needed)
Ploidy screening in Musa using flow cytometry
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html Doležel et al., Biol. Plant. 36: 351, 1994

http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
An integrated system for
production of polyploids has
been developed and applied
in banana and cassava
The protocol combines in
vitro induction of polyploidy
and ploidy screening using
flow cytometry
The advantage of the
protocol is the production of
solid (non-mixoploid)
polyploids
Ploidy manipulations
Van Duren et al., Euphytica 88: 25, 1996

0
100
200
300
400
500
0 50 100 150 200 250
Mixoploid (2x + 4x)
2C 4C
0
100
200
300
400
500
0 50 100 150 200 250
Mixoploid (4x + >5x)
4C
>5C
0 50 100 150 200 250
Standard (2x)
2C
0
100
200
300
400
500
4C
Relative nuclear DNA content (channel number)
Nu
mb
er
of n
ucle
i
Plant body consists of three histological layers (L1, L2 and L3),
which may differ in ploidy (= chimerism, mixoploidy)
Chromosome counting in roots (only L3 layer) cannot be used for
reliable identification of mixoploid individuals
Flow cytometry allows rapid and reliable detection of mixoploidy
Identification of mixoploids
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

Shoot-tips treated
with colchicine
Mixoploid
selected
shoot
M1V1 M1V2 M1V3 M1V4
3x + 6x
3x + 6x
3x + 6x
3x + 6x
3x + 6x
3x + 6x
3x + 6x
3x
3x
3x
3x
3x
6x
6x
6x
M1V0
A1B
A
A1
A1A
A2B
2A
2B
1A
2A
2A
1B
2
A2A
1
A1B
1
A1A
2
A1A
1
A2
A2A
A2B
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
Dissociation of mixoploids
Roux et al., PCTOC 66: 189, 2001

0
100
200
300
400
500
0
100
200
300
400
0 100 200 300 400 0 100 200 300 400 500
Relative nuclear DNA content
Nu
mb
er
of
nu
cle
i
A B
C D
PisangMas
PisangMas
PisangMas
PisangMas
Kluai Tiparot
Pisang Jambe
Balonkawe
(Kluai) Ngoen
3x, NOT 4x 3x, NOT 4x
3x, NOT 4x 4x, NOT 3x
Germplasm characterization
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html Horry et al., Infomusa 7: 5, 1998

ITC, KU Leuven
Characterization of Musa germplasm (ploidy)
Mixoploidy
(0.79%)
Other ploidy
(7.65%)
Determined for
the first time
(7.04%)
Confirmed
(83.3%)
Mixed ploidy
(1.22%)
Flow cytometry was used to verify the classification of Musa germplasm
held at the INIBAP Transit Centre (KU Leuven, Belgium)
Ploidy analysis of 1150 out of 1175 accessions
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html Doleželová et al., Infomusa 14: 34, 2005

4x
2x
3x
Empetrum
2x + 3x + 4x
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
Population biology
Distribution of Empetrum cytotypes in the Giant Mountains
(Czech Republic)
Ploidy screening of large populations (cytotype distribution,
hybrid zones, …)

F1 hybrids may be conveniently detected based on
intermediate DNA content
XCR
BC
G1
G2
L. multiflorum (2n = 14)
CR
BC G1
G2
F1 hybrid C
RB
C
G1
F. arundinacea (2n = 42)
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
Identification of hybrids

THE USE OF EUPLOID PLANT OF
THE SAME SPECIES AS AN INTERNALSTANDARD
Discrimination is possible when the coefficient of variation of G1 peaks islower than half of the difference in DNA content (2.4% in this example)
THE USE OF A DIFFERENT SPECIESAS AN INTERNAL STANDARD
Relative difference in DNA content (D):
G1 Peak Ratio 1
G1 Peak Ratio 2D = * 100 [%]
0 100 200 300 400 5000
30
60
90
120
0 100 200 300 400
SE S A
G1 Peak Ratio 1 G1 Peak Ratio 2
RELATIVE NUCLEAR DNA CONTENT
0 100 200 300 400 5000
30
60
90
120
0 100 200 300 40 0
CV = 1.2% CV = 1.0%
E EA A
RELATIVE NUCLEAR DNA CONTENT
- G1 Peak Ratio 1
E = euploid, A = aneuploid, S = standard
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
Aneuploidy

0
50
100
150
200
250
300
1 21 41 61 81 101 121 141 161 181 201 221 241
Relative DNA content
Nu
mb
er
of
nu
cle
i
Peak DI CV%
3x 0.79 1.58
CRBC 1.00 1.74
CRBC3x
0
50
100
150
200
250
300
1 21 41 61 81 101 121 141 161 181 201 221 241
Relative DNA content
Nu
mb
er
of
nu
cle
i
Peak DI CV%
3x-1 0.76 0.99
CRBC 1.00 1.25
CRBC
3x-1
0
50
100
150
200
250
300
1 21 41 61 81 101 121 141 161 181 201 221 241
Relative DNA content
Nu
mb
er
of
nu
cle
i
Peak DI CV%
3x-2 0.74 1.02
CRBC 1.00 1.26
CRBC3x-2
Triploid (2n = 3x = 33)
2n = 33 - 1
2n = 33 - 2
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
Aneuploidy in Musa
Roux et al., Plant Cell Rep. 21: 483, 2003

a: antipodals; c: central cell with two polar nuclei; e: egg apparatus with egg cell
and two synergids.
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
Reproduction mode (FCSS)
C-values of unreplicated embryo and endosperm nuclei depend on
whether the female and/or male gametes were reduced or unreduced,
and whether the embryo and/or endosperm developed autonomously
or after fertilization:
Matzk et al., Plant J. 21: 97, 2000

Distribution of DNA content of nuclei isolated from field bean meristem
root tip cells. A non-parametric curve-fitting method was used for
histogram deconvolution for cell cycle phases.
Relative DNA content
G1 = 47.4%
S = 30.5%
G2 = 22.1%
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
Cell cycle
G1
G2
S

Flow cytometry allows to analyse the
degree of endopolyploiy and the
frequency of endopolyploid cells
G2
M
G1
S
ER
4C
8C
2C
S S G2G2 G1G1
ENDOREDUPLICATION
00
200
400
600
800
1000
50 100 150 200 250
2C
4C8C
16C
32C
Mammillaria san angelensis
(parenchym)
Nuclear DNA content
Nu
mbe
r of
nu
cle
i
Endoreduplication (polysomaty)
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html Palomino et al., Plant Sci. 19: 191, 1999

Applications:
• Relative DNA content
• DNA content in absolute units (genome size)
• Nuclear DNA base content (AT/GC ratio)
Flow cytometry of plant cell nuclei
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

Nuclear genome
size (Mb)Musa acuminata (591 - 615)Musa balbisiana (534 - 540)
65
1200
4400
7800
16400
120000
2700
H. sapiens
The size of nuclear genome
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

The method
Ratio of G1 peak positions Glycine
to Musa is 1.984 => 2C nuclear
DNA content of M. acuminata
errans is 2.50 / 1.984 = 1.26 pg
DNA (or 608 Mbp / 1C*)
SAMPLE
20 mg of Musa leaf
tissue
STANDARD
5 mg of G. max
(2C = 2.50 pg DNA)
leaf tissue
Simultaneous
isolation and
staining of
nuclei
Nuclei isolation
buffer + propidium
iodide
Relative nuclear DNA content
Num
ber
of
nucle
i
Musa G1
nuclei
Glycine G1
nuclei
Removal of large
debris by filtration
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
*) 1pg DNA = 0.978 Mbp
Doležel et al., Biol. Plant. 36: 351, 1994

http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
Germplasm characterization for genome size
Accession name Section Genome size
(Mbp/1C)*
Calcutta 4 Eumusa 627
Galeo 626
Pisang Mas 635
M. acuminata ssp. banksii 646
Guyod 647
M. balbisiana type Cameroun 578
Honduras 579
M. schizocarpa 704
M. laterita Rhodochlamys 624
M. velutina 635
M. mannii 649
Kluai Bou 609
M. ornata 664
M. beccarii Callimusa 798
M. peekelii ssp. peekelii Australimusa 791
M. textilis 734
M. maclay type Hung Si 755
Kawaputa 766
Ensete gilletii Related genus 619
Musa genomes
differ by size:
- A ~ 630 Mbp
- B ~ 580 Mbp
- S ~ 700 Mbp
- T ~ 730 Mbp
- C ~ 790 Mbp
*) 1pg DNA = 0.978 Mbp
Bartoš et al., Cytogenet.
Genome Res. 109: 50, 2005

0 50 100 150 200 2500 50 100 150 2000 50 100 150 200
Propidium iodide
maizeleukocytes
leukocytes
DAPI Mithramycin
FR = 0.817 FR = 0.601 FR = 1.083
Relative nuclear DNA content (channel number)
0
200
400
600
800
1000
Num
ber
of
nucle
i
leu
ko
cyte
s
maiz
e
maiz
e
Histograms of relative nuclear DNA content of maize and human
leukocytes obtained using fluorescent dyes with different DNA base
preferences (FR = ratio of DNA peaks - maize / leukocytes)
Due to different AT/GC ratio of human and maize, peak ratios are different
for each DNA fluorochromehttp://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
DNA base content
Doležel et al., Physiol. Plant. 85: 625, 1992

Acknowledgements
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
David Galbraith (Tucson)
Jan Suda (Prague)
Fritz Matzk (Gatersleben)
Nicolas Roux (Montpellier)
Pietro Pifanelli (Genoa)
Rony Swennen (Leuven)
Jean-Pierre Horry (Montpellier)

The first
book
on plant
flow
cytometry


• Musa balbisiana
cv. Pisang Klutuk Wulung
• Genome size: 530 Mbp
• Scientific interest: BSV,
B genome structure
NucleiIntact cells Cellular debris
Nuclei isolation
Nuclei sorting
Relative DNA content
G1 nuclei
G2 nucleiDebris
Sort window
Purification of cell nuclei
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

The use of flow-sorted nuclei avoids problems with secondary
metabolites and eliminates cytoplasmic DNA contamination. Isolated
DNA of high quality and ideal for cloning.
Library screening with a cp probe
0.09%8.27%
Standard procedure Flow sorting
BAC library from
Musa balbisiana PKW
- Number of clones: 36,864
- Average insert size: 135 kb
- Genome equivalents: 9x
- Clones with cp DNA: 3%
- Clones with mt DNA: 0.004%
Purification of cell nuclei
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html

Complex interactions of many genes
Gene expression patterns specific for particular tissues
RNA isolation from heterogeneous organs:
- Difficult interpretation
Solution
- Isolation of particular cell types
• Microdissection
• Cell sorting using flow cytometry
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
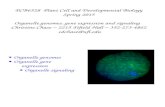
Gene expression

(A) Wild-type plant
(B) - (F) Transgenic
plants expressing
nuclear GFP
regulated by:
(B) p35S
(C) pRPL16B
(D) pSHR
(E) pSCR
(F) pSultr2-1
Simultaneous analysis of GFP (FL1) a DAPI (FL4)
http://www.ueb.cas.cz/Olomouc1/LMCC/lmcc.html
Gene expression in root of Arabidopsis thaliana