Adv in Cementum Devt[1]
-
Upload
johanna-cristancho-riveros -
Category
Documents
-
view
43 -
download
2
Transcript of Adv in Cementum Devt[1]
![Page 1: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/1.jpg)
3 ____________________________________________________________________________
Advances in Defining Regulators of CementumDevelopment and Periodontal Regeneration
Brian L. Foster,* Tracy E. Popowics,{ Hanson K. Fong,{ andMartha J. Somerman*,{
*Department of Periodontics, School of Dentistry
University of Washington, Seattle, Washington 98195{Department of Oral Biology, School of Dentistry
University of Washington, Seattle, Washington 98195{Department of Materials Science and Engineering
University of Washington, Seattle, Washington 98195
I. I
Curre
Copy
ntroduction
II. Q
uestion 1. What Are the Unknowns That Must Be Considered in Order to Replicate theEnamel (Crown) and How Do the Proteins Involved in Crown Development Relate to
Root Development?
A
. Ent T
right
namel Structure
B
. E namel Biomineralization: Role of ProteinsC
. F uture Prospects for Enamel RegenerationIII. Q
uestion 2. What Do We Know About the Cells Required for Periodontal Developmentand Regeneration?
A
. D evelopmental CellsB
. D erivation of Cementum: Competing Theories of Cementoblast OriginC
. D iVerences Between Cementoblasts and OsteoblastsD
. T ooth Stem Cell PopulationsIV. Q
uestion 3. What Genes and Associated Proteins Are Important for Root/PeriodontalTissue Formation?
A
. F actors Associated with the Putative Epithelial Niche (HERS and ERM)and Surrounding Mesenchyme
B
. B one Morphogenetic ProteinsC
. P eriostin and Nuclear Factor I‐C/CAAT Box Transcription FactorD
. R egulators of Phosphate and Pyrophosphate MetabolismE
. F actors Known to Regulate Osteoprogenitor Cells and OsteoblastsF
. E merging and Other Factors to ConsiderV. C
onclusions and Future DirectionsA
cknowledgmentsR
eferencesSubstantial advancements havebeenmade in defining the cells andmolecular
signals that guide tooth crown morphogenesis and development. As a result,
very encouragingprogress has beenmade in regenerating crown tissuesbyusing
opics in Developmental Biology, Vol. 78 0070-2153/07 $35.002007, Elsevier Inc. All rights reserved. 47 DOI: 10.1016/S0070-2153(06)78003-6
![Page 2: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/2.jpg)
48 Foster et al.
dental stem cells and recombining epithelial andmesenchymal tissues of specific
developmental ages.Todate, attempts to regenerate a complete tooth, including
the critical periodontal tissues of the tooth root, have not been successful. This
may be in part due to a lesser degree of understanding of the events leading to
the initiation and development of root and periodontal tissues. Controversies
still exist regarding the formation of periodontal tissues, including the origins
and contributions of cells, the cues that direct root development, and the
potential of these factors to direct regeneration of periodontal tissueswhen they
are lost to disease.
In recent years, great strides have been made in beginning to identify and
characterize factors contributing to formation of the root and surrounding
tissues, that is, cementum, periodontal ligament, and alveolar bone. This
review focuses on the most exciting and important developments over the
last 5 years toward defining the regulators of tooth root and periodontal
tissue development, with special focus on cementogenesis and the potential
for applying this knowledge toward developing regenerative therapies. Cells,
genes, and proteins regulating root development are reviewed in a question‐answer format in order to highlight areas of progress as well as areas of
remaining uncertainty that warrant further study. � 2007, Elsevier Inc.
I. Introduction
During the last decade, we have gained substantial insights into the mechanisms
and factors controlling formation of many organs and tissues and with this, new
ideas on how to regenerate tissues lost as a consequence of pathologies, injuries,
and genetic disorders. These insights, based on new technologies and on the
exponential growth in defining the factors/genes/proteins regulating tissue and
organ development, have allowed us to enjoy more rapid discoveries than in the
past. Technological advances have resulted in increased eVorts to develop im-
provements in existing therapies targeted at replacement of lost tissues/organs/
body parts. An area of focus has been the oral cavity, with improvements seen in
(1) materials used to restore decayed, damaged tooth structure; (2) prosthetic
devices to replace missing teeth—full dentures, partial dentures, bridges, and
implants; and (3) materials/agents used to regenerate periodontal tissues—for
example, root cementum, periodontal ligament attachment, and alveolar bone.
In fact, with regard to developing clinical products that promote and/or protect
against bone loss, some of the newer products (on the market within the last
3 years) were first appreciated for their role in regulating key events during
formation and diVerentiation of hard tissues, including teeth. These include
bone morphogenetic protein [(rhBMP‐2): INFUSE; Medtronic Sofamor
Danek, Minneapolis, MN)] approved for clinical use in open fracture of long
bones, nonunions and vertebral arthrodesis, and parathyroid hormone (Fortio;
![Page 3: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/3.jpg)
3. Regeneration of the Periodontium 49
teriparatide (rhDNA origin) injection contains human PTH (1‐34), Eli Lilly &
Co.) given intermittently to promote bone formation in individuals with severe
osteoporosis and in whom antiresorptive therapies have proven insuYcient.
To date, attempts to regenerate a complete tooth—crown, root, PDL,
bone—have not been successful; however, progress has been made in regen-
erating crown tissues. Because of the parallel of epithelial‐mesenchymal
(E‐M) signaling in crown formation, that is, ameloblasts‐enamel; odontoblasts‐dentin, withE‐Msignaling in other tissues during development, rodentmodels of
tooth crown development have been studied extensively, resulting in a wealth of
information as to the cells/factors and events controlling crown development
(Chai and Slavkin, 2003; Fong et al., 2005; ThesleV, 2003; ThesleV andMikkola,
2002; Zhang et al., 2005). Yet, there is still more information needed in order to
mimic enamel‐dental formation as a way to restore lost tissue structure. What is
known and was first realized decades ago is that appropriately timed mixing of
cells obtained from tooth epithelium with tooth mesenchyme simulates cell
diVerentiation toward ameloblasts and odontoblasts with subsequent crown
formation (the development of tooth germs in tissue culture, 1965; Duailibi
et al., 2004; Harada et al., 1999; Huggins et al., 1934; Kollar and Baird, 1969,
1970a,b; Kollar and Fisher, 1980;Mina and Kollar, 1987; Nieminen et al., 1998;
Ohazama et al., 2004b; Tucker and Sharpe, 2004; Young et al., 2002).
In contrast, the events/factors leading to formation of the root and surrounding
tissues, that is, cementum, alveolar bone and a functional periodontal ligament
(PDL) are just beginning to unfold.
This review focuses on the most exciting developments over the last 5 years
toward defining the regulators of root development, that is, cementogenesis
and the significance of this knowledge toward developing therapies targeted
at regeneration of a whole tooth and surrounding support structures.
We recognize that modulators of PDL and bone formation are key for root
formation and thus at times address these tissues, but the emphasis for this
review is on cementum. Further, based on the recent emphasis on defining the
possible role of epithelial‐derived factors during root development, a discussion
on the enamel‐associated factors produced by ameloblasts, and their known
and putative roles in formation of enamel and cementum, are discussed.
As the knowledge of factors that influence development of the period-
ontium increases in coming years, developing an eVective platform for the
delivery of these known factors will become increasingly important for pur-
poses of tissue regeneration. In the areas of drug delivery and tissue engi-
neering, advances have been made in the development of materials that can
serve as a vehicle to deliver proteins/genes/cells in vivo. Agents identified with
regenerative potential may then be partnered with delivery systems to local-
ize and regulate release of cells and factors at sites of repair/regeneration of
lost periodontal structures. Much exciting work has been done to advance
the design and fabrication of delivery systems, and several excellent reviews
![Page 4: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/4.jpg)
Development
Diseased
Enamelbiomineralization
(#1)
Cells(#2) Genes/
proteins(#3)
Deliverysystem
Healthy
Figure 1 Progression of root development and regeneration. The tooth root develops as a
result of complex interactions of cells, signals, and matrix proteins, now just beginning to be
understood. The question‐answer format used in this review addresses recent progress in
defining key modulators of root development, and defines areas warranting further study.
Therapies targeted at regenerating the whole tooth will necessarily incorporate factors relating
to crown development and possibilities for enamel regeneration (Question 1), as well as cells
(Question 2) and genes/proteins (Question 3) that regulate periodontal development. Lastly,
cells and factors to be used in regenerative therapies should be partnered with eVective delivery
systems that serve as a scaVold for cells and/or function in controlled release of bioactive factors
to the local area.
50 Foster et al.
and primary publications may be consulted for detailed information on this
work (Abukawa et al., 2006; Bartold et al., 2006b; Jin et al., 2003; Nakahara,
2006; Taba et al., 2005).
A question‐answer format has been used to address progress in ascertain-
ing the key modulators of root development over the past 5 years, as well
as to recognize areas of remaining uncertainty that warrant further study.
A model visualizing the progression, from the stage of initiation of root
development and from a diseased periodontal state to a functional tooth is
shown in Fig. 1 to visually demonstrate the questions being posed.
1. What are the unknowns that must be considered in order to replicate
the enamel (crown) and how do the proteins involved in crown
development relate to root development?
2. What is known about the cells required for development and regeneration
of cementum?
3. What are the genes and associated proteins required for root development
and regeneration?
![Page 5: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/5.jpg)
3. Regeneration of the Periodontium 51
II. Question 1. What Are the Unknowns That Must BeConsidered in Order to Replicate the Enamel (Crown) andHow Do the Proteins Involved in Crown DevelopmentRelate to Root Development?
Enamel is the hardest biological tissue in the body. Although the primary
component of enamel, hydroxyapatite (HAP), does not compare favorably
with most known structural ceramics in terms of mechanical properties, it
exhibits remarkable durability. The key to enamel’s durability despite re-
peated attrition in the bacteria‐laden environment of the oral cavity lies in its
intricate microstructure; hence, regenerative strategies with the aim of suc-
cessful replication of the crown must involve not only the chemical makeup
of enamel, but the structural makeup as well. Current materials engineer-
ing technology has yet to find a way to fabricate the complex 3‐D enamel
structure. To complicate matters, mature enamel is a nonliving tissue, as the
ameloblasts that synthesize enamel matrix are lost on tooth eruption. A key
to enhancing progress toward regenerating enamel includes understanding
the cellular and molecular mechanisms regulating formation of this tissue.
The following discussion focuses on our current understanding of enamel
structure as it relates to mechanical functions, and on the genes/proteins
regulating enamel biomineralization. Also discussed are future approaches
to consider for designing regenerative enamel.
A. Enamel Structure
The organization of enamel can be imagined as a hierarchical structure,
starting at the smallest scale with HAP crystals approximately 50‐nm wide.
These crystals are bundled into a few micrometers wide which are referred
to as enamel rods or prisms, representing the next scale of hierarchy. The
interweaving of enamel rods builds the bulk of enamel tissue. The crystal
rod/interrod organization has been investigated carefully, and beautiful,
illustrative images can be found in textbooks such as Ten Cate’s Oral Histo-
logy (Nanci, 2003). Increasing evidence suggests that orientation and decus-
sation of enamel rods are important properties for preserving the
mechanical integrity of mature enamel (Marshall et al., 2001; Xu et al.,
1998). For example, rod organization is important for preserving the overall
enamel structural integrity by directing microcracks traveling through the
dentin‐enamel junction (DEJ) into the dentin, where they are then arrested
(Imbeni et al., 2005). Despite the extensive body of data characterizing the
enamel structure, several questions remain regarding key structural details
![Page 6: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/6.jpg)
52 Foster et al.
that must be elucidated in order to understand the development and regener-
ation potential for enamel. Two of these critical questions include: (1) What
are the directional changes in enamel rods extending from the DEJ to enamel
surface, and (2) How are interrods structurally related to enamel rods?
Answering these questions is essential to achieve an adequate understanding
of the mechanical properties of enamel, as well as to gain insight toward
regeneration of a functional crown.
B. Enamel Biomineralization: Role of Proteins
1. Amelogenin
Amelogenin is the most abundant and best characterized protein in develop-
ing enamel. The amino acid sequence of amelogenin protein is highly con-
served across many species, suggesting physiologic relevance and common
functional properties across species (Paine and Snead, 2005). Amelogenin’s
eVect on enamel development has been aptly studied in both in vivo and
in vitro systems. Several lines of evidence support proper self‐assembly of
amelogenin proteins is essential for facilitating directional nucleation
of hydroxyapatite minerals. Human phenotypes for the condition amelogen-
esis imperfecta (AI) demonstrate lack of or altered amelogenin, resulting
in inferior enamel characterized by hypoplasticity or hypomineralization,
and often associated with disorganized enamel rods (Gibson et al., 2001b,
2005; Wright et al., 2003). Likewise, a severe form of AI, similar to humans
with amelogenin defects, was observed in amelogenin knockout (KO) mice
(Gibson et al., 2001a).
The current understanding of amelogenin’s role in enamel mineralization
has come to light through characterization of enamel in situ and isolated
recombinant amelogenin, in normal and defective forms. The first indication
of amelogenin’s ability to self‐assemble and dictate mineral organization
came from investigations focused on analyzing developing mouse enamel
where arrays of nanospheres were observed to approximate the sides of
needle‐like HAP crystallites (Moradian‐Oldak et al., 1995; Robinson et al.,
1981). Later, atomic force microscopy (AFM) and dynamic light scattering
measurements on M‐180 (mouse full‐length) amelogenin revealed that the
protein assembled into 20 nm nanospheres (Moradian‐Oldak et al., 2000).
Furthermore, dynamic light scattering measurements performed on M‐180with altered C‐terminal and N‐terminal domains indicated disruption of self‐assembly, resulting in smaller nanospheres with a wider size distribution
(Moradian‐Oldak et al., 2000). These findings revealed that C‐ and
N‐terminal domains were essential for proper amelogenin self‐assembly.
![Page 7: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/7.jpg)
3. Regeneration of the Periodontium 53
When transgenic mice were developed to express the same altered C‐ or N‐terminal domains of amelogenin, similar disruption in the nanosphere self‐assembly was observed, resulting in disruption in crystal organization of the
mineral phase during the secretory stage of enamel formation (Paine et al.,
2001). The resulting mature enamel was found to be hypomineralized with
disorganized rods, a direct eVect of altered C‐ and N‐terminal domains
manifested in a disorganized mineral phase in the nucleation stage of enamel
formation (Paine et al., 2001).
Details of the interactions between amelogenin and mineral are still
emerging, however the evidence to date indicates that amelogenin has a
strong binding aYnity to HAP via the hydrophilic C‐terminal domain.
Several investigators have demonstrated controlled HAP growth in the
presence of amelogenin using in vitro systems (Beniash et al., 2005; Iijima
et al., 2002). In one example, HAP crystals with a long ribbonlike morphol-
ogy resulted from growth in the presence of amelogenin (Iijima et al., 2002).
Another solution precipitation experiment showed formation of needle‐likeHAP crystals when amelogenin was introduced into the system (Beniash
et al., 2005). In both of these examples, the long axis of the crystals was the
crystallographic [001] direction (normal to the crystallographic (001) plane),
similar to that found in physiological enamel HAP, suggesting amelogenins
bind to the crystal surface(s) perpendicular to the (001) plane, limiting the
growth direction in [001] only. Additional binding studies by Hablitz et al.
showed that when amelogenins were introduced to a composite that exposed
fluoroapatite and glass, both having hydrophilic surfaces, amelogenins
bound only to fluoroapatite (Habelitz et al., 2004). NMR studies further
revealed that the binding site was through the C‐terminal domain of amelo-
genin (Shaw et al., 2004).
2. Non‐amelogenin Proteins
Although present in minor amounts relative to amelogenin, additional
enamel matrix proteins (EMPs) identified in developing enamel have been
shown to play a role in regulation of crystal growth. These non‐amelogenin
EMPs include enamelin, ameloblastin, tuftelin, biglycan, decorin, and ame-
lotin. The specific roles of these proteins in influencing biomineralization of
enamel are not fully understood and are currently under active investigation
(Table I). In both humans and mice, an AI‐like phenotype is the result of
mutation or KO of genes associated with some of these proteins, suggesting
that they play an important role in biomineralization.
Mutations in the enamelin gene in humans and mice result in AI char-
acterized by hypoplastic enamel (Hu and Yamakoshi, 2003; Kim et al., 2005;
![Page 8: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/8.jpg)
Table I Factors Found in Developing Enamel
Factor Cells/Tissues Function/Putative Function Models References
Amelogenin Ameloblasts, HERS,
odontoblasts, periodontal
tissues (see also Table II)
Directs hydroxyapatite crystal
habit during developmental stage
of enamel formation by assembling
into extracellular protein matrix in
which mineral nucleates. Amelogenin
is also considered a potential
signaling molecule in dentin and
cementum development (Table II)
Human X‐linked amelogenesis
imperfecta (AI) (reduction
or elimination of amelogenin
expression by X‐chromosome):
partially hypomineralized enamel
Beniash et al. (2005);
Gibson et al. (2001a,b),
(2005); Iijima et al. (2002);
Lagerstrom ‐Fermer and
Landegren (1995);
Paine et al. (2001);
Wright et al. (2003)
Transgenic mice: altered A domain
(AA 1‐42) and altered B domain
(AA 157‐173) of M‐180amelogenin—hypomineralization
of enamel in both cases
Amelogenin KO mice:
hypomineralized enamel
(Table II regarding root
resorption)
In vitro mineralization: mineral
morphology control—short
needlelike or long ribbonlike
crystal shapes depending on
mineralization condition
Leucine‐rich amelogenin
peptide (LRAP)
Ameloblasts—alternative
splice product of
amelogenin
Suggested functions include:
responsible for binding to
hydroxyapatite, and implicated
as a signaling molecule in
periodontal tissue formation
Transgenic LRAP: expressed in
amelogenin null mice did not
rescue hypomineralized enamel
Boabaid et al. (2004b);
Chen et al. (2003)
LRAP overexpression in mice:
enamel pitting; in vitro, aVects
genes associated with PDL cells
and cementoblasts, and some
studies suggest proliferative
eVects
![Page 9: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/9.jpg)
Tyrosine‐rich amelogenin
peptide (TRAP)
Ameloblasts—cleavage
product of amelogenin
Byproduct of amelogenin generated
by protease; no known eVect on
enamel; Implicated as signaling
molecule in periodontal tissue
formation
TRAP overexpression in mice:
no eVect on enamel, regulates
cementoblast behavior in vitro
Paine et al. (2004);
Swanson et al. (2006)
Enamelin Ameloblasts Regulates mineralization of enamel,
but its role has not been determined
Enamelin mutation in humans:
hypoplastic enamel
Kim et al. (2005);
Masuya et al. (2005);
Rajpar et al. (2001)Enamelin mutation in mice:
hypoplastic enamel
Ameloblastin Ameloblasts, ERM
(see also Table II)
Acts as a repressor of amelogenin,
limits ameloblast proliferation,
may regulate crystal nucleation
(see also Table II)
Ameloblastin KO mice:
Amelogenesis imperfecta
Ameloblastin overexpression in
mice: partially disrupted
enamel rod structure
Fukumoto et al. (2004,
2005); Paine et al. (2003)
Tuftelin Found concentrated in
dentin‐enamel
junction (DEJ)
May contribute to amelogenesis Tuftelin overexpression in mice:
disrupted rod/interrod
morphology
Luo et al. (2004)
Amelotin Ameloblasts Unknown Not reported Iwasaki et al. (2005)
Biglycan Bone, dentin, enamel Repressor of amelogenin Biglycan KO mice: transient
eVect included increased enamel
formation, interrod as primary
enamel structure while rod
structure was minimally affected
in newborns.
Adult teeth appeared normal
Goldberg et al.
(2002, 2005)
Decorin Bone, dentin, enamel Unknown Decorin KO mice: transient
eVect included decreased
enamel formation and
disorganized rod structure in
newborns. Adult teeth
appeared normal
Goldberg et al. (2005)
(Continued )
![Page 10: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/10.jpg)
Dentin
sialophosphoprotein
(DSPP)
re‐secretoryameloblasts, DEJ
AVects DEJ morphology via
regulation of predentin formation
DSPP KO mice: irregular DEJ Sreenath et al. (2003)
Enamelysin (MMP‐20) entin, enamel Proteolytically breaks down enamel
proteins, e.g., amelogenin, in order
to facilitate mineral growth. MMP‐20is expressed in secretory and transition
stages of enamel development
MMP‐20 KO mice: disrupted rod
pattern, hypoplastic enamel
MMP‐20 mutation in mice: heavily
pigmented, hypoplastic enamel
Bartlett et al. (2004);
Bartlett et al. (2006);
Caterina et al.
(2002); Hu et al. (2002)
Kallikrein‐4 (KLK4) dontoblasts,
ameloblasts,
prostate
Proteolytically breaks down enamel
proteins, e.g., amelogenin, in order to
facilitate mineral growth. KLK4 is
expressed in transition and maturation
stages of enamel development
KLK4 mutation in human:
yellow‐brown discoloration, lower
mineral content in enamel
Hart et al. (2004);
Hu et al. (2002)
Table I Continued
Factor Cells/Tissues Function/Putative Function Models References
P
D
O
![Page 11: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/11.jpg)
3. Regeneration of the Periodontium 57
Masuya et al., 2005; Rajpar et al., 2001). However, how enamelin interacts
with HAP mineral and/or the major matrix protein, amelogenin, remains
largely unknown.
Similarly, ameloblastin, whose expression by ameloblasts decreases from
secretory stage to maturation stage of amelogenesis, has been found to aVectmineralization. Ameloblastin KO mice were reported to develop hypocalci-
fied enamel with no recognizable rod structure (Fukumoto et al., 2004,
2005). In transgenic mice overexpressing (O/E) ameloblastin, Paine et al
observed disruption of the rod/interrod structure in localized regions of
enamel (Paine et al., 2003). Studies on ameloblastin null mice have also
suggested that the protein functions as a cell adhesion molecule and regula-
tor of cell growth (Fukumoto et al., 2004, 2005), however the specific role
and mechanism for ameloblastin in mineral formation remains unclear.
Tuftelin, another enamel protein with reported self‐assembly properties,
may be an important protein in enamel mineralization, but its physiological
function has yet to bewell characterized (Deutsch et al., 1998, 2002; Paine et al.,
1996, 1998). Tuftelin O/E in mice resulted in disruption of the enamel rod/
interrod structure, and the loss of the characteristic ribbonlike enamel crystallite
morphology within rods (Luo et al., 2004).
Other nonamelogenin proteins identified with enamel formation include
biglycan, decorin, and amelotin. Biglycan and decorin are leucine‐rich pro-
teoglycans, implicated in regulation of mineralized tissues. Loss of biglycan
or decorin expression in KO mice resulted in an increase or decrease in
enamel tissue formation, respectively (Goldberg et al., 2002). In both cases,
the mineral structure was initially disrupted, but recovered with maturation
of enamel. The reader is directed to Section IV.F for further information
on the proteoglycans decorin and biglycan, and their role in regulating
mineralized tissues of the tooth.
Amelotin, discovered and reported to be an ameloblast‐specific gene, hasbeen identified in humans and mice (Iwasaki et al., 2005). Expression of
amelotin mRNA was restricted to maturation stage ameloblasts in mice.
Amelotin’s potential role in enamel development is under investigation.
3. Proteases
While amelogenins are critical in the nucleation step of enamel biominerali-
zation, degradation of amelogenins is important for providing the space
for HAP mineral crystals to expand during the growth stage of amelogen-
esis. Two proteolytic enzymes, matrix metalloproteinase‐20 (MMP‐20) andkallikrein‐4 (KLK‐4), have been identified as enzymes required for breaking
down amelogenins (Bartlett et al., 1998; Fukae et al., 1998; Simmer et al.,
1998). MMP‐20, secreted into the enamel extracellular matrix by amelo-
blasts during the secretory stage, is responsible for the proteolytic cleavage
![Page 12: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/12.jpg)
58 Foster et al.
of amelogenin protein into smaller fragments. A study by Ryu and co-
workers showed that incubation of MMP‐20 with recombinant porcine
amelogenin (rP172) produced the same cleaved amelogenin fragments that
are found in vivo (Ryu et al., 1999). KLK‐4, secreted during early maturation
stage, functions to further digest matrix proteins and cleavage products
incompletely digested by MMP‐20, facilitating nearly complete removal of
proteins from mature enamel (Simmer and Hu, 2002). The latest data using
in vitro models support the notion of diVerences in the way MMP‐20 and
KLK‐4 digest 32‐kDa enamelin. While MMP‐20 cleaved enamelin only after
it was deglycosylated, KLK‐4 readily cleaved enamelin into nine cleavage
products (Yamakoshi et al., 2006). Human phenotypes carrying mutated
MMP‐20 or KLK‐4 exhibit autosomal recessive hypomaturation AI (Hart
et al., 2004; Kim et al., 2005; Ozdemir et al., 2005). Likewise, hypoplastic
enamel with a disrupted rod pattern was reported in MMP‐20 null mice
(Caterina et al., 2002). The MMP‐20 null mice demonstrated that complete
elimination of EMPs from the enamel space failed to occur in the absence of
this proteolytic enzyme, resulting in limited space required for expansion of
the mineral phase.
C. Future Prospects for Enamel Regeneration
The knowledge accumulated to date on the structure and biomineralization
of enamel is abundant. However, there is much more to learn about these
two aspects of enamel before we can fully describe the structure–function
relationship of mature enamel and the processes involved in enamel forma-
tion. In terms of structure–function relationships, there is yet to be a clear
model describing the true 3‐D rod architecture throughout the tissue. The
ability to precisely describe directional changes of rods and their decussation
pattern from the DEJ to the crown surface is critical for understanding
the ability of the crown to distribute masticatory loads. From observation
of biological models, the building of the enamel structure has proven to be
a complex process. It requires an orchestration of protein–protein and
protein–mineral interactions that occur in a temporally and spatially co-
ordinated manner. Proper assembly and elimination of amelogenin has been
shown to be critical in nucleation and growth of the mineral phase during
formation of enamel. However, much is unknown with regard to the specific
functions of other proteins in the enamel biomineralization process. Fur-
thermore, emerging data have revealed that enamel proteins may serve a
critical role as signaling molecules in tooth root development (see Section
IV.A and Table II for details on the potential roles of enamel matrix proteins
in root formation). Continued investigations targeted at understanding the
detailed structure of enamel and the functions of individual EMPs, in enamel
![Page 13: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/13.jpg)
Table II Factors Associated with the Putative Epithelial Niche (HERS and ERM) and Surrounding Mesenchyme
Factor Cells/Tissues Function/Putative Function Models References
Msx2 (homeobox
containing
transcription
factor known to
play a role in
crown formation)
HERS (not in
apical
mesenchyme),
and present in
many other cell
types including
dental pulp and
PDL (limited)
Products from the mesenchymal cells in
the local region, e.g., BMPs, may
regulate HERS production of Msx2
and/or other factors. One outcome
of this interaction may be control of
root patterning
Msx2 KO: presence of
irregularly shaped molar
roots and increased
expression of periostin
Satokata et al.
(2000) ;
Yamamoto et al.
(2004a)
BMP‐2 and 4 (bone
morphogenetic
protein 2 and 4)
Apical mesenchyme/
follicle region
(not HERS)
BMP‐2/4 and possibly BMP‐3, alsofound in high concentrations in
the follicle region, regulate products
produced by the HERS cells; this
interaction controls growth and
morphogenesis of the root sheath,
and thus root patterning
BMP‐4 KO: arrested at
earlier stages of tooth
development, therefore
specific root defects are
unknown
Yamashiro et al.
(2003) ; Yamamoto
et al. (2004a)
FGF‐10 (fibroblast
growth factor 10)
Apical mesenchyme/
follicle region
(not HERS)
Continuous FGF‐10 expression by apical
mesenchyme maintains epithelial stem
cell population (as in continuously
erupting rodent incisors). Cessation of
FGF‐10 expression necessary for
transition to root formation in teeth of
limited eruption (e.g., rodent molars)
Mouse, FGF‐10 deficiency
and overexpression:
Deficiency: defect of
epithelial stem cell
(apical bud) compartment.
Harada et al. (1999),
(2002a,b);
Yokohama‐Tamaki
et al. (2006)
Overexpression: formation
of apical bud in mouse
molars, inhibiting HERS
formation and root
development
(Continued )
![Page 14: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/14.jpg)
Ameloblastin Ameloblasts, HERS,
cementocytes
(low levels)
A known product of ameloblasts thought
to regulate enamel crystal size
Ameloblastin produced by HERS cells is
hypothesized by some to promote
acellular cementum formation
Ameloblastin KO: exhibits
an enamel phenotype but
no root deformities have
been reported
Simmer and Fincham
(1995); Zeichner ‐ David
et al. (2003)
Amelogenina Ameloblasts, HERS
region, odontoblasts,
PDL/cementum, and
possibly in other
tissues
A major protein of developing enamel and
a known product of ameloblasts involved
in regulating crystal structure. Suggested
functions in non ‐ epithelial tissues includeacting as a signaling molecule to regulate
diVerentiation of odontoblasts and
cementoblasts b. Hatakeyama et al. (2003,
2006) suggest that amelogenin acts to
protect the root from osteoclast‐mediated
root resorption
Amelogenin KO: exhibits
defective, chalky enamel
similar to that observed in
humans with amelogenesis
imperfecta (Gibson et al. ,
2001a). After root formation
is completed, root resorption
is enhanced in association
with osteoclasts and
cementicles in the
periodontal region
Boabaid et al. (2004b);
Bosshardt and Nanci
(2004); Bosshardt (2005);
Gibson et al. (2001b);
Hatakeyama et al. (2003);
Hatakeyama et al. (2006);
Nebgen et al. (1999);
Shimizu et al. (2005);
Veis et al. (2000);
Viswanathan et al. (2003)
Shh (sonic
hedgehog)
HERS, dental
mesenchyme, inner
enamel epithelium,
enamel knot
Involved in epithelial‐mesenchymal
interactions during tooth morphogenesis.
May contribute to root elongation through
signaling with Ptc1 and Gli1 genes and
proliferation of dental mesenchyme
Shh null mice: not viable
Ptc1 mes mutants: reduced
proliferation of mesenchyme
adjacent to HERS and
shorter roots
Nakatomi et al. (2006)
Table II Continued
Factor Cells/Tissues Function/Putative Function Models References
![Page 15: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/15.jpg)
IGF ‐1 (insulin ‐likegrowth factor ‐ I)
HERS May contribute to elongation of the HERS,
IGF receptors are present in vivo , and
elongation of HERS/increased cell
proliferation occurred in the outer
epithelial layer when IGF was added in vitro
IGF ‐ 1 localized to HERS in
5‐ day‐ old mice. In vitro
experiments supported a
role for IGF ‐ 1 in regulatingmitotic activity in HERS cells
Fujiwara et al. (2005)
OPN, BMP‐ 2,ameloblastin
Epithelial cell rests
of Malassez (ERM)
May assist in repair of cementum by increasing
cell proliferation; alternatively, may be
vestigial products of HERS with no function
in the mature tissues of the periodontium
No animal models with
defective ERM have
been developed
Hasegawa et al. (2003);
Yamashiro et al. (2003)
aNote: Amelogenin has several isoforms ( Bartlett et al ., 2006):
� LRAP (6.9 kDa); also called [A ‐ 4]/M59 and suggested to be a signaling molecule for odontoblasts and cementoblasts ( Tompkins and Veis, 2002 ). LRAP KO and
LRAP overexpression in mice do not appear to have a root phenotype.
� [Aþ 4]/M73 (8.1 kDa) has also been proposed as a signaling molecule ( Tompkins and Veis, 2002).
� TRAP: Similarly, suggested as a signaling molecule ( Swanson et al. , 2006).bStudies by Wang et al. (2005a) and Tompkins et al. (2006) have identified LAMPs as possible regulators of amelogenins, either involved in assisting with breakdown of
amelogenins (LAMP‐3, Wang et al.) or possibly serving as cell surface receptors (LRAP‐LAMP‐1, Tompkins et al.). Further, Tompkins et al. reported that A‐4/LRAP binds
to murine LAMP‐1, a lysosomal associated membrane protein, also present on cell surfaces, in a saturable fashion in murine myoblasts (C2C12 cells).
![Page 16: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/16.jpg)
Hypothesized activitiesDifferentiationPrecursor cells
Origins of cementoblasts and cementum
Epithelial
Papilla
Follicle
IEE
OEE
Pulp/Odontoblast
HERS
Osteoblast
PDL Cell
Cementoblast
ERM
HERS dislocates from developing rootsurface and forms ERM (Cho and Garant,2000; Diekwisch, 2001; Luan et al., 2006)
HERS secretes acellular cementum (Bosshardt,2005; Zeichner-David et al., 2003)
HERS cells e-m transform and secretecellular cementum (Bosshardt, 2005; Bosshardtand Nanci, 2004; Lezot et al., 2000; Thomas,1995)
Follicle-derived cementoblastssecrete acellular and cellular
cementum (Cho and Garant, 2000;Diekwisch, 2001; Luan et al., 2006)
Follicle-derived cementoblastssecrete (only) cellular cementum(Chai et al., 2000; Zeichner-David et al., 2003)
HERS secretes proteins that inducecementogenesis (Fong and Hammarstrom,2000; Fukae et al., 2001; Gestrelius et al., 2000;Hu et al., 2001)
Generally established Proposed hypothesis
Induction of cementogenesis (Alatli-Kutet al., 1994; Takano et al., 2003)
Ectomesenchymal
![Page 17: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/17.jpg)
3. Regeneration of the Periodontium 63
as well as in root development, will require concentrated research eVorts in the
next decade.Knowledge built from these research findingswill enhance our basis
for regenerating the crown as well as the ‘‘whole’’ tooth.
III. Question 2. What Do We Know About the Cells Requiredfor Periodontal Development and Regeneration?
While the origins for cells and tissues of the tooth crown have been fairly
well established, much remains unclear about cells involved with forming the
periodontium, and this has been a subject of speculation for at least five
decades, arguably with no authoritative statement yet made. The following
section will discuss the cells involved in periodontal development, their
potential contribution to regeneration, as well as controversies regarding
their origins.
A. Developmental Cells
Odontogenesis is characterized by sequential, reciprocal, reiterative signaling
between tissues of the epithelium (dental lamina) and mesenchyme (ecto-
mesenchyme derived from cranial neural crest) and ultimately, both epithelial
and ectomesenchymal cells are involved in periodontal tissue formation
(Fig. 2). Tooth development continues with ectomesenchymal cells develop-
ing into the dental follicle and surrounding the epithelial enamel organ and
the mesenchymal dental papilla (Nanci and Somerman, 2003). Cells within
the follicle region have been proposed to be the origin for tissues of the
periodontium, namely cementum, periodontal ligament (PDL) and alveolar
bone. But this hypothesis has not been accepted without challenge, as will be
discussed in some detail below. The exact origin of cementum and cemento-
blasts remains a matter of debate; current hypotheses are summarized in
Fig. 2 and described in the following text.
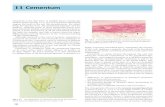
Figure 2 Origins of cementoblasts and cementum. This figure reviews competing hypotheses on
origins of cementoblasts and cementum tissue by considering possible fates for cells of
ectomesenchymal (top panel) and epithelial (bottom panel) origin, and hypothesized roles
in tooth root formation. The primary division lies between a proposed ‘‘classical’’ mesenchymal
origin (represented in top panel) and an ‘‘alternative’’ epithelial origin (represented in bottom
panel). However, several variations exist within each hypothesis, and these diVerences need not be
mutually exclusive. IEE ¼ inner enamel epithelium; OEE ¼ outer enamel epithelium; HERS ¼Hertwig’s epithelial root sheath; ERM ¼ epithelial cell rests of Malassez; PDL ¼ periodontal
ligament; e‐m ¼ epithelial‐mesenchymal transformation.
![Page 18: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/18.jpg)
64 Foster et al.
1. Ectomesenchymally Derived Cells
During the cap stage of tooth development, the epithelial enamel organ takes
on a concave form and is bordered by two ectomesenchymal tissues, papilla
and follicle, descended from cranial neural crest (CNC) cells. The dental
papilla is composed of densely packed cells that during the subsequent bell
stage become increasingly sequestered within the developing enamel organ,
eventually giving rise to the pulp and dentin tissue. The mesenchymal cells
surrounding the developing enamel organ and papilla compose the dental
follicle (sometimes called the dental sac), a collagenous tissue separating the
nascent tooth bud from surrounding oral tissues. Dental follicle has been
proposed to be the common origin for supportive tissues of the tooth (i.e.,
the periodontium), including cementum, PDL, and alveolar bone (Cho and
Garant, 2000; Nanci and Somerman, 2003; Saygin et al., 2000). Cells within
the follicle region are also essential for signaling associated with tooth
eruption, through regulation of osteoclasts in the coronal portion of the
bony crypt via CSF‐1, RANKL, and OPG expression, signaled in turn by
PTHrP and other factors still to be identified (Liu et al., 2005a; Wise et al.,
2002, 2005). During tooth eruption and root elongation, the formative
dental follicle gives rise to the mature structure of the PDL, a highly vascular
and innervated region that provides attachment of the tooth to the sur-
rounding alveolar bone via collagen fibers. The PDL is also home to a
heterogeneous population of cells, including stem cells with potential for
regeneration of periodontal tissues, which will be discussed in more detail at
the end of this section (Cho and Garant, 2000; Nanci and Somerman, 2003;
Seo et al., 2004).
2. Hertwig’s Epithelial Root Sheath Cells
Root initiation begins after the crown dentin and enamel have formed, and
before tooth eruption. The cervical loop, the most apical extension of the
enamel organ, extends into the bilayered Hertwig’s epithelial root sheath
(HERS), composed of the outer enamel epithelium (OEE) and inner enamel
epithelium (IEE). The HERS layers proliferate and extend apically, outlining
the future shape of the nascent tooth root (Luan et al., 2006). In mammalian
root formation, dislocation from the root and disintegration of the double‐layered HERS is considered a key event, allowing access of the underlying
dentinal surface to cementum‐forming cells (Cho and Garant, 1988;
Diekwisch, 2001). This general sequence of events has been further supported
by in vitro tissue recombination experiments (MacNeil and Thomas, 1993). As
root formation continues, the dislocated HERS cells break up into epithelial
‘‘nests’’ and ‘‘cords,’’ which may be subsequently reduced to epithelial cells
rests of Malassez (ERM) (Wentz et al., 1950). In addition to the possibility
![Page 19: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/19.jpg)
3. Regeneration of the Periodontium 65
of HERS cells migrating away from the root surface to contribute to ERM,
it has also been documented that some HERS cells undergo apoptosis
(Kaneko et al., 1999) or become incorporated into the cellular cementum
(Lezot et al., 2000). Developmental studies, as well as a review of evolution-
ary evidence (Luan et al., 2006), provide information indicative of a role
for ERM in regulating PDL homeostasis, protecting against resorption
and ankylosis, and perhaps contributing to cementum repair (Hasegawa
et al., 2003).
It has been proposed that HERS plays an active role in induction
or secretion of acellular and/or cellular cementum, and this hypothesis is
described in detail below (under the ‘‘alternative’’ epithelial hypothesis of
cementogenesis). Potential signals of HERS that may stimulate cementum
formation are discussed under Section III and listed in Table II.
B. Derivation of Cementum: Competing Theories of Cementoblast Origin
1. Acellular Versus Cellular Cementum
Acellular cementum covers approximately two‐thirds of the root, and
around the time the tooth comes into occlusion, cementum development
shifts from acellular to cellular. Acellular cementum (acellular extrinsic fiber
cementum, AEFC) forms first on the coronal and mid‐portion of the root at
a slow rate, while cellular cementum (cellular intrinsic fiber cementum,
CIFC), a more bone‐like tissue, forms more apically and more rapidly,
incorporating cells into the mineralized matrix that become cementocytes.
Acellular cementum seems to be more dependent on alkaline phosphatase
activity (Jayawardena et al., 2002), as it may be more severely aVected than
cellular cementum in hypophosphatasia (Beertsen et al., 1999; van den Bos
et al., 2005).
The cause or mechanism of the shift from acellular to cellular cementum is
not well understood, though hypotheses to explain this transition include the
possibilities that occlusal mechanical forces somehow cue the shift, cells
producing each type of cementum are from diVerent populations, or diVer-ent extracellular factor(s) regulate(s) acellular versus cellular cementum.
Potential regulators that have been considered include the dentin matrix of
the root, enamel matrix proteins, and other components of the ECM.
In experiments modeled after those performed by Hammarstrom (Alatli‐Kut et al., 1994; Hammarstrom et al., 1996), Takano et al. treated rats and
guinea pigs with bisphosphonate to delay dentin matrix mineralization, and
observed that acellular cementum was precluded by formation of a cellular
type of cementum on the nonmineralized dentin along the entire surface of
the root (Takano et al., 2003). While dentin sialoprotein (DSP) was localized
![Page 20: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/20.jpg)
66 Foster et al.
to the border between dentin and cellular cementum (but not acellular
cementum) in untreated rats, in the bisphosphonate‐treated rats DSP pene-
trated the soft dentin matrix along much of the root, even diVusing into
surrounding tissues. The authors hypothesize that the timing of mineraliza-
tion of mantle dentin in conjunction with dentin matrix proteins influences
the type of cementum forms.
2. The ‘‘Classical’’ Mesenchymal Hypothesis
The ‘‘classical’’ hypothesis, nearly 50 years old (Paynter and Pudy, 1958),
proposes cementoblasts are cells descended from the dental follicle that
migrate to the developing root surface and are triggered to diVerentiate intocementum matrix‐secreting cells, that is, cementoblasts (Bosshardt and
Selvig, 1997; Cho and Garant, 2000; Diekwisch, 2001; Luan et al., 2006;
Saygin et al., 2000). This hypothesis fits into an overarching proposition of a
common developmental origin (i.e., the dental follicle) for the three forma-
tive cell populations of the periodontium, namely cementoblasts, PDL cells,
and alveolar osteoblasts (Melcher, 1985; Ten Cate, 1997).
During rat molar root development, mesenchymal cells of the follicle were
reported tomigrate to theHERS, disrupt the epithelial structure, and begin to
lay down cementum matrix via cellular processes, as interpreted from studies
employing light and electron microscopy (Cho and Garant, 1988, 2000).
Similar observations of mesenchymal cells accessing the developing root
surface were reported in the mouse molar, with the exception that HERS cells
may themselves contribute to the disruption of the HERS structure prior to
root formation, while the first matrix secreting cells in cementum formation
were the migrating mesenchymal (follicle) cells (Diekwisch, 2001; Luan et al.,
2006; Ten Cate, 1997). Migratory capabilities of dental follicle cells were
supported in mouse molar organ culture, where fluorescently tagged follicle
cells migrated apically and were found in PDL and alveolar bone (Diekwisch,
2002). Human and porcine specimens in the extensive Bernard Gottlieb
collection (Baylor College of Dentistry, Dallas, TX) yielded similar observa-
tions that HERS cells departed the root surface prior to initiation of cemen-
tum, forming a loose network of cells that subsequently disintegrated, with
some presumably contributing to the population of ERM, islands of
epithelial‐derived cells that remain in the PDL into adulthood with uncertain
function (Diekwisch, 2001).
In support of the ‘‘classical’’ hypothesis, in a well‐executed developmental
study in mice, Chai et al.were able to track cells of cranial neural crest (CNC)
origin, from embryogenesis to 6 weeks of age, using a two‐component
(Wnt1‐Cre, R26R) genetic system for cell lineage tracking through develop-
ment (Chai et al., 2000). In this way, CNC progeny were identified by
�‐galactosidase activity (only present in cells expressing Wnt1 and constitutive
![Page 21: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/21.jpg)
3. Regeneration of the Periodontium 67
R26R, but marked indelibly, even when Wnt1 expression is shut oV). CNC‐derived cells contributed to formation of cementum and periodontal
ligament, as well as to condensed dental mesenchyme, dental papilla, odonto-
blasts, and other tissues. While cementum showed strong lacZ expression,
indicating a CNC origin, these results need not preclude an epithelial
contribution.
Some species‐specific diVerences in cementum development are worth
noting, one being that in rodents the sequence of events is muddied by
HERS initially covering the entire root surface and remaining in close
proximity as cementum is formed, as opposed to humans where HERS is
more completely divorced from the developing root prior to any observable
cementum.
Supposing the classical hypothesis of common origin for cellular and acellu-
lar cementum, PDL, and alveolar bone, the question naturally arises, ‘‘What
factors direct a common precursor cell to become a cementoblast, osteoblast,
or PDL cell?’’ This is a valid question worthy of future study, with some
potential regulators discussed under Question 3 and presented in Tables II–V.
3. The ‘‘Alternative’’ Epithelial Hypothesis
An alternative hypothesis that has been proposed (Slavkin and Boyde, 1975)
questions the evidence for a mesenchymal origin and instead considers an
epithelial contribution from HERS to cementogenesis (Bosshardt, 2005;
Bosshardt and Nanci, 1997, 2004; Bosshardt and Schroeder, 1996; MacNeil
and Somerman, 1999; Thomas, 1995; Zeichner‐David, 2006; Zeichner‐David
et al., 2003). Under this proposal, cementoblasts are thought to be derived
from an epithelial‐mesenchymal transformation of HERS cells, which then
secrete cementum matrix proteins. DiVerences of opinion exist regarding
origins of acellular and cellular cementum, as delineated below.
In a careful observation of cementogenesis in pigs using light microscopy
and TEM with immunogold labeling, Bosshardt and Nanci found a lack of
compelling evidence for a mesenchymal migration of follicle cells, but rather
observed a potential phenotypic epithelial‐mesenchymal transformation of
outer enamel epithelium (OEE) cells to a secretory, connective tissue cell‐likemorphology in the vicinity of initiation of cementogenesis (Bosshardt and
Nanci, 2004). Studies using a Dlx‐2/LacZ reporter construct in transgenic
mice localized Dlx‐2 expression to root epithelium (HERS) during root
development, and also to a limited population of cementoblasts during
acellular and cellular cementum formation, but failed to detect Dlx‐2 in
dental follicle and papilla (Lezot et al., 2000). During acellular cementum
formation, Dlx‐2 was identified in diVerentiated cementoblasts, and during
cellular cementum formation in innermost cementoblasts and cementocytes.
![Page 22: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/22.jpg)
68 Foster et al.
Some of the Dlx‐2 positive cementoblasts also stained positive for amelo-
blastin. The authors concluded a complex origin for cementum‐forming
cells, in other words, suggesting that a select population of cementoblasts
were derived from the HERS. Another interpretation of these results could
be that HERS cells are passively incorporated within the forming cementum
matrix being synthesized by mesenchymally derived cementoblasts.
Evidence for an epithelial origin for acellular cementum also lies in the
demonstration that these cells can produce proteins characteristic of mesen-
chymal cells, and cementum in particular (Bosshardt and Nanci, 1997;
Mouri et al., 2003; Zeichner‐David, 2006; Zeichner‐David et al., 2003).
If HERS cells transform to contribute to acellular cementum formation,
the possibility of cellular cementum derived from HERS may also be con-
sidered. A hypothesis based on morphological examinations in human and
porcine teeth proposes that HERS is the origin not only for both types of
cementum, but also for subpopulations of periodontal ligament fibroblasts
(Bosshardt, 2005). This hypothesis would explain the diVerent phenotypes ofcementoblast versus osteoblast, and the heterogeneity of cells populating the
PDL region. While strides are being made toward describing the origins of
cementum, and a great dialogue of diVering viewpoints has been cultivated
in the literature, the origin of cementum is still under debate.
4. Involvement of Epithelial‐Derived Products in Cementum Formation
Apart from ideas about the transformation of HERS to cementoblasts, it has
been suggested that HERS may induce cementogenesis by secretion of enamel
matrix proteins (EMPs) (e.g., amelogenin, ameloblastin, and enamelin) or
other proteins that influence cell migration, attachment, and/or matrix secre-
tion leading to cementogenesis (Gestrelius et al., 2000; Hammarstrom et al.,
1996; Slavkin, 1976; Zeichner‐David, 2001, 2006).
Evidence supporting a role for EMPs in cementogenesis has been ac-
cumulating from investigations employing immunohistochemistry, in situ
hybridization, and in vitro assays, all supporting EMP expression by HERS
cells in several species (Bosshardt and Nanci, 1998; Fong and Hammarstrom,
2000; Fukae et al., 2001; Hamamoto et al., 1996; Hammarstrom, 1997; Hu
et al., 2001; Luo et al., 1991; Slavkin et al., 1989a,b). However, serious dis-
crepancies in these collective reports remain unresolved. Reports conflict with
one another on several points, including: which EMPs are or are not ex-
pressed, how much protein is present and if levels are suYcient to play an
important role in root formation, the region of localization on the root, and
what cells produce EMPs. In studies of porcine cementogenesis, little evidence
was found to support a significant role of enamel matrix derivatives (in this
case, amelogenin and ameloblastin) based on absence of significant quantities
of these ameloblast products in the HERS and on the developing root surface
![Page 23: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/23.jpg)
3. Regeneration of the Periodontium 69
(Bosshardt and Nanci, 2004). In mouse molars, immunocytochemistry and
in situ hybridization failed to detect any trace of amelogenin in HERS cells,
and amelogenin was also absent in porcine cementum extracts assayed by
Western blot (Diekwisch, 2001). Janones et al. used microwave processing in
conjunction with immunocytochemistry to demonstrate that in developing
rat molars, amelogenin was present in early tooth formation, but gone before
initiation of cementogenesis (Janones et al., 2005). These conflicting results,
both old and new, should then be considered carefully for possibilities such as
false positives and specificity or cross‐reactivity of antibodies. Ultimately, to
confirm that EMPs are functionally important in cementogenesis, a consistent
and regular expression of these proteins would be expected in association with
developing cementum, and up to now, this standard remains to be met in a
convincing way. Better probes, antibodies, etc., should assist in solving this
puzzle.
For example, an immortalized murine HERS cell line expressed amelo-
blastin (but not amelogenin or enamelin) in vitro. HERS conditioned media
was found to induce BSP and OCN expression, as well as in vitro minerali-
zation (Zeichner‐David, 2006). Further, theseHERScellswere also observed to
undergo an apparent phenotypic transformation to a morphologically distinct
fibroblastic cell expressing cementum‐associated transcripts BSP, OCN, and
OPN, supporting the potential not only for HERS to induce cementogenesis,
but also secrete cementum matrix proteins directly (Zeichner‐David et al.,
2003).
Furthermore, if EMPs play an important role in tooth root formation,
a cementum phenotype might be expected in animals deficient in these
proteins. While a root phenotype has been suggested in amelogenin knock-
out mice, it is unclear whether this is a direct or indirect result (Hatakeyama
et al., 2003, 2006), and this will be addressed in the next section under
Question 3, as well as in Table II focusing on HERS‐ and ERM‐associatedproducts. Though the role of epithelial proteins in root formation remains
controversial, a treatment derived from porcine enamel organ known as
EmdogainÒ is currently used clinically with the aim to promote periodontal
regeneration. The applications of EmdogainÒ will be discussed in more
detail under Question 3.
C. Differences Between Cementoblasts and Osteoblasts
A common origin for cementum and alveolar bone has been proposed in the
form of the dental follicle and perifollicular cells. Yet in the absence of a
clear understanding of cementum origins, how can progress be made toward
improving tissue engineering and promoting periodontal regeneration?
Cementoblasts and osteoblasts and their respective tissues may be compared
![Page 24: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/24.jpg)
70 Foster et al.
with respect to cells and regulators of their diVerentiation, and structural
and functional properties of the cementum versus bone matrices. It is outside
the scope of this review to exhaustively catalog points of comparison be-
tween cementoblasts and cementum versus osteoblasts and bone; for this the
reader is recommended to excellent reviews on the topic (Bosshardt, 2005;
Diekwisch, 2001; Nanci and Bosshardt, 2006; Saygin et al., 2000; Zeichner‐David, 2006). Potential areas for progress in characterizing cementoblasts
including identification of cementum marker proteins, performing compar-
isons to other cell types, and using in vitro models of cementoblasts and
precursor cells in conjunction with in vivo observations.
1. Cementum‐Specific Markers
Attempts have been made to identify unique cementum‐specific marker pro-
teins that would distinguish cementum from bone. In the study of dental
tissues, many ‘‘specific markers’’ have even been declared, later to be reported
in other tissues as well. For example, DSPP and DMP‐1, formerly thought
dentin‐specific, have subsequently been localized to bone and cementum and
their respective cells, in vivo and in vitro (Baba et al., 2004a; Foster et al., 2006;
Qin et al., 2002). Amelogenin, thought to be an ameloblast‐specific product, isexpressed by pulp cells and odontoblasts during tooth development (Nagano
et al., 2003; Oida et al., 2002; Papagerakis et al., 2003; Veis et al., 2000).
There is a history of putative cementum‐specific factors aswell. A cementum‐derived growth factor (CGF) originally isolated from a human cementoblas-
toma and posited to be a novel growth factor and mitogen (Yonemura et al.,
1992, 1993) was identified in human and bovine cementum, as well as in PDL
cells and furthermore, on detailed analysis recognized as being very similar in
composition to IGF1 (Narayanan et al., 1995). Cementum attachment protein
(CAP) was identified from a human cementum tumor and proposed to be an
extracellular matrix protein functioning in migration and attachment of ce-
mentoblast precursors to the root surface (Arzate et al., 1992; Bar‐Kana et al.,
2000; Pitaru et al., 1995, 2002; Saito et al., 2001); CAP was later found to be
expressed in PDL cells and alveolar bone cells, and to share homology with
some collagen domains (BarKana et al., 1998;Wu et al., 1996). Another protein
identified from cementum tumor was termed cementum‐protein 23 (CP‐23)(Alvarez‐Perez et al., 2006). Antibodies made to this protein cross‐reacted with
a cartilage type collagen, type X collagen, and CP‐23 was identified within the
PDL region, cementum and around blood vessels in the PDL. While CGF,
CAP, and CP‐23 may play roles in periodontal development, they are not,
strictly speaking, markers of cementoblasts or cementum. Importantly, these
proteins were identified from a human cementoma and cementomas by defi-
nition are composed of a variety of cells, for example, fibroblasts, osteoblasts,
and cementoblasts. Additional examples include lumican and fibromodulin,
![Page 25: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/25.jpg)
3. Regeneration of the Periodontium 71
reported to be more highly expressed in cementum than bone (Bosshardt,
2005). Glucose transporter‐1 (GLUT‐1) was suggested to be a factor separat-
ing cementoblasts from osteoblasts (Koike et al., 2005), and though this
protein is widely expressed, it is tenfold higher in human cementoblasts versus
osteoblasts in vitro.
While these proteins may not be unique cementum markers, they may still
be useful in defining cementum matrix versus bone. These and other proteins
are thought to be enriched or relatively highly expressed in cementum versus
bone and have potential to be used to assemble a panel of markers charac-
teristic or suggestive of cementum. As of yet, there is not any marker by itself
that is unique or specific to this tissue.
2. Comparisons of Cementoblasts to Other Cell Types
As no conclusive study demonstrating cementoblast origin has yet been
reported and no cementum‐specific marker is likely, a very practical option
may be direct cell‐to‐cell comparison, as between cementoblasts and osteo-
blasts. In vivo studies are limited by the need for specific probes and anti-
bodies and the laborious nature of screening, while in vitro studies make
many aspects of analysis easier, but results must be analyzed cautiously
because of removal of cells from the natural milieu. Head‐to‐head compar-
isons of cells have yielded valuable insights when confirmed by other meth-
ods such as in situ hybridization and immunohistochemistry. Examples of
such comparison technologies include laser capture, microarray analysis,
proteomics, and subtractive hybridization. All, except laser capture, have
been used to begin to define markers for dental cells (Hao et al., 2005; Koike
et al., 2005; Lallier et al., 2005; Reichenberg et al., 2005; Shi et al., 2001).
Care must be taken in the choice and preparation of cells to be compared in
such experiments, as this sort of analysis may result in misleading conclu-
sions if precautions are not used. For example, the cell populations being
compared may be derived from diVerent developmental stages, which would
strongly influence gene and protein profiles expressed.
The logical comparison for cementoblasts would be osteoblasts lining the
surrounding alveolar bone. Although alveolar bone is generally thought to be
consistent with other bone tissues in cell and matrix components, it is a local,
specialized bone tissue with unique features, including proximity to the tooth
and the cellular/molecular influence of the tooth tissues, and a very high rate
of remodeling relative to other bone tissues of the body (Sodek and McKee,
2000). There is some evidence that bonemarrow stromal cells (BMSCs) within
the same individuals diVer in a skeletal site‐specific fashion, and that orofacialstem cells may represent a unique population (Akintoye et al., 2006).
If cementoblasts and alveolar osteoblasts share a direct precursor cell, it is
![Page 26: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/26.jpg)
72 Foster et al.
possible that they share a more similar genetic profile than cementoblasts
versus other osteoblast or osteoblast precursor populations.
A cleverly designed experiment by Kaneda et al. used a strategy of
consecutive enzymatic digestions of extracted mouse molars to explore
diVerences between subpopulations of PDL cells, from cells obtained mid-
way across the PDL space to those closest to the root surface, including
cementum‐lining cells (Kaneda et al., 2006). As subpopulations were char-
acterized closer to the root, their alkaline phosphatase activity and potential
for promoting in vitro mineralization increased, as well as expression of BSP
mRNA. Further studies employing a similar approach should yield insights
into characteristics of subpopulations of cells located in the PDL region,
and into the potential of these various subtypes to diVerentiate toward a
cementoblast phenotype.
3. In Vitro Cell Models for Cementoblasts and Precursor Cells
Establishment of in vitro cementoblast models in parallel with studying
in vivo cementum development can be a powerful way to progress our
understanding of the origins and characteristics of this tissue. To date,
cementoblast cell lines for use in vitro have been prepared from mice (Berry
et al., 2003; D’Errico et al., 2000; MacNeil et al., 1998), rats (Kitagawa et al.,
2005), cows (Saito et al., 2005), human (Grzesik et al., 1998), and human
cementoblastoma (Arzate et al., 1992). These cells express high levels of BSP,
OCN, and OPN, and can produce mineralized nodules in vitro and ectopic
ossification in an in vitro SCID mouse model. Additionally, putative cemen-
toblast precursors, dental follicle cells, have been isolated and cultured from
mice (Zhao et al., 2001), rats (Yao et al., 2004), and humans (Morsczeck
et al., 2005), and these may provide clues as to potential mechanisms
required cementoblast diVerentiation. An immortalized HERS‐derived cell
line has been established from mice, and has been characterized as producing
enamel‐related proteins prior to a phenotypic shift toward a mesenchymal
cell type that produces a mineralized matrix resembling acellular cementum
(Zeichner‐David et al., 2003). While species diVerences, phenotypic drift,
and secondary eVects of immortalizationmust be considered, these approaches
have already yielded considerable insight into the nature of ‘‘cementoblasts’’
and will continue to do so in future research.
D. Tooth Stem Cell Populations
The nature and regenerative capacities of stem cell populations in tooth
tissues have been one of the most exciting revelations in dental research
in the last five years, with enormous potential for future application in
![Page 27: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/27.jpg)
3. Regeneration of the Periodontium 73
designing regenerative therapies and tooth engineering in the future (Bartold
et al., 2006a; Chai and Slavkin, 2003; Fong et al., 2005; Ohazama et al.,
2004b; Risbud and Shapiro, 2005; ThesleV and Tummers, 2003). Embryonic
stem cells are pluripotent, that is, they have the capability to diVerentiateinto all cell types with appropriate conditions and stimulation. Stem cell
research eVorts focus on the sizeable probability for such cells to be used in
adult tissue regeneration and gene therapy. However, the current number of
embryonic stem cell lines is limited and their use is controversial and subject
to government regulation. As a result, there has been great interest in
exploring stem cell populations in adults. Adult stem cells are undiVeren-tiated cells that remain in developed tissues of the adult organism and are
multipotent, meaning they have the capability to diVerentiate into multiple
cell types within a tissue, organ, or system. Adult stem cells have been
identified in several locations including bone marrow, blood, neural and
muscle tissue, and tooth environment (Fuchs and Segre, 2000). While the
breadth of potential for diVerentiation, or potency, for most of these adult
stem cell types remains to be fully explored, the therapeutic possibilities for
an adult‐derived, unlimited population of multipotent stem cells are quite
exciting (Robey, 2000). The identification and characterization of these adult
stem cell populations in the tooth region has been one of the most exciting
and promising discoveries of the last five years.
1. Dental Pulp Stem Cells
The dental pulp holds promise for regeneration of dentin in response to
trauma (Goldberg and Smith, 2004), and this has been recognized for many
years. This knowledge, coupled with advances in technology, has enhanced
our understanding of the underlying mechanisms involved in pulp cell
maturation. A human adult stem cell population was identified and isolated
from pulp chambers of impacted third molars. In cell culture, these dental
pulp stem cells (DPSCs) were demonstrated to be clonogenic, rapidly pro-
liferative, able to diVerentiate and form mineralized nodules in vitro, and
produce a structure resembling a dentin/pulp complex in ex vivo transplan-
tation experiments with SCID mice (Gronthos et al., 2000). In the same
experiment, bone marrow stromal stem cells (BMSSCs) formed a more
distinctly bone‐like tissue. In subsequent studies, the DPSC profile was
further developed by identifying mesenchymal stem cell markers STRO‐1and CD146, and transplant experiments were performed with DPSCs, with
cells exhibiting odontoblast‐like gene and protein expression, and produc-
ing dentin‐like tissues (Batouli et al., 2003; Shi et al., 2001). The DPSC niche
was hypothesized to be a perivascular location within the pulp. Subsequent
work demonstrated the ability to harvest similar mesenchymal stem cells
from human exfoliated deciduous teeth, cells that were termed SHED
![Page 28: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/28.jpg)
74 Foster et al.
(Miura et al., 2003). Sorting and ex vivo expansion of pulp stem cells from
exfoliated deciduous teeth allowed for the cells to be directed to adipocyte
and myotube phenotypes, as well as osteoblast‐like cells that produced a
mineralized tissue consistent with woven bone (Laino et al., 2006).
2. Periodontal Ligament Stem Cells
The PDL demonstrates some limited potential for repair of periodontal
tissues should they be damaged by trauma or disease, however, while there
are currently several strategies aimed at regenerating periodontal tissues,
sometimes successful, they are not predictable (Grzesik and Narayanan,
2002; Taba et al., 2005; Wang et al., 2005b; Zohar and Tenenbaum, 2005).
This repair potential of the PDL is thought to result from the presence of
a population of multipotent stem cells within the local region or recruited
from the vasculature that are capable of regenerating cementum, bone, and
PDL fibers (Bartold et al., 2000; Gould et al., 1980; McCulloch, 1985, 1995;
Melcher, 1985). Although several groups have demonstrated the regene-
rative potential of a compartment of periodontal cells, recent studies
have confirmed a stem cell population and characterized the nature of these
cells.
Human postnatal PDL stem cells (PDLSCs) were isolated, cultured, and
characterized in vitro (Seo et al., 2004). PDLSCs were fibroblast‐like, clono-genic and rapidly proliferative, and were positive for mesenchymal stem cell
markers STRO‐1 and CD146, similar to DPSCs and BMSSCs, indicating a
possible common perivascular origin. Interestingly, expanded PDLSCs also
expressed relatively high levels of a tendon‐associated transcription factor,
scleraxis. In vitro studies showed that after incubation in diVerentiationmedia, PDLSCs expressed proteins characteristic of cementoblasts, including
BSP, OCN, MEPE, ALP, and TGF�R1, and had the ability to promote the
formation of mineralized nodules. PDLSCs transplanted into SCID mice
produced collagen fibers suggestive of the PDL and amineralized tissue consis-
tent with cellular cementum. It remains unclear what signals may be necessary
to cue precursor cells to a cementum versus bone phenotype, and at present, no
specificmarkers have been established for cementum versus bone (as described
in detail above). Subsequent studies added to this work by showing that
viable PDLSCs could be retrieved from frozen PDL tissues (Seo et al., 2005),
increasing the practical potential for these stem cells to be used clinically
(Bartold et al., 2006a; Shi et al., 2005). While the origins of cementoblasts
remain in question, studies with periodontal ligament stem cells (PDLSCs)
showing ability to produce cementum‐like tissues in SCID mouse transplant
experiments lend some support to the mesenchymal origin of cementum, or
at least cellular cementum. Indeed, expression of common transcription
factors, cell surface markers, growth factors, and matrix proteins in postnatal
![Page 29: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/29.jpg)
3. Regeneration of the Periodontium 75
stem cell populations suggests common regulatory pathways for cementum,
dentin, and bone.
3. Epithelial Stem Cells of the Continuously Erupting Incisor
The postnatal stem cells of the bone marrow, dental pulp, and PDL are
multipotent mesenchymal stem cells with a capacity for limited generation of
mesenchymally derived tissues. In the teeth of humans and the molars of
rodents (teeth of limited eruption), the ameloblasts that form the tooth
enamel are lost on tooth eruption, and there seems to be no epithelial self‐renewing stem cell population remaining. However, in the continuously
erupting incisor of rodents, new enamel (as well as dentin and cementum)
are constantly generated apically to compensate for attrition on the incisal
edge (Harada et al., 2002a). Therefore, new ameloblasts must be available
from postnatal epithelial stem cell populations in order to synthesize enamel
in the adult. A specialized apical bud structure proposed to be the epithelial
stem cell niche was identified at the apical end of incisors of mice and guinea
pigs (Ohshima et al., 2005). The apical bud was characterized by large
amounts of stellate reticulum and basal epithelium, and a candidate mole-
cule for maintenance of the apical bud niche was identified as fibroblast
growth factor 10 (FGF‐10) by adjacent mesenchymal cells, with epithelial
Notch signaling also implicated as playing a role (Harada et al., 2002b).
Ameloblast diVerentiation and patterning in the rodent incisor was shown
to be dependent on downregulation of follistatin in the epithelium on the
labial edge, and subject to regulation by the antagonistic actions of BMP‐4fromodontoblasts and activin fromdental follicle (Yamashiro et al., 2004). In a
primary cell culture study, apical bud stem cells were shown to require mes-
enchymal cell interaction to be prompted to diVerentiate to an ameloblast‐likephenotype (Morotomi et al., 2005).
4. Crown and Root Developmental Fates
The developmental diVerence between teeth of limited eruption versus con-
tinuous eruption may lie in the ‘‘choice’’ between maintenance of the epithe-
lial stem cell niche as opposed to loss of this niche and development of a
root. The developmental fate of the continuously erupting incisor is deter-
mined by maintenance of an apical bud epithelial stem cell population
(Ohshima et al., 2005), while if crown development is arrested, a root fate
is pursued (Tummers and ThesleV, 2003). The root fate is characterized by
transformation and flattening of the stellate reticulum into the double‐layeredHERS,which lengthens to define the root shape outline, and fenestrates
just prior to cementum formation. The fate of the HERS is a matter of debate,
potentially becoming ERMs, secreting pro‐cementum factors, or contributing
![Page 30: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/30.jpg)
76 Foster et al.
to acellular and/or cellular cementum formation. Crown and root fates were
investigated in an elegant study of the molar of the sibling vole, which intrigu-
ingly develops root analog areas while remaining continuously erupting. In the
root‐like regions of vole molars, FGF‐10, Notch 1 and 2, and BMP‐4 signals,
thought to contribute to the epithelial stem niche, disappear coincident with
development of root‐like tissues, similar to in mouse molars (Harada et al.,
2002b; Ohshima et al., 2005; Tummers and ThesleV, 2003; Yokohama‐Tamaki
et al., 2006). Therefore, mechanisms that downregulate signals specific for
crown formation may be required for initiation of root formation. These
findings are intriguing to consider in light of the failure of tissue recombination
experiments to form tooth roots.
IV. Question 3. What Genes and Associated Proteins AreImportant for Root/Periodontal Tissue Formation?
In the last 5 years, newly discovered factors and new roles for already known
factors regulating root/periodontal development have emerged and have
changed how we view odontogenesis. Much of the recent progress in under-
standing factors that regulate root/periodontal tissue (R/PT) development
has naturally arisen from studies of molecules that control mineralized tissue
formation. The development of transgenic mice, designed to over‐ or under-express specific genes, and the study of mice with well‐defined mutations has
provided much insight into potential factors required for R/PT development.
Tables II–V highlight factors that have been reported within (approximately)
the last 5 years to play a role in R/PT development, including genes/
proteins that result specifically in a root phenotype when deficient or over-
expressed. In addition, factors already established to play a role in root/
periodontal development will be briefly discussed, with an update of relevant
references.
It is well established that specific genes and associated proteins are re-
quired for patterning, proliferation, and diVerentiation of cells during crown
development, that is ameloblasts for enamel and odontoblasts for dentin.
Much attention has been given to factors involved in epithelial–mesenchymal
interactions, including fibroblast growth factors (FGFs), sonic hedgehog
(SHH), bone morphogenetic proteins (BMP)s, Wnts and associated recep-
tors, as well as downstream transcription factors such as distal‐less homeo-
box (Dlx), Msx, AP‐1 factors, Pax‐9, and runt‐related transcription factor 2
(RUNX 2). Many of the knockout (KO) models developed to understand the
specific roles for these genes during tooth development have resulted in
severe phenotypes (and sometimes death in utero) because of the critical role
these genes/proteins play during early development. This has in some cases
![Page 31: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/31.jpg)
3. Regeneration of the Periodontium 77
prevented any detailed analysis of the teeth, because if the animals survived,
tooth development was arrested at stages prior to root formation. Conse-
quently, there is limited direct evidence for the role of such genes and their
products in R/PT formation (Zhao, 2003). Several excellent reviews on the
roles of specific genes and signaling molecules during crown development are
available (Jernvall and ThesleV, 2000; ThesleV and Aberg, 1999; ThesleV and
Mikko la, 2002; Thes leV et al. , 1995 , 2 003; Tucker and Sha rpe, 2004; Zhanget al., 2005). One major finding from research targeting crown development
has been the recognition of niche areas, specifically the enamel knot, and
cervical loop/apical bud regions, where a plethora of genes crucial for reg-
ulating crown development are expressed.
While the aforementioned studies have focused primarily on molecules
regulating crown development, they have also raised new questions related
to investigation into R/PT development. Specifically, do ‘‘niche’’ regions for
R/PT development exist, similar to the enamel knot region? And can we
identify the signaling molecules that regulate cell diVerentiation toward a
cementoblast, PDL cell, and osteoblast cell fate? Discussed below and
described in Tables II–V are factors, both well‐established and putative, that
have been implicated in regulating R/PT development, with an emphasis on
cementum formation. Some molecules that have been reviewed previously
(Bosshardt, 2005; Bosshardt and Nanci, 1997; Diekwisch, 2001; Popowics
et al., 2005; Saygin et al., 2000) or implicated in R/PT formation with limited
evidence to support their function in root development are mentioned below,
but not included in the tables.
A. Factors Associated with the Putative Epithelial Niche (HERS and ERM)and Surrounding Mesenchyme
After crown formation is completed, but prior to eruption, the outer and
inner epithelia form a double‐layered sheath called Hertwig’s epithelial root
sheath (HERS), and proliferate apically to outline the form the root will
take, as detailed in the previous section. It is generally accepted that HERS
cells of the inner enamel epithelium (IEE) regulate cells of the dental papilla
to diVerentiate into odontoblasts and secrete matrix proteins required for
forming root dentin, yet the events and molecular factors directly responsi-
ble for this sequence of events remain unknown (Thomas, 1995). With
further studies, specific factors critical for directing root dentin formation
may be identified (Table II).
The role of HERS with regard to cementum formation is even less clear,
and hypotheses are discussed in the previous section and summarized in
Fig. 2. Hypotheses discussed above include the induction of cementogenesis
by epithelial products from HERS, epithelial–mesenchymal transformation
![Page 32: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/32.jpg)
78 Foster et al.
of HERS cells into a cementoblasts, and potential for diVerent contributionsin the development of acellular versus cellular cementum. After development
of the R/PT, remnant epithelial cells may reside in mature PDL as epithelial
cell rests of Malassez (ERM).
Several groups have begun to characterize HERS cells and their proteins
to determine the contribution of these cells/factors during tooth root devel-
opment. These investigations have demonstrated unique signaling molecules
within the HERS region, with preliminary evidence of a unique niche region.
A specialized structure termed the ‘‘apical bud’’ in the continuously erupting
rodent incisor has been identified as an attractive candidate for consider-
ation as an epithelial stem cell niche region for the crown (labial) side, with a
less well‐defined region on lingual side, that still may serve as a niche region
(Harada and Ohshima, 2004). In order to confirm that the HERS and labial
epithelial regions are stem cell niche regions, specific gene signals and target
cells need to be further characterized, and the possibility for epithelial‐mesenchymal transformation in some select group of cells considered. Some
of the investigations to date that support a role for HERS/epithelial pro-
ducts in R/PT formation are discussed below and also presented in Table II.
Signaling between cervical dental tissues directs the behavior of cells and
decision to act toward formation of crown versus root tissues. In the case of
continuous crown formation, dental papilla cells surrounding the cervical
loop express BMP‐4 and FGF‐10 (Table II). By regulating cell division,
FGF‐10 promotes survival of epithelial stem cells within the cervical loop
and allows continuous growth of rodent incisors. The data to date provide
evidence that the absence of these signals from the cervical region of mouse
molars, teeth of limited eruption, switches cellular activities from crown
formation to root formation (Tummers and ThesleV, 2003; Yokohama‐Tamaki et al., 2006).
Signaling between HERS cells and adjacent mesenchyme occurs during
root formation and appears to regulate the proliferation and diVerentiationof both epithelial and mesenchymal cells. Because the BMP–Msx pathway is
known to elicit reciprocal interactions between epithelial and mesenchymal
cells during early tooth development, Yamashiro and colleagues examined
the expression of BMP–Msx signaling pathway molecules within the HERS
region (Yamashiro et al., 2003). During tooth morphogenesis, expre-
ssion of BMP‐4 in the apical mesenchyme precedes Msx2 expression in the
root sheath. None of the BMPs (i.e., BMP‐2, 3, 4, or 7) were detected in the root
sheath epithelium, nor transcripts for Msx1 or 2 in the mesenchyme. In con-
trast, Yamamoto and colleagues found BMP‐2 and 4 expression in HERS
cells (Yamamoto et al., 2004a), suggesting that these signaling molecules
may play a role in developing root shape. In relation to cell diVerentiation,BMP‐2 and 7 are transiently expressed in both preodontoblasts and diVer-entiating odontoblasts, and may signal epithelial diVerentiation and/or have
![Page 33: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/33.jpg)
3. Regeneration of the Periodontium 79
autocrine or paracrine eVects on diVerentiating odontoblasts (Yamashiro et al.,
2003). Msx2 null mice have been reported to have irregularly shaped molar
roots, although the extent of these alterations is not clear (Satokata et al., 2000).
The expression of Msx2 in cells within the HERS region and of BMPs in
the surrounding mesenchyme/follicle cells, coupled with the root pheno-
type in Msx2 KO mice, suggests that these molecules play a role in regulating
root patterning and cell diVerentiation similar to their function during crown
development.
Additional signals between HERS cells and mesenchyme control root
elongation. Sonic hedgehog (SHH) expression within HERS cells is thought
to signal target genes Patched1 (Ptc1) and Gli1 and promote proliferation of
dental mesenchyme (Nakatomi et al., 2006). PTC mutants show reduced cell
division within dental mesenchyme, shortened tooth roots, and disturbances
in tooth eruption. Autocrine or paracrine expression of insulin‐like growth
factor‐1 (IGF‐1) by HERS cells has also been implicated in regulating root
elongation. IGF‐1 receptors are present on HERS cells, and application of
IGF‐1 to mouse HERS organ cultures, in vitro, resulted in elongation of the
root sheath, possibly from increased cell proliferation from the OEE
(Fujiwara et al., 2005).
Some enamel proteins, including amelogenins and ameloblastin, in addi-
tion to their roles in crown formation (Section II and Table I), have been
proposed as regulators of R/PT formation (Table II). Reports have indicated
expression of these molecules in the HERS region and in the pulp region,
although at low levels and with a great deal of variability between species
(Bosshardt and Nanci, 1997, 2004; Fong and Hammarstrom, 2000; Janones
et al., 2005; Nebgen et al., 1999; Oida et al., 2002; Papagerakis et al., 2003;
Zeichner‐David et al., 1997). Amelogenin KO mice exhibit a tooth pheno-
type resembling amelogenesis imperfecta (AI) in humans, namely hypoplas-
tic enamel characterized by poorly organized hydroxyapatite crystals,
resulting in chalky‐white, fragile teeth (Gibson et al., 2001a). Additional
studies of amelogenin KO mice have implicated a role for amelogenin in
root development or maintenance. Increased osteoclastic root resorption
(Hatakeyama et al., 2003) and decreased BSP expression (Viswanathan
et al., 2003) by root surface cells have been reported in amelogenin KO mice
compared to controls. It has been further proposed that amelogenin and the
alternatively spliced product LRAP may be involved in regulating levels of
receptor activator of NF‐�B ligand (RANKL) within the local tooth root
environment, thereby acting as a protector of against osteoclast‐mediated
resorption of the root surface (Hatakeyama et al., 2006). In amelogenin KO
mice, RANKL on the tooth root surface was increased, as determined by
immunohistochemistry. Binding of RANKL (secreted from osteoblasts,
PDL cells, cementoblasts, etc.) to RANK on osteoclast precursor cell sur-
faces results in maturation/activation to functioning osteoclasts. Further,
![Page 34: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/34.jpg)
80 Foster et al.
it was reported that adding LRAP to cocultures of PDL/cementum cells and
bone marrow cells resulted in decreased RANKL expression. Boabaid et al.
additionally report that LRAP increased OPG and had a slight eVect (notstatistically significant) on decreasing RANKL expression in immortalized
cementoblasts, in vitro (Boabaid et al., 2004b).
The epithelial cell rests of Mallasez (ERM) that correspond with the
remnants of the root sheath within the periodontal ligament have been
known to express transcription factors and signaling molecules including
OPN, and BMP‐2 and 4 (Mouri et al., 2003; Rincon et al., 2005). These
findings have led some researchers to suggest that ERMs aVect periodontaltissues within the local environment (Table II). Additional evidence that
ERMs may have a role in repair of root tissues has been provided by
Hasegawa et al., who reported that in early stages of cementum repair and
adjacent to sites of root resorption, ERM cells express OPN, ameloblastin,
and BMP‐2, molecules associated with regulation of mesenchymal cell
behavior (Hasegawa et al., 2003).
While the potential role of epithelial products in root development
warrants further study, EmdogainÒ (Straumann Biologics, Waltham, MA,
USA), an epithelial protein derivative aimed at regenerating periodontal
tissues has been in use clinically for many years. EmdogainÒ, an extract of
porcine tooth germs, promotes periodontal regeneration with varied reports
of successful outcomes (Bartlett et al., 2006; Esposito et al., 2005; Giannobile
and Somerman, 2003; Heden and Wennstrom, 2006; Venezia et al., 2004).
In vitro studies have suggested that EmdogainÒ may preferentially promote
proliferation, matrix production, and diVerentiation/mineralization in PDL
fibroblasts (Gestrelius et al., 1997; Lyngstadaas et al., 2001), with increased
proliferation but varying influence on gene expression in dental follicle cells
(Hakki et al., 2001), osteoblasts, and putative cementoblasts (Tokiyasu
et al., 2000).
While the predominant protein in EmdogainÒ is amelogenin, several other
factors have been reported, including alternatively spliced and proteolytically
cleaved amelogenins, LRAP and TRAP respectively, as well as amelo-
blastin, TGF‐�, and BMPs (Kawase et al., 2001, 2002; Maycock et al.,
2002; Suzuki et al., 2005; Takayama et al., 2005). Both in vitro and in vivo
EmdogainÒ and the amelogenins have been confirmed to have bioactive
signaling properties (Bartlett et al., 2006; Boabaid et al., 2004b; Esposito
et al., 2005;Giannobile and Somerman, 2003; Swanson et al., 2006; Tompkins
and Veis, 2002; Tompkins et al., 2005; Veis, 2003; Venezia et al., 2004;
Viswanathan et al., 2003). Evidence for a signaling role for amelogenins has
been bolstered by identification on myoblast cells of an LRAP‐interactive cellsurface binding protein, lysosomal adhesion membrane protein‐1 (LAMP‐1)(Tompkins et al., 2006). Using a yeast two hybrid system, enamel matrix
proteins were also found to interact with a large number of secreted and
![Page 35: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/35.jpg)
3. Regeneration of the Periodontium 81
membrane proteins, notably LAMP‐3 (Wang et al., 2005a). Further studies of
these enamel matrix proteins will help to clarify the significance of these
putative receptors and interacting proteins, and subsequently elucidate their
signaling role, if any, in root/periodontal tissue formation.
B. Bone Morphogenetic Proteins
The importance of BMPs and BMP antagonists for contolling crown devel-
opment is well established (Bei et al., 2000; Ferguson et al., 1998; Iwata et al.,
2002; Kratochwil et al., 1996; Laurikkala et al., 2003; Maas and Bei, 1997;
Zhang et al., 2000). More recent studies have provided insights into the
interactions of various BMPs and their associated antagonists during bone
and crown development, for example, negative regulators ectodin and follis-
tatin. However, the significance of these interactions during root develop-
ment warrants further investigation (Kassai et al., 2005), especially with the
awareness that BMPs have very complex interactions in other tissues. For
example, BMP‐2, 4, 7 can induce ectopic bone formation individually, but
BMPs also form heterodimers, for example, BMP‐2/7, and 4/7, with greater
mineral stimulating ability than their constituents (Franceschi, 2005)
(Tables II and III).
As one approach to defining the role of BMPs in tooth development,
Plikus and colleagues evaluated the eVects of downregulating expression of
BMP signaling in oral and dental tissues by creating keratin 14‐Noggin
transgenic mice (Plikus et al., 2005). These mice developed a wide spectrum
of tooth phenotypes that included abnormal histogenesis and diVerentiationof ameloblasts and odontoblasts, a decrease in the number of teeth devel-
oped, reduction and/or alteration in size and shape of teeth, as well as
changes in the size and shape of roots. Root alterations included failure of
molar teeth to form multiple roots and lack of definition in the cementum‐enamel junction (CEJ) region. Using a similar strategy to better define the
role of gremlin, a BMP antagonist, in osteoblastic diVerentiation and func-
tion, Gazzero and colleagues generated mice with conditional gremlin over-
expression by employing an osteocalcin promoter driven—gremlin construct
(Gazzerro et al., 2005). Mice overexpressing gremlin exhibited an osteopenic
bone phenotype that included impaired bone formation, bone fractures,
disorganized collagen bundles at the endosteal cortical surface, a marked
decrease in osteoblast numbers, and reduced mineral apposition and bone
formation rates versus WT littermate controls. In addition, although not
detailed, incisor teeth were observed to be fragile in gremlin overexpressing
mice compared to WT controls.
BMP‐3 has emerged as a signaling factor that unlike other BMPs that
promote osteoblast/cementoblast, odontoblast diVerentiation, antagonizes
![Page 36: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/36.jpg)
Table III Factors Associated with Reported Root Phenotypes
Factor Cells/Tissues Function/Putative Function Models References
Periostin Preferentially expressed in
cells associated with bone,
lung, kidney, heart valve,
although found in many
other tissues, including
cancerous tissues. Also
expressed at high levels in
embryonic periosteum
Teeth: PDL region (restricted
to PDL after postnatal day
7 in mice)
Epithelia
Papillae cells
Odontoblasts
Follicle cells
Alveolar bone region
Periostin is a secreted 90‐kDa protein
with strong homology to the insect
cone guidance protein fasciclin I
family (includes �ig‐h3). Periostinis thought to be involved in cell
adhesion via �v�3 and �5 integrins,
although periostin does not contain
an RGD motif. Exact function for
this protein remains to be
established
Suggested general functions include:
(1) Induction of angiogenesis, (2)
Regulation of hard‐soft tissueinterfaces, (3) Regulation of
deposition and organization of
other ECM molecules, (4)
Protective function during stress/
mechanical load (e.g., in teeth:
chewing/tooth movement/ tooth
eruption), and (5) promotion of cell
migration and adhesion. Periostin
gene and protein are reported to be
induced by several factors including
BMP‐2, TGF‐�, PDGF, and
angiotensin II
In humans, five alternatively spliced
forms have been identified, but
functions not established
Periostin KO: exhibits a strong tooth/
periodontal phenotype: widening of
PDL; root resorption; increased
osteoclasts all suggestive of
aggressive periodontal disease.
Further, the incisors exhibit
compression of enamel and dentin
suggested to be related to a
proposed role for periostin in
controlling shear force/collagen
degradation
At birth, KO mice appear normal but
many die before weaning and those
that survive have growth
retardation. Trabecular bone is
decreased, although this phenotype
is not as dramatic as that of the
tooth. Partial correction of defects,
most notably incisor enamel, is
noted when the animals are given a
soft diet
Mice with periostin overexpression
exhibit cardiac dilation and
dysfunction
BMP‐4 KO mice have decreased levels
of periostin in mesenchymal tissues
and MSX2 KO mice have increased
evels of periostin
Gillan et al. (2002); Horiuchi
et al. (1999); Kii et al.
(2006) ; Kruzynska ‐ Frejtaget al. (2004); Li et al.
(2005a); Rios et al. (2005);
Suzuki et al. (2004); Wilde
et al. (2003)
![Page 37: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/37.jpg)
NF1‐C/CTF (nuclear
factor I‐C)NF1 protein family
of site‐specificDNA‐bindingproteins (also
known at CTF or
CAAT box
transcription
factor)
General: found in many
tissues during development
and in mature tissues as
well
Tooth associated include:
dental papilla region
Ameloblasts Odontoblasts/
preodontoblasts (strong
expression during root
formation)
Mesenchymal cells
Stellate reticulum region
PDL region
Bone region
HERS
Suggested function: in NF1‐C KO
mice, HERS cells fail to proliferate
and/or fail to induce odontoblast
diVerentiation required for root
formation. However, the specific
transcription factors and cell‐signaling pathways disrupted in
cells from NF1‐C KO mice remain
to be defined
NF1 protein family: functions both in
viral DNA replication and in the
regulation of gene expression
NF‐1C KO: defective root
development
Steele‐Perkins et al. (2003,2005)
Incisors:
Maxillary: KO mice fail to form roots
but enamel and dentin appear
normal
Mandible: more severely aVected;
histologically, disorganized tissues
occur in place of incisors
Molars: crowns form, but no root
development is seen. Jaw bones
seem normal although during
preparation of heads for histology,
the teeth fall out and sockets are
shallow with an organized mesh of
bony spicules vs. WT tissues with
deep sockets and intact teeth. Also,
mandibles appeared approx. 10%
smaller vs. WT, but maxillary size
diVerences were not reported
Gene expression:
Noted decreased expression (50%) of
tooth‐associated genes dentin
sialoprotein, ameloblastin,
amelogenin in mandibles of KO vs.
WT; but normal transcripts for �1
type I col and Nfia, b, x
(Continued )
![Page 38: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/38.jpg)
DMP‐1 (dentin
matrix protein‐1)Odontoblasts, osteoblasts,
osteocytes, hypertrophic
chondrocytes,
cementoblasts,
cementocytes, cementum
matrix, brain neurons,
other tissues (salivary
glands, muscle)
Role in mineral formation: dentin
matrix assembly and crystal growth
Multifunctional: attachment,
diVerentiation, activation of MMP‐9, role in osteocyte response to
mechanical stress
DMP‐1 KO:
Bone and cartilage: decrease in
mineral to matrix ratio, increase in
crystal size in bones, osteomalacia,
disordered canalicular network,
cartilage defect, chondrodysplasia‐like phenotype
Newborn DMP‐1 KO mice have
slightly expanded hypertrophic
zones and modest increase in bone
diameter vs. WT—DMP‐1 not
essential for early bone and tooth
development
Tooth: reduced dentin, increase in
predentin; reduced rate of dentin
formation; abnormal dentin
tubules; delay in/absent 3rd molars;
cementum—cementocytes not
healthy, but details on root defects
have not been reported
Overexpression of DMP‐1 in
pluripotent and mesenchymal cells
promotes an odontoblast
phenotype
Almushayt et al. (2006); Baba
et al. (2004b); Feng et al.
(2003); Foster et al. (2006) ;
Kalajzic et al. (2004); Ling
et al. (2005); Ye et al. (2005)
Table III Continued
Factor Cells/Tissues Function/Putative Function Models References
![Page 39: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/39.jpg)
BMP‐3 (bone
morphogenetic
protein 3)
Osteoblasts
Follicle cells
Cementoblasts
PDL cells
BMP‐2/4 are expressed in all tissues
involved in tooth formation, while
BMP‐3 expression is limited to the
follicle region and later in
development to the PDL region.
Existing data suggest that BMP‐3acts as an antagonist to BMP‐2/4.Based on this data it has been
suggested that BMP‐3 acts as a
regulator of soft–hard tissue
interfaces
BMP‐3 KO: increased bone mass,
however no specific tooth
phenotype has been reported
BMP‐3 overexpression: a 2006
abstract from Gagari et al. reported
decreased mass of dentin and
cementum, enlarged pulp
chambers, and widened PDL in
BMP‐3 transgenic mice vs. WT
littermate controls
Aberg et al. (1997); Chen et al.
(2004); Daluiski et al.
(2001); Gagari et al. (2006);
Gamer et al. (2005); Zhao
(2003)
BMPs/BMP
antagonists
All cells associated with tooth
development and
maturation
Known to have major roles in
controlling patterning and
diVerentiation of odontoblasts and
ameloblasts, BMPs have been
shown to enhance regeneration of
periodontal tissues (Nifusojeckii,
other reviews), but their specific
role during development of root/
periodontal tissues has not been
reported
Keratin14‐Noggin transgenic mice:
significant tooth phenotype with
alterations in number, size, shape,
and cell diVerentiation
Root specific: no mandibular molars,
maxillary molars—fail to form
multiple roots, poor or no CEJ,
small teeth with limited roots,
HERS forms, but proliferation of
cells in this region is limited
Osteocalcin‐gremlin transgenic mice:
General: impaired bone formation
and osteopenia
Tooth Phenotype: not explored in
depth but reported tooth fragility
Aberg et al. (1997); Gazzerro
et al. (2005) ; Nadiri et al.
(2004); Nifuji and Noda
(1999); Plikus et al. (2005);
Ripamonti (2005); Yanagita
(2005)
![Page 40: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/40.jpg)
86 Foster et al.
these BMPs and so inhibits cementoblast and osteoblast function
(Bahamonde and Lyons, 2001). Gagari et al. (to date in abstract form only)
have reported that collagen type 1 promoter‐driven BMP‐3 transgenic mice
exhibited a tooth phenotype that included decreased cementum and dentin
mass, enlarged pulp chambers, and a widened PDL region (Gagari et al.,
2006). In contrast, BMP‐3 null mice have been reported to have decreased
bone density, but a tooth phenotype was not reported (Daluiski et al., 2001).
Additionally, Gamer and colleagues found that BMP‐3 interferes with both
activin and BMP signaling by binding to AcTRIIB, the common type II
receptor for BMPs (Gamer et al., 2005). These data, coupled with previous
studies demonstrating high expression for BMP‐3 in the follicle/PDL region,
support a role for BMP‐3 in regulating hard–soft tissue interfaces in the
periodontium (Aberg et al., 1997; Ripamonti and Reddi, 1997; Takahashi
and Ikeda, 1996; Yamashiro et al., 2003). The existing evidence is strong that
BMPs and associated antagonists are critical for R/PT development and
future studies in this area may contribute to novel therapies for regenerating
tooth structures.
C. Periostin and Nuclear Factor I‐C/CAAT Box Transcription Factor
While many of the molecules described in this section play a role in miner-
alized tissue development, two molecules that may have a more specific
critical role in root/periodontal tissue development have been identified from
their respective KOmouse phenotypes, which were remarkable for the condi-
tion of their periodontia. These molecules include the extracellular matrix
protein, periostin, and the nuclear factor I‐C/CAAT box transcription factor,
NFI‐C/CTF.Periostin has been detected in many tissues (Nakamura et al., 2005), but
mice null for periostin exhibit a very specific tooth phenotype (Rios et al.,
2005) (Table III). Mice lacking the periostin gene appear relatively normal at
birth but develop a condition resembling an aggressive form of periodontal
disease by 3 months of age. The defect seems to be selective to the root/
periodontal region, with odontoblasts and dentin only mildly altered when
compared with the periodontal apparatus. Some enamel defects are obser-
ved, but are limited to incisors and suggested to be associated with increased
enamel stress due to a weakened PDL (Rios et al., 2005), or possibly due to
disruption of the shear zone important for continuously erupting teeth (Kii
et al., 2006). These data suggest the periostin may play a key role in protecting
the root surface from root resorption, as well as for maintaining a functional
periodontal ligament. The knowledge gained from continued studies directed at
defining the factors regulating periostin expression during root formation, and
the relationship between periostin and other root/PDL‐associated extracellular
![Page 41: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/41.jpg)
3. Regeneration of the Periodontium 87
matrix molecules should prove valuable long‐term for designing regenerative
therapies.
The nuclear factor I‐C/CAAT box transcription factor (NFI‐C/CTF) KO
mice have provided new insights into molecules that may be involved in
directly regulating root development. There are four genes encoding nuclear
factor I transcription‐replication proteins in mammals, NFI‐A, ‐B, ‐C, and ‐X.
Members of the NFI protein family of site‐specific DNA‐binding proteins
function both in viral DNA replication and in the regulation of gene expres-
sion. NFI‐C/CTF contains a prototypical proline‐rich transcription activation
domain and a heptamer repeat that is homologous to the C‐terminal domain
of RNA polymerase II (Gronostajski, 2000).
To elucidate the physiological roles for this family of nuclear transcription
factors, Gronostajaski’s laboratory has focused on disrupting their expres-
sion (Steele‐Perkins et al., 2003, 2005). Although NFI‐C is expressed in
many organ systems, including developing teeth, disruption of the Nfic gene
in mice resulted primarily in a unique tooth phenotype: molars lacking roots,
thin and brittle mandibular incisors, and weakened and abnormal maxillary
incisors. Molar crown development is normal and animals on a soft diet are
fertile and live as long as their littermates (Steele‐Perkins et al., 2003).
D. Regulators of Phosphate and Pyrophosphate Metabolism
1. Progressive Ankylosis Protein, Plasma Cell Membrane Glycoprotein 1,
and Tissue Nonspecific Alkaline Phosphatase
Regulators of phosphate metabolism have received considerable attention
within the last 5 years, with convincing evidence that inorganic phosphate
(Pi), beyond its known role as an important component of hydroxyapatite
mineralization, may also regulate cell behaviors and mineralization as a
signaling molecule. Conversely, pyrophosphate (PPi) functions as a well‐known and potent inhibitor of hydroxyapaptite formation. Table IV pro-
vides information on the role of Pi and PPi associated genes and their protein
products in regulating mineralized tissues. These include mouse progressive
ankylosis gene (ANK, as well as human homolog, ANKH), a putative
transporter of PPi from the intracellular compartment to the extracellular
space (Ho et al., 2000), the PPi‐generating nucleoside triphosphate pyropho-
sphohydrolase plasma cell membrane glycoprotein‐1 (PC‐1) (Goding et al.,
1998), and tissue nonspecific alkaline phosphatase (TNAP), an enzyme
proposed to cleave PPi substrate to its Pi constituents (Whyte et al., 1995).
Further information regarding the functions of these proteins is provided in
Table IV, including implications of their roles based on mouse models, for
example, mutation or KO. Results from studies to date suggest that local
![Page 42: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/42.jpg)
Table IV Regulators of Phosphate (Pi) and Pyrophosphate (PPi) Metabolism
Factor Cells Function/Putative Function Models References
PC ‐1: (NPP1—nucleotide
pyrophosphatase
phosphodiesterase ‐1)gene symbol: Enpp1
ANK: proteins of mouse
progressive ankylosis
(ank) gene. (ANKH,
human homologue) gene
symbol: Ank
Expressed by a diverse
group of cells including
osteoblasts, PDL cells,
odontoblasts, follicle
cells, and
cementoblasts, among
others
PC‐ 1: increases intra/extracellular and matrix
vesicle PPi ; inhibits apatite
deposition
ANK: transporter/
cotransporter of PPi from
intracellular to
extracellular matrix;
inhibits apatite deposition
PC ‐1/ANK mutations:
In animals/humans with mutations in
these genes, ectopic calcification and
decreased extracellular PPi occur. In
murine models with mutations in either
PC‐ 1 or Ank, there is a marked increase
(� 10‐ fold) in cementum formation
(appears to be cellular), while dentin,
enamel, PDL region, and surrounding
alveolar bone appear normal (Fong et al.,
2005; Nociti et al., 2002). There have been
no reports of a tooth phenotype in humans
Fedde et al. (1999); Fong
et al. (2005) ; Harmey et al.
(2004); Ho et al. (2000);
Johnson et al. (2003);
Nociti et al. (2002);
Nurnberg et al. (2001) ;
Okawa et al. (1998);
Reichenberger et al. (2001) ;
Rutsch et al. (2000); Rutsch
et al. (2001); Terkeltaub
(2001)
TNAP: (TNSALP, ALKP,
ALK) tissue nonspecific
alkaline phosphatase
gene symbol: Akp2
Expressed by a diverse
group of cells including
osteoblasts, PDL cells,
odontoblasts, follicle
cells, and
cementoblasts, among
others
A marker of cell (osteoblast/
cementoblast)
di V erentiation; catalytic
function in mineralization;
may transport ions across
membrane; hydrolyzes the
mineralization inhibitor,
PPi
TNAP mutations: Chapple (1993); Beertsen
et al. (1999); van den Bos
et al. (2005); Whyte, (2002)
In animals/humans there are a variety of
forms of TNAP‐associatedhypophosphatasia, which result in locally
increased levels of PPi and osteopenia. In
the area of the tooth root, cementum
deficiency severely compromises anchoring
of the PDL between bone and the tooth,
with severe periodontal disease and
eventually tooth loss
TNAP KO: appears to aVect cementum
formation selectively vs. dentin/enamel.
There is no or minimal cementum
formation and hence no PDL attachment
and teeth are exfoliated
![Page 43: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/43.jpg)
3. Regeneration of the Periodontium 89
control of PPi/Pi is critical for normal root/periodontal tissue development,
and further that cementum may be a uniquely sensitive tissue to PPi and Pi in
the local area. In cases of TNAP deficiency (TNAP mutation or KO, the
condition hypophosphatasia in humans), bones are osteopenic and root
cementum is disrupted, generally with a lack of acellular cementum and
severely disrupted cellular cementum (Beertsen et al., 1999; van den Bos
et al., 2005). Lack of cementum prevents insertion of PDL (Sharpey’s) fibers,
leading to lack of attachment and exfoliation of teeth. In contrast, humans
and animals with loss of function of PC‐1 or ANK exhibit low levels of PPi
in the local extracellular environment, resulting in ectopic calcifications in
joints, with mice exhibiting an arthritis‐like condition (Ho et al., 2000;
Terkeltaub, 2001). Humans withmutations in these genes also present pathol-
ogies resulting from deficient PPi, including craniometaphyseal dysplasia
(CMD) and idiopathic infantile arterial calcification (IIAC) (Nurnberg
et al., 2001; Reichenberger et al., 2001; Rutsch and Terkeltaub, 2005; Rutsch
et al., 2001). An unexpected and intriguing tooth phenotype has been reported
inmice withmutations in either PC‐1 or ANK. Rather than observing ectopic
calcification in the PDL, a marked increase in cementum formation was
observed, while PDL, dentin, and alveolar bone appeared unaVected (Nociti
et al., 2002). Ongoing studies are directed at examining the mechanical and
structural properties of all of these mineralized tissues, under situations in
mice, where PPi and/or Pi have been altered.
The importance of maintaining the appropriate concentration of Pi in the
extracellular environment for regulation of mineralization was highlighted
by the elegant studies of Murshed and colleagues (Murshed et al., 2005).
By studying a variety of KO mice, the critical nature of modulating extra-
cellular Pi concentration both for regulating physiological mineralization
and preventing pathological calcification was described. However, details
on R/PT regulation and development were not included, and require further
analysis using the animal models developed by this group. Studies focusing
on teeth and surrounding regions may provide insight into mechanisms
leading to altered cementum versus apparently normal enamel and dentin
phenotypes in cases of altered Pi/PPi homeostasis.
In addition to ANK, PC‐1, and TNAP, several other Pi‐regulating pro-
teins have been demonstrated to be important in controlling mineralization,
although tooth phenotypes in some cases have not yet been reported. These
are described in the following sections.
2. Phosphate‐Regulating Gene with Homology to Endopeptidase on the
X‐Chromosome
X‐linked hypophosphatemic rickets (XLH/HYP), the most common form of
rickets in humans, is caused by a mutation in the PHEX gene. The murine
![Page 44: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/44.jpg)
90 Foster et al.
homologue (Hyp mice) of the human disease is marked by renal phosphate
wasting, abnormal regulation of vitamin D metabolites, rickets, osteomala-
cia, growth retardation, resistance to vitamin D therapy, hypophosphate-
mia, and high levels of FGF‐23 and MEPE (Quarles, 2003; Rowe, 2004).
Additionally, cartilage abnormalities were reported in Hyp mice, resulting
potentially from participation of PHEX in regulation of growth plate carti-
lage (Miao et al., 2004). Dentin and bone hypomineralization in Hyp mice
may result from not only low serum Pi, but also some intrinsic osteoblast/
odontoblast defect (Ogawa et al., 2006). A tooth root phenotype has not
been reported.
3. Fibroblast Growth Factor 23
FGF‐23 regulates phosphate homeostasis, and FGF‐23 mutation in humans
causes autosomal dominant hypophosphatemic rickets (ADHR) (Rowe,
2004; White et al., 2006; Yu and White, 2005). FGF‐23 null mice exhibit
growth retardation, hyperphosphatemia, increased levels of 1, 25 vitamin D
levels, increased total‐body Bone Mineral content but decreased Bone
mineral density of limbs, and premature death by 13 weeks of age (Razzaque
et al., 2006; Sitara et al., 2004). Although a general increase in mineralization
resulted, there was also an accumulation of unmineralized osteoid associated
with limb deformities and excessivemineralization of soft tissues such as heart
and kidney. Crossing of FGF‐23 null with Hyp mice (PHEX mutation,
equivalent to X‐linked hypophosphatemia) resulted in a mouse phenotype
resembling the FGF‐23 null in skeletal phenotype and serum phosphate,
suggesting that FGF‐23 is upstream of PHEX (Sitara et al., 2004). There
has been some evidence for PHEX involvement in degradation of FGF‐23(Bowe et al., 2001), and results from the Hyp/FGF‐23 null mice are consistent
with the hypothesis that increased FGF‐23 in PHEX mutated mice and
humans may be responsible for the observed Pi disorder (Sitara et al., 2004).
4. Matrix Extracellular Phosphoglycoprotein
Matrix extracellular phosphoglycoprotein, a member of the SIBLING ex-
tracellular matrix protein family (Fisher and Fedarko, 2003) is expressed by
several mineralized tissue‐associated cells, including osteoblasts and osteo-
cytes, hypertrophic chondrocytes, dental pulp cells, and odontoblasts, as
well as other tissues (Argiro et al., 2001; Liu et al., 2005b; Lu et al., 2004;
MacDougall et al., 2002; Nampei et al., 2004; Rowe et al., 2000). Increased
expression of MEPE protein has been noted in humans with XLH and their
Hyp mouse counterparts (Argiro et al., 2001; Guo et al., 2002; Liu et al.,
2005c; Rowe, 2004). MEPE is thought to control renal phosphate excretion
and to modulate mineralization. MEPE contains an acidic serine aspartic
![Page 45: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/45.jpg)
3. Regeneration of the Periodontium 91
rich motif, ASARM, that is cleaved by cathepsin B, and PHEX inhibits this
activity (Rowe et al., 2004). One hypothesis is that the ASARM motif
inhibits crystal growth, with MEPE KO mice therefore exhibiting acceler-
ated mineralization and bone formation. Interestingly, it was reported that
dentonin, a fragment of MEPE isolated from erupted human molars pro-
moted the proliferation of dental pulp stem cells, in vitro, and it was specu-
lated that such molecules may have potential to participate in repair of lost
or damaged dentin (Liu et al., 2004).
E. Factors Known to Regulate Osteoprogenitor Cells and Osteoblasts
1. Wnt, Hedgehog, Osterix, and Nuclear Factor of Activated T Cells
Pluripotent mesenchymal stem cells (MSCs) have the potential to diVerenti-ate into several diVerent cell types, and specific transcription factors that
have been found to commit MSC diVerentiation to the osteoblast lineage
require further study for their potential role in root and periodontal tissue
development. Sequential expression of Indian hedgehog (Ihh) and canonical
Wnt signals at progressive stages of osteoblast development has been found
to coordinate the expression of transcription factors directing osteoblast
diVerentiation (Hu et al., 2005). For example, osterix (Osx) regulates down-
stream genes that commit MSCs to an osteoblast lineage, while NFAT was
found to cooperate with Osx to accelerate osteoblast diVerentiation and
bone formation (Koga et al., 2005; Tai et al., 2004, 2005). Further, the expres-
sion of p53 had been found to repress Osx expression, inhibiting osteoblast
diVerentiation and favoring osteoblast contributions to osteoclastogenesis
(Wang et al., 2006) (Table V).
2. Runt‐Related Transcription Factor 2 and TaVazin
Runt‐related transcription factor 2 (Runx2) expression is also necessary for
osteoblast diVerentiation and function and a role in developing tooth crown
has been identified (Aberg et al., 2004a,b; D’Souza et al., 1999). In osteo-
blasts, Runx2 directly stimulates transcription of osteoblast‐related genes
such as OCN, type I collagen, OPN, and collagenase type III (Ducy et al.,
1997; Franceschi et al., 2003; Kern et al., 2001). Canonical Wnt signaling
upregulates Runx2 expression (Gaur et al., 2005), and Runx2 subsequently
coordinates diverse signals involved in osteoblast diVerentiation and activity
(Franceschi et al., 2003). For example, the transcription factor, taVazin(TAZ), is an endogenous co‐activator of Runx2 in cells, and therefore an
endogenous regulator of osteoblast diVerentiation (Cui et al., 2003). Inter-
estingly, TAZ simultaneously represses gene transcription associated with
![Page 46: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/46.jpg)
92 Foster et al.
the adipocyte diVerentiation pathway (Hong et al., 2005). Stimuli that
promote bone formation via regulation of transcription have been found
to upregulate both TAZ and Runx2 (Hong et al., 2005). Runx2 expression
during tooth development also has several tooth‐specific downstream targets
(Gaikwad et al., 2001). The formation of successional teeth is inhibited by
Runx2 activity (Wang et al., 2005c), and during tooth morphogenesis Runx2
mediates FGF signaling between epithelium and mesenchyme (Aberg et al.,
2004b). Runx2 has also been identified in periodontal ligament cells; however,
its function appears to be suppressed, preventing diVerentiation of PDL cells
toward osteoblasts (Saito et al., 2002).
3. Activating Transcription Factor 4
Activating transcription factor 4 (ATF4) was identified as a factor for
osteoblast diVerentiation, and it is in turn the substrate for p90 ribosomal
S6 kinase 2 (RSK2), a growth factor‐regulated kinase (Yang et al., 2004).
A mutation in RSK2 was mapped as the cause for CoYn‐Lowry Syndrome
(CLS), which is associated with skeletal abnormalities in addition to mental
retardation. Mice null for ATF4 displayed a phenotype indicative of osteo-
blast defects, namely delayed bone formation in early development and low
bone mass. In addition to regulating type I collagen, ATF4 was found to act
cooperatively with Runx2 in regulating the OCN promoter in osteoblasts
(Xiao et al., 2005). No details on a tooth phenotype were provided.
4. Receptor Activator of NF‐kB Ligand and Osteoprotegerin
Receptor activator of NF‐�B ligand (RANKL) and osteoprotegerin (OPG)
have emerged as the primary factors in the axis of regulation of osteoclasts
and their precursors (Takahashi et al., 1999; Tsuda et al., 1997), and parallel
roles in tooth development and eruption have been described (Ohazama
et al., 2004a; Rani and MacDougall, 2000; Wise et al., 2002). Little is known
of the role of cementoblasts in osteoclast‐mediated turnover. Cementoblasts,
as well as cells of the nearby PDL and their precursors, express RANKL and
OPG (Boabaid et al., 2004a; Liu et al., 2005a; Sakata et al., 1999) and so may
be considered to take part in the regulation of osteoclastogenesis, though it is
currently not clear how these tissues participate in conditions of health and
disease. Cementum itself does not undergo significant physiological remo-
deling unlike the nearby alveolar bone that undergoes rapid turnover
throughout life. Additionally, though cementum resorption is not unknown,
it is relatively infrequent compared to osteoclast‐mediated resorption of
bone. Even in advanced periodontal disease, alveolar bone may be severely
eroded while cementum remains intact. This indirect evidence supports a pro-
tective role for cementum against biological resorption, a hypothesis supported
![Page 47: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/47.jpg)
3. Regeneration of the Periodontium 93
by results from in vitro (Boabaid et al., 2004a; Hatakeyama et al., 2006; Nociti
et al., 2004) and in vivo investigations (Hatakeyama et al., 2003). Further study
of the involvement of cementoblasts and PDL in osteoclastogenesis should
elucidate their role in osteoclastogenesis in the periodontium.
5. Dlx Transcription Factors
Members of the Dlx family of transcription factors, a subfamily of divergent
homeobox genes related to the Drosophila distal less (Dll) gene, have been
implicated as key regulators of tissue development and cell diVerentiation(Stock et al., 1996). Currently, six Dlx genes have been identified, in both
humans and mice, with convincing evidence that they play critical roles
in diVerentiation of bone‐forming cells. Dlx KO mice have profound cranio-
facial defects and absence of molars (Thomas et al., 1997). In humans,
tricho‐dento‐osseous (TDO) syndrome, characterized by enamel defects,
enlarged pulp chambers, and distorted roots (taurodontism), has been linked
to a mutation in Dlx3 (Price et al., 1998). Interestingly, in a very preliminary
study, Morsczeck reported that Dlx3 increased in dental follicle cells during
‘‘osteogenic’’ diVerentiation, in vitro.
F. Emerging and Other Factors to Consider
1. Proteoglycans
Another important group of molecules present in cementum and known to
play critical roles in tooth development are the proteoglycans (PGs). Proteo-
glycans are macromolecules composed of core proteins and glycosaminogly-
cans (GAGs). Small leucine rich proteoglycans (SLRPs) including decorin,
biglycan, lumican, osteoadherin/osteomodulin, and fibromodulin have been
suggested to play important roles in collagen‐linkedmineralization (Buchaille
et al., 2000; Couble et al., 2004; Embery et al., 2001; Iozzo, 1998). The im-
portance of PGs for appropriate crown development has been highlighted
(Goldberg et al., 2005) and indicates that further studies are warranted to
determine their respective roles during root formation. Several studies have
implicated SLRPs as being important for controlling mineralization of dental
tissues, and it was demonstrated that defective enamel and dentin formation
resulted from loss of biglycan and decorin in KOmice (Goldberg et al., 2005).
Although roles for PGs in root development have not been clarified, SLRPs
are apparently present in all mineralized tissues, and it can therefore be
safely asserted that there is some important, conserved function for this class
of PGs in mineralized tissues. Immunohistochemical studies have shown that
![Page 48: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/48.jpg)
94 Foster et al.
cementum in several species (rat, human, mouse) is immunoreactive to various
PGspecies (Yamamoto et al., 2004b). PGpresence and involvement in develop-
ment was further supported in a rat molar model by intense immunoreactivity
for chondroitin 4‐sulfate, chondroitin 6‐sulfate, and unsulfated chondroitin,
during early phases of a cellular cementogenesis. GAGs and PGs have been
detected inmature PDL, and taken together these data provide strong evidence
that PGs are important for regulating collagen fibril formation during cemen-
togenesis and in the mature periodontium (Buchaille et al., 2000; Hakkinen
et al., 1993, 2000; Kaneko et al., 2001; Matias et al., 2003).
2. Small Integrin‐Binding Ligand N‐Linked Glycoprotein Family
The SIBLING (Small Integrin‐Binding Ligand N‐Linked Glycoprotein)
family is composed of genes located on human chromosome 4 (mouse
chromosome 5) encoding noncollagenous extracellular matrix‐associatedproteins associated with bones and teeth. While sequence homology among
SIBLINGs is not high, their relatedness is suggested by common organiza-
tional features and similar, functionally important post‐translational mod-
ifications (Fisher and Fedarko, 2003; Huq et al., 2005; Qin et al., 2004).
Genes in this family include bone sialoprotein (BSP), osteopontin (OPN),
dentin matrix protein‐1 (DMP‐1), dentin sialophosphoprotein (DSPP), and
matrix extracellular phosphoglycoprotein (MEPE). The DSPP transcript is
processed and results in two proteins, namely dentin sialoprotein (DSP) and
dentin phosphoprotein (DPP). SIBLINGs have common sequences includ-
ing an arginine‐glycine‐aspartate (RGD) integrin‐binding domain that likely
functions in signaling and cell attachment (Ganss et al., 1999; Sodek et al.,
2000), and an ASARM or similar motif (in all except BSP) that may be a
mineral inhibitory domain. SIBLINGs typically undergo extensive post‐translational modification, including enzymatic cleavage (in DSPP and
DMP‐1), phosphorylation, glycosylation, and likely more complex proces-
sing such as polymerization, in vivo (Gericke et al., 2005; He et al., 2005b;
Kaartinen et al., 2005; Qin et al., 2004). Several lines of evidence support a
role for SIBLINGs in root development (Bosshardt, 2005; Diekwisch, 2001;
Saygin et al., 2000), including the timed and spatial expression of BSP and
OPN during development and repair of root/periodontal tissue (Bosshardt
et al., 1998; D’Errico et al., 1997; MacNeil et al., 1994; Shigeyama et al.,
1996), coupled with their suggested roles in nucleation and regulation of
crystal growth (Boskey et al., 2000; Gericke et al., 2005; He et al., 2005a;
Tartaix et al., 2004). However, mice null for OPN, BSP, or MEPE have not
been reported to exhibit a root/periodontal phenotype, suggestive of some
redundancy with other molecules. DMP‐1 and DSPP, originally identified in
dentin and thought to be specific for this tissue, have been shown to not only
be critical for dentin development, but also present in other mineralized
![Page 49: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/49.jpg)
Table V Factors Known to Regulate Osteoprogenitor cells and Osteoblasts (Role in Cementogenesis Unknown)
Factor Cells/Tissues Function/Putative Function Models References
Wnt: wingless int
Hh: hedgehog
Ihh: Indian
hedgehog
Mesenchymal stem cells
(MSCs), many others
Osteoblast diVerentiation; Hh and
Wnt signals control osteoblast
development in a sequential
manner
Ihh is expressed in prehypertrophic
and early hypertrophic
chondrocytes and signals to
immature chondrocytes and
perichondrial cells. Canonical Wnt
signaling is downstream of Ihh
signaling
During tooth development Hh and
Wnt signals are emitted from the
cap stage enamel knot and are
considered to have a role in crown
morphogenesis
Many participants in the Wnt pathway
have been associated with mineralized
tissues, in health and disease:
Dickkopfs (Dkks), secreted frizzled‐related proteins (sFRPs), Wnt
inhibitory factor 1 (Wif1), LDL
receptor protein 5 (LRP5), and Wnts
4, 10b, and others
Ihh KO:
Shows dysregulation of chondrocyte
maturation and absence of expression
of target genes for the Wnt canonical
pathway in the perichondrium
Lrp5 KO:
Lrp5 is a transmembrane protein that
forms part of the cell surface receptor
complex binding Wnt within the Wnt
canonical pathway. Lrp5 deficient
mice exhibit osteopenia with fewer
total osteoblasts per bone area, and a
50% reduction in bone formation
Canalis et al. (2005); Hu et al.
(2005) ; Kato et al. (2002);
Li et al. (2005b); Nusse
(2005); St ‐Jacques et al.(1999); Thesle V et al.
(2001); Vaes et al. (2005);
Westendorf et al. (2004)
Osx: osterix
NFAT: nuclear
factor of activated
T cells
MSCs, osteoblasts Osterix is a transcription factor
required for osteoblast
diVerentiation, operating
downstream of Runx2; NFAT
cooperates with Osx to accelerate
osteoblast diVerentiation and bone
formation
Osx null mice:
Homozygotes for the mutation are not
viable, and lack both endochondral
and intramembranous bone
formation; developing tooth germs
appear unaVected
NFAT KO:
Mice are embryonically lethal
Koga et al. (2005) ;
Nakashima et al. (2002);
Tai et al. (2005)
(Continued )
![Page 50: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/50.jpg)
x2: runt‐relatedanscription
ctor 2
In bone, MSCs; in
developing teeth: dental
mesenchyme, including
papilla, follicle cells,
and periodontal
ligament during pre‐eruptive tooth
development
Transcription factor necessary for
osteoblast diVerentiation; focal
point for integration of a variety of
signals aVecting osteoblast activity
Runx2 KO:
Functional osteoblasts, mineralized
bone, and hypertrophic cartilage are
absent
Tooth morphogenesis arrested in the
transition from the bud to cap stages
Aberg et al. (2004a,b) ;
Bronckers et al. (2001);
D’Souza et al. (1999);
Franceschi and Xiao
(2003); Franceschi et al.
(2003); Komori et al.
(1997); Otto et al. (1997)
Mediates epithelial‐mesenchymal
interactions during tooth
development
Z: TaVazin MSCs Acts as a transcriptional modulator
during osteoblast diVerentiation;
endogenous coactivator of Runx2;
promotes osteoblast formation,
inhibits adipocyte formation
No mouse models reported. In humans,
mutations in the TAZ gene are
responsible for Barth’s syndrome
(BTHS), X‐linked endocardial
fibroelastosis (EFE), X‐linked fatal
infantile dilated cardiomyopathy
(CMD3A), and familial isolated
noncompaction of left ventricular
myocardium (INVM)
Brady et al. (2006); Hong and
YaV e (2006); Hong et al.
(2005)
F4 activating
anscription
ctor 4
Osteoblasts ATF4 is a transcription factor that
regulates osteoblast diVerentiation
and function; cooperates with
Runx2 in stimulating osteoblast‐specific Ocn expression
ATF4 KO:
Delayed osteoblast diVerentiation
throughout the skeleton, and a
reduction in the area of mineralized
tissue visible in frontal and parietal
bones, clavicles and long bones; no
tooth phenotype reported
Xiao et al. (2005); Yang et al.
(2004)
le V Continued
tor Cells/Tissues Function/Putative Function Models References
Run
tr
fa
TA
AT
tr
fa
Tab
Fac
![Page 51: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/51.jpg)
RANKL:
Receptor activator
of NF‐�B ligand
RANKL:
Osteoblasts,
cementoblasts, PDL
cells, dental follicle
cells, and many others
(e.g., cells of immune
system)
In bone RANK/RANKL receptor/
ligand expression in osteoblasts
promotes osteoclastogenesis; OPG
operates as a decoy receptor for
the RANK receptor, and inhibits
osteoclastogenesis
RANKL KO:
Severe osteopetrosis and lack of
osteoclasts, absence of tooth eruption
Amizuka et al. (2003); Bucay
et al. (1998); Katagiri and
Takahashi (2002); Kong
et al. (1999); Ohazama
et al. (2004a); Yasuda et al.
(1998); Yao et al. (2004)
Osteoprotegerin OPG:
Osteoblasts, PDL cells,
dental follicle cells,
dental epithelial cells,
dental papilla cells, and
many others (e.g., cells
of immune system)
Expression in dental tissues may
coordinate bone and tooth
development
OPG KO:
Increased osteoclastic activity and
bone remodeling, severe bone loss,
destruction of growth plate cartilage
and increased vascular calcification
OPG overexpression:
Osteopetrosis with normal tooth
eruption
p53 MSCs Osteoblast/osteoclast diVerentiation
(negatively regulates osteoblast
diVerentiation/function by
repressing expression of Osx), p53
deficiency confers osteoblasts with
an increased ability to promote
osteoclastogenesis
p53 KO:
Largely viable with a small proportion
with defects in neural tube closure.
Early onset of tumors, such as
lymphomas and sarcomas. High bone
mass, increased Osx expression, more
rapid diVerentiation in osteoblasts,
increased tendency for osteoblasts to
promote osteoclastogenesis
Armstrong et al. (1995);
Attardi and Donehower
(2005); Sah et al. (1995);
Wang et al. (2006)
![Page 52: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/52.jpg)
98 Foster et al.
tissues and cells, including cementum (Baba et al., 2004a,b; Massa et al.,
2005; Qin et al., 2002, 2003). Cementum/root phenotypes have been indi-
cated in mice null for DMP‐1 and DSPP, but at present phenotypes have not
been fully reported (Sreenath et al., 2003; Ye et al., 2004). See Table III for
more details on the DMP‐1 deficient mouse model.
SIBLING family members also share a tendency for an astonishing degree
of multifunctionality, perhaps best embodied by the ubiquitous OPN.
In addition to well‐established roles as mineral regulators, SIBLINGs have
been identified as regulators of matrix metalloproteinase (MMP) function.
The MMPs are an extensive family of secreted or cell surface enzymes that
are critical in physiological development and remodeling of the extracellular
matrix, as well as certain pathologies. Several MMPs have been specifi-
cally associated with tooth development and remodeling (Apajalahti et al.,
2003; Bourd‐Boittin et al., 2005; Fanchon et al., 2004; Goldberg et al., 2003;
Maruya et al., 2003; Randall and Hall, 2002; Takahashi et al., 2003; Tsubota
et al., 2002), as well as periodontal and other oral diseases (Sorsa et al.,
2004). SIBLING proteins, BSP, OPN, and DMP‐1 were shown to specifically
activate MMP‐2, MMP‐3, and MMP‐9, respectively, even in the presence of
tissue inhibitors ofMMPs (TIMPs) (Fedarko et al., 2004). The significance of
SIBLING–MMP interaction in bone and tooth development is not yet clear,
but a model for localized interaction of matrix proteins and enzymes in tooth
development and eruption will surely be an important subject for further
study. An additional function for DMP‐1 as an intracellular transcriptional
regulator involved in diVerentiation has been proposed, indicating additional
mechanisms for SIBLING influence on tooth formation (Almushayt et al.,
2006; Narayanan et al., 2003).
3. Cementum Protein‐23
Cementum protein 23 was identified as a potential cementum marker by
screening a human cementum tumor cDNA library (Alvarez‐Perez et al.,
2006). However, cementum tumors are reported to exhibit a mixed cell type
so it is diYcult to determine the specificity of this protein/gene to cementum/
cementoblasts. Antibodies made to CP‐23 cross‐reacted with a cartilage type
collagen, type X collagen. CP23 has been identified in cementum, subpopu-
lations of cells in the PDL region, and specifically in PDL cells located
around blood vessels.
4. Betaig‐h3
Betaig‐h3 (�ig‐h3) is a collagen‐associated protein containing an RGD motif
that has been identified in several tissues and cells (Ohno et al., 2002). High
concentrations are found in cartilage and in the PDL region. A function
![Page 53: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/53.jpg)
3. Regeneration of the Periodontium 99
proposed is a negative regulator of osteogenesis, acting to maintain a struc-
tural balance between PDL and bone‐tooth interface. Further, there is
evidence for mechanical induction of �ig‐h3 and potential for this protein
to regulate chondrocyte diVerentiation via the TGF‐� pathway (Doi et al.,
2003; Ohno et al., 2005).
5. Brain‐Derived Neurotrophic Factor
BDNF, a member of the neurotrophin family, is considered to play a role in
survival and diVerentiation of central and peripheral neurons (Ebendal,
1992). In addition to being expressed in neural cells, BDNF is found in
many non‐neural cells/tissues including tooth germ, mature PDL, bone,
cartilage, heart, spleen, placenta, osteoblasts, immune cells, prostate, and
kidney (Nakanishi et al., 1994; Nosrat et al., 1998; Yamashiro et al., 2001).
Takeda et al., using a dog model of periodontal disease, reported that
BDNF promoted periodontal regeneration, that is, new bone, connective
tissue fibers, and new cementum (Takeda et al., 2005).
6. Bono 1
Bono 1 has been identified in bone cells, in secretory odontoblasts coexpressed
with DSPP, but not in pre‐secretory ameloblasts (where one does see DSPP)
and follicle cells (James et al., 2004). Bono 1 is associated with regions of
mineralization in bone, dentin and cementum, leading James et al. to propose
involvement in controlling mineral formation.
7. Connective Tissue Growth Factor
Connective tissue growth factor (36–38 kDa) (Asano et al., 2005; Shimo et al.,
2002; Yamaai et al., 2005) belongs to CCN family, that is, CTGF, CEF10,
and Nov. CTGF is found in several cells, including PDL cells, fibroblasts,
chondrocytes, dental mesenchyme cells, epithelial cells, vascular endothelial
cells of the enamel knot, pre‐ameloblasts and dental lamina (Friedrichsen
et al., 2003, 2005; Nakanishi et al., 2001). Studies to date indicated that
expression of CTGF is regulated by TGF‐�1/BMP‐2. Interestingly, CTGF
promotes expression of gene/proteins associated with PDL homeostasis, for
example, increased expression of type I collagen, periostin, andALP, but with
no eVect on OPN or OCN gene expression (Asano et al., 2005). Other studies
indicate that CTGF is involved in a chemotactic and mitogenic eVect in
fibroblast‐like cells in vitro, and further enhances cell proliferation andmatrix
synthesis in connective tissues linked towound healing (Lin et al., 2003, 2005).
KO animals have abnormal growth plates, while CTGF mutant mice have
impaired endochondral ossification (Ivkovic et al., 2003), though no tooth
![Page 54: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/54.jpg)
100 Foster et al.
phenotype has been reported. Other studies using tooth germ implants in vitro
with CTGF antibodies report severe inhibition of proliferation of both
epithelial and mesenchymal cells, and a delay in cytodiVerentiation of amelo-
blasts and odontoblasts (Shimo et al., 2002). Intriguingly, CTGF is not
expressed in Cbfa1‐null mice embryos (Yamaai et al., 2005). Based on these
studies, there is growing evidence that CTGF may have a significant role in
the development of mineralized tissues and in promotion of endochondral
ossification.
8. Ectodysplasin (Tabby/Downless)
Ectodysplasin is associated with ectodermal tissues (Pispa and ThesleV, 2003;Sharpe, 2001). The tabby gene (Ta) encodes the soluble tumor necrosis factor
(TNF) ligand ectodysplasin (Eda). Eda binds to the TNF receptor (EdaR),
encoded by the downless gene (dl), and this interaction leads to NF�Bactivation via the cytoplasmic death domain adapter, Edaradd, encoded by
the crinkled locus gene (Cr) (Courtney et al., 2005). Mice with a mutation in
Ta, dl, or Cr display an ectodermal dysplasia phenotype characterized by
abnormal development of ectoderm derived structures, including teeth
(Courtney et al., 2005; Drogemuller et al., 2001; Risnes et al., 2005; Tucker
et al., 2000). Additionally, studies manipulating levels of signaling molecules
in the Eda axis support a critical role for these signals in determining tooth
shape and cusp number (Kangas et al., 2004; Pispa et al., 2004). Altered
signals from the enamel knot have been demonstrated in mutant mice, and
the most dramatic defects are seen in molars with reduced size and abnormal
shape (including roots). Specific root/PDL targeted signals related to these
genes (Ta, dl or Cr) have not been identified.Humanswithmutations in EDA,
EDAR or EDARADD genes have hypohidroitic ectodermal dysplasia, with
more markedly aVected individuals exhibiting severe tooth deformities and
tooth loss (Courtney et al., 2005).
9. Osteocrin
Osteocrin was initially identified using a virus‐based signal‐trap proteomic
approach, and further characterized as a bone‐selective molecule (MoVattet al., 2002; Thomas et al., 2003). Data suggest expression of this molecule in
developing mineralized tissues, osteoblasts, osteocytes, hypertropic chon-
drocytes, and additionally the PDL region (Bord et al., 2005). Currently,
it is thought that osteocrin may play a role in regulating osteoblast matura-
tion, and subsequently mineral formation. The role of osteocrin in tooth
development, if any, is speculative and requires further investigation.
![Page 55: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/55.jpg)
3. Regeneration of the Periodontium 101
10. Matrix Gla Protein
Matrix gla protein (MGP), a mineral‐binding extracellular matrix protein,
was originally identified from bone matrix (Price et al., 1983). MGP expres-
sion has subsequently been reported in several types of cells and tissues,
including early in development in lung and limb buds and cells of chondro-
cytic lineage (Luo et al., 1995), and later in mineralized tooth‐associated areas
including dentin and the PDL/cementum region (Camarda et al., 1987; Hale
et al., 1988; Hashimoto et al., 2001).MGPwas also found in tissues producing
unmineralized matrices, cartilage and vascular smooth muscle. MGP has
been proposed to be a negative regulator of mineralized tissues (Mori et al.,
1998), and mice lacking MGP exhibit pathological calcification in arteries,
aortic valves, and cartilage (Luo et al., 1997). MGP expression in the period-
ontium may regulate hard–soft tissue interactions during tooth root develop-
ment, as well as in mature tissues, however, no tooth phenotype has been
found in MGP null mice. It is possible that MGP has a role in root/PDL
development, though its function may overlap that of other mineralization
regulators, for example OPN. Mice deficient in both MGP and OPN had
three times as much arterial calcification by age 4 weeks, and died earlier from
vascular rupture, supporting a shared role of MGP and OPN as inhibitors of
calcification in the vasculature (Speer et al., 2002). A tooth phenotype in
MGP and OPN null mice has not been reported, and it remains to be seen
whether they operate similarly in the periodontal region.
V. Conclusions and Future Directions
In recent years, rapid advances in technologies and molecular biology have
propelled all areas of biomedical research forward at an exciting pace. The
exquisitely regulated, sequential, reciprocal, reiterative cell signaling that
defines morphogenesis and diVerentiation during the development of a tooth
has been well characterized and reported (Jernvall and ThesleV, 2000; ThesleV,2003). Considered together, advances of the last 5–6 years, including increased
understanding of molecular signaling in tooth development, characterization
and employment of tooth stem cell populations, and recombination of cells and
tissues of specific developmental ages to generate tooth structures in vitro, have
truly shown the way to a bright future for tooth engineering.
With this progress in mind, it is now time to delve into how the tooth root
develops and explore the possibilities for regeneration of these tissues, for
the success of bioengineering a ‘‘whole tooth’’ depends on it. Characteriza-
tion of root and associated periodontal apparatus formation lags behind
that of crown: what cells are involved, what signaling drives morphogenesis
![Page 56: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/56.jpg)
Figure 3 Root/PDL formation: key regulators and attractive candidates to consider. While
mechanisms for tooth crown formation have been well established, cells and signals required for
periodontal and tooth root formation are only now beginning to unfold. Naturally occurring
mutations (mut) and knockout (KO) and gene overexpressing (O/E) animal models have driven
the identification of some key regulators, while elucidation of mechanisms controlling root and
periodontal formation is currently underway. During Late Bell stage, root formation is
initiated, preceded by the conversion of the cervical loop to the bilayer Hertwig’s epithelial root
sheath (HERS). A. With proper signaling, the healthy root with associated periodontal
apparatus forms, composed of root dentin (D), cementum (C), periodontal ligament (P), and
alveolar bone (B). B. Alterations in several genes/proteins are known to contribute to cementum
phenotypes, including regulators of Pi/PPi homeostasis (ank, PC‐1, TNAP) and BMP signal-
ing (BMP‐3). C. The development and maintenance of the periodontal ligament (PDL)
is dramatically altered as a result of BMP‐3 overexpression (O/E) and loss of periostin.
D. Alterations on the level of the whole root often lead to tooth loss, including phenotypes
marked by absence of roots (NF1c KO) and distorted roots (Msx2 and DMP‐1 KO, noggin
O/E). Text: Pi ¼ inorganic phosphate; PPi ¼ pyrophosphate; BMP ¼ bone morphogenetic
protein; FGF ¼ fibroblast growth factor; HERS ¼ Hertwig’s epithelial root sheath; NF1c ¼nuclear factor 1c; Shh ¼ sonic hedgehog; KO ¼ knockout; mut ¼ mutation; ank ¼ progressive
ankylosis protein; PC‐1 ¼ plasma cell membrane glycoprotein 1; TNAP ¼ tissue nonspecific
alkaline phosphatase; O/E ¼ overexpressing; PDL ¼ periodontal ligament; DMP‐1 ¼ dentin
matrix protein 1. Insets: IEE ¼ inner enamel epithelium; OEE ¼ outer enamel epithelium;
D ¼ dentin; C ¼ cementum; P ¼ periodontal ligament; B ¼ bone.
102 Foster et al.
![Page 57: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/57.jpg)
3. Regeneration of the Periodontium 103
and diVerentiation, how is the extracellular matrix synthesized, and how are
cells dynamically aVected by the local environment? This chapter attempted
to pose some of these questions and provide updates regarding current
knowledge on the roles of cells/factors/genes in regulating root and peri-
odontal tissue development, as well as directions for future research. While it
is clear that there is yet much to accomplish, some clues have begun to
emerge regarding tooth root formation. Animal models with periodontal
phenotypes have provided a starting point by indicating transcription fac-
tors, signals and signaling pathways, and matrix proteins that are important
in root formation. Attractive candidates required for controlling root/
periodontal tissue formation, homeostasis, and regeneration have been pre-
sented, and are summarized in Fig. 3. These last years have provided new
insights into the possible triggers for cementum/periodontal tissue develop-
ment and regeneration, and new directions for investigation that should
result ultimately in improved clinical approaches for regeneration of lost
periodontal tissues.
Acknowledgments
Support for this research was provided from grants DE05932 and DE15109 (MJS), and T32
DE0 7023–29 (HKF), from the National Institute of Dental and Craniofacial Research,
National Institutes of Health.
References
Aberg, T., Wozney, J., and ThesleV, I. (1997). Expression patterns of bone morphogenetic
proteins (Bmps) in the developing mouse tooth suggest roles in morphogenesis and cell
diVerentiation. Dev. Dyn. 210(4), 383–396.
Aberg, T., Cavender, A., Gaikwad, J. S., Bronckers, A. L., Wang, X., Waltimo‐Siren, J.,ThesleV, I., and D’Souza, R. N. (2004a). Phenotypic changes in dentition of Runx2 homo-
zygote‐null mutant mice. J. Histochem. Cytochem. 52(1), 131–139.
Aberg, T., Wang, X. P., Kim, J. H., Yamashiro, T., Bei, M., Rice, R., Ryoo, H. M., and
ThesleV, I. (2004b). Runx2 mediates FGF signaling from epithelium to mesenchyme during
tooth morphogenesis. Dev. Biol. 270(1), 76–93.
Abukawa, H., Papadaki, M., Abulikemu, M., Leaf, J., Vacanti, J. P., Kaban, L. B., and
Troulis, M. J. (2006). The engineering of craniofacial tissues in the laboratory: A review
of biomaterials for scaVolds and implant coatings. Dent. Clin. North Am. 50(2), 205–216, viii.
Akintoye, S. O., Lam, T., Shi, S., Brahim, J., Collins, M. T., and Robey, P. G. (2006). Skeletal
site‐specific characterization of orofacial and iliac crest human bone marrow stromal cells in
same individuals. Bone [Epub ahead of print].
Alatli‐Kut, I., Hultenby,K., andHammarstrom, L. (1994). Disturbances of cementum formation
induced by single injection of 1‐hydroxyethylidene‐1,1‐bisphosphonate (HEBP) in rats: Light
and scanning electron microscopic studies. Scand. J. Dent. Res. 102(5), 260–268.
Almushayt, A.,Narayanan,K., Zaki,A.E., andGeorge,A. (2006).Dentinmatrix protein 1 induces
cytodiVerentiation of dental pulp stem cells into odontoblasts. Gene Ther. 13(7), 611–620.
![Page 58: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/58.jpg)
104 Foster et al.
Alvarez‐Perez, M. A., Narayanan, S., Zeichner‐David, M., Rodriguez Carmona, B., and
Arzate, H. (2006). Molecular cloning, expression and immunolocalization of a novel human
cementum‐derived protein (CP‐23). Bone 38(3), 409–419.
Amizuka, N., Shimomura, J., Li, M., Seki, Y., Oda, K., Henderson, J. E., Mizuno, A., Ozawa,
H., and Maeda, T. (2003). Defective bone remodelling in osteoprotegerin‐deficient mice.
J. Electron Microsc. (Tokyo) 52(6), 503–513.
Apajalahti, S., Sorsa, T., and Ingman, T. (2003). Matrix metalloproteinase ‐2, ‐8, ‐9, and ‐13in gingival crevicular fluid of short root anomaly patients. Eur. J. Orthod. 25(4), 365–369.
Argiro, L., Desbarats, M., Glorieux, F. H., and Ecarot, B. (2001). Mepe, the gene encoding a
tumor‐secreted protein in oncogenic hypophosphatemic osteomalacia, is expressed in bone.
Genomics 74(3), 342–351.
Armstrong, J. F., Kaufman, M. H., Harrison, D. J., and Clarke, A. R. (1995). High‐frequencydevelopmental abnormalities in p53‐deficient mice. Curr. Biol. 5(8), 931–936.
Arzate, H., Olson, S. W., Page, R. C., and Narayanan, A. S. (1992). Isolation of human tumor
cells that produce cementum proteins in culture. Bone Miner. 18(1), 15–30.
Asano, M., Kubota, S., Nakanishi, T., Nishida, T., Yamaai, T., Yosimichi, G., Ohyama, K.,
Sugimoto, T., Murayama, Y., and Takigawa, M. (2005). EVect of connective tissue growth
factor (CCN2/CTGF) on proliferation and diVerentiation of mouse periodontal ligament‐derived cells. Cell Commun. Signal. 3(Epub), 1–11.
Attardi, L. D., and Donehower, L. A. (2005). Probing p53 biological functions through the use
of genetically engineered mouse models. Mutat. Res. 576(1–2), 4–21.
Baba, O., Qin, C., Brunn, J. C., Jones, J. E., Wygant, J. N., McIntyre, B. W., and Butler, W. T.
(2004a).Detectionof dentin sialoprotein in rat periodontium.Eur. J.Oral Sci. 112(2), 163–170.
Baba, O., Qin, C., Brunn, J. C., Wygant, J. N., McIntyre, B. W., and Butler, W. T. (2004b).
Colocalization of dentin matrix protein 1 and dentin sialoprotein at late stages of rat molar
development. Matrix Biol. 23(6), 371–379.
Bahamonde, M. E., and Lyons, K. M. (2001). BMP3: To be or not to be a BMP. J. Bone Joint
Surg. Am. 83‐A(Suppl. 1, Pt. 1), S56–S62.Bar‐Kana, I., Savion, N., Narayanan, A. S., and Pitaru, S. (1998). Cementum attachment
protein manifestation is restricted to the mineralized tissue forming cells of the period-
ontium. Eur. J. Oral Sci. 106(Suppl. 1), 357–364.
BarKana, I., Narayanan, A. S., Grosskop, A., Savion, N., and Pitaru, S. (2000). Cementum
attachment protein enriches putative cementoblastic populations on root surfaces in vitro.
J. Dent. Res. 79(7), 1482–1488.
Bartlett, J. D., Ryu, O. H., Xue, J., Simmer, J. P., and Margolis, H. C. (1998). Enamelysin
mRNA displays a developmentally defined pattern of expression and encodes a protein
which degrades amelogenin. Connect. Tissue Res. 39(1–3), 101–109; discussion 141–149.
Bartlett, J. D., Beniash, E., Lee, D. H., and Smith, C. E. (2004). Decreased mineral content in
MMP‐20 null mouse enamel is prominent during the maturation stage. J. Dent. Res. 83(12),
909–913.
Bartlett, J.D.,Ganss,B.,Goldberg,M.,Moradian‐Oldak, J., Paine,M.L., Snead,M.L.,Wen,X.,
White, S. N., and Zhou, Y. L. (2006). Protein‐protein interactions of the developing enamel
matrix. Curr. Top. Dev. Biol. 74, 57–115.
Bartold, P. M., McCulloch, C. A., Narayanan, A. S., and Pitaru, S. (2000). Tissue engineering:
A new paradigm for periodontal regeneration based on molecular and cell biology.
Periodontol. 2000 24, 253–269.
Bartold, P. M., Shi, S., and Gronthos, S. (2006a). Stem cells and periodontal regeneration.
Periodontol. 2000 40, 164–172.
Bartold, P.M.,Xiao,Y., Lyngstaadas, S. P., Paine,M. L., and Snead,M.L. (2006b). Principles and
applications of cell delivery systems for periodontal regeneration.Periodontol. 2000 41, 123–135.
![Page 59: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/59.jpg)
3. Regeneration of the Periodontium 105
Batouli, S., Miura, M., Brahim, J., Tsutsui, T. W., Fisher, L. W., Gronthos, S., Robey, P. G.,
and Shi, S. (2003). Comparison of stem‐cell‐mediated osteogenesis and dentinogenesis.
J. Dent. Res. 82(12), 976–981.
Beertsen, W., VandenBos, T., and Everts, V. (1999). Root development in mice lacking
functional tissue non‐specific alkaline phosphatase gene: Inhibition of acellular cementum
formation [In Process Citation]. J. Dent. Res. 78(6), 1221–1229.
Bei, M., Kratochwil, K., and Maas, R. L. (2000). BMP4 rescues a non‐cell‐autonomous
function of Msx1 in tooth development. Development 127(21), 4711–4718.
Beniash, E., Simmer, J. P., and Margolis, H. C. (2005). The eVect of recombinant mouse
amelogenins on the formation and organization of hydroxyapatite crystals in vitro. J. Struct.
Biol. 149(2), 182–190.
Berry, J. E., Zhao, M., Jin, Q., Foster, B. L., Viswanathan, H., and Somerman, M. J.
(2003). Exploring the origins of cementoblasts and their trigger factors. Connect. Tissue
Res. 44 (Suppl. 1), 97–102.
Boabaid, F., Berry, J. E., Koh, A. J., Somerman, M. J., and McCcauley, L. K. (2004a). The role
of parathyroid hormone‐related protein in the regulation of osteoclastogenesis by
cementoblasts. J. Periodontol. 75(9), 1247–1254.
Boabaid, F., Gibson, C. W., Kuehl, M. A., Berry, J. E., Snead, M. L., Nociti, F. H.,
Katchburian, E., and Somerman, M. J. (2004b). Leucine‐rich amelogenin peptide:
A candidate signaling molecule during cementogenesis. J. Periodontol. 75(8), 1126–1136.
Bord, S., Ireland,D.C.,MoVatt, P., Thomas,G. P., andCompston, J. E. (2005). Characterization
of osteocrin expression in human bone. J. Histochem. Cytochem. 53(10), 1181–1187.
Boskey, A., Spevak, L., Tan, M., Doty, S. B., and Butler, W. T. (2000). Dentin sialoprotein
(DSP) has limited eVects on in vitro apatite formation and growth. Calcif. Tissue Int. 67(6),
472–478.
Bosshardt, D. D. (2005). Are cementoblasts a subpopulation of osteoblasts or a unique phenotype?
J. Dent. Res. 84(5), 390–406.
Bosshardt, D. D., and Nanci, A. (1997). Immunodetection of enamel‐ and cementum‐related(bone) proteins at the enamel‐free area and cervical portion of the tooth in rat molars.
J. Bone Miner. Res. 12(3), 367–379.
Bosshardt, D.D., andNanci, A. (1998). Immunolocalization of epithelial andmesenchymalmatrix
constituents in association with inner enamel epithelial cells. J. Histochem. Cytochem. 46(2),
135–142.
Bosshardt, D. D., and Nanci, A. (2004). Hertwig’s epithelial root sheath, enamel matrix proteins,
and initiation of cementogenesis in porcine teeth. J. Clin. Periodontol. 31(3), 184–192.
Bosshardt, D. D., and Schroeder, H. E. (1996). Cementogenesis reviewed: A comparison
between human premolars and rodent molars. Anat. Rec. 245(2), 267–292.
Bosshardt, D. D., and Selvig, K. A. (1997). Dental cementum: The dynamic tissue covering of
the root. Periodontol. 2000 13, 41–75.
Bosshardt, D. D., Zalzal, S., McKee, M. D., and Nanci, A. (1998). Developmental appearance
and distribution of bone sialoprotein and osteopontin in human and rat cementum. Anat.
Rec. 250, 13–33.
Bourd‐Boittin, K., Fridman, R., Fanchon, S., Septier, D., Goldberg,M., andMenashi, S. (2005).
Matrix metalloproteinase inhibition impairs the processing, formation and mineralization of
dental tissues during mouse molar development. Exp. Cell Res. 304(2), 493–505.
Bowe, A. E., Finnegan, R., Jan de Beur, S. M., Cho, J., Levine, M. A., Kumar, R., and Schiavi,
S. C. (2001). FGF‐23 inhibits renal tubular phosphate transport and is a PHEX substrate.
Biochem. Biophys. Res. Commun. 284(4), 977–981.
Brady, A. N., Shehata, B. M., and Fernhoff, P. M. (2006). X‐linked fetal cardiomyopathy
caused by a novel mutation in the TAZ gene. Prenat Diagn. 26(5), 462–465.
![Page 60: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/60.jpg)
106 Foster et al.
Bronckers, A. L., Engelse, M. A., Cavender, A., Gaikwad, J., and D’Souza, R. N. (2001). Cell‐specific patterns of Cbfa1 mRNA and protein expression in postnatal murine dental tissues.
Mech. Dev. 101(1–2), 255–258.
Bucay, N., Sarosi, I., Dunstan, C. R., Morony, S., Tarpley, J., Capparelli, C., Scully, S., Tan,
H. L., Xu, W., Lacey, D. L., Boyle, W. J., and Simonet, W. S. (1998). Osteoprotegerin‐deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 12(9),
1260–1268.
Buchaille, R., Couble, M. L., Magloire, H., and Bleicher, F. (2000). Expression of the small
leucine‐rich proteoglycan osteoadherin/osteomodulin in human dental pulp and developing
rat teeth. Bone 27(2), 265–270.
Camarda, A. J., Butler, W. T., Finkelman, R. D., and Nanci, A. (1987). Immunocytochemical
localization of gamma‐carboxyglutamic acid‐containing proteins (osteocalcin) in rat bone
and dentin. Calcif. Tissue Int. 40(6), 349–355.
Canalis, E., Deregowski, V., Pereira, R. C., and Gazzerro, E. (2005). Signals that determine the
fate of osteoblastic cells. J. Endocrinol. Invest. 28(Suppl. 8), 3–7.
Caterina, J. J., Skobe, Z., Shi, J., Ding, Y., Simmer, J. P., Birkedal‐Hansen, H., and Bartlett,
J. D. (2002). Enamelysin (matrix metalloproteinase 20)‐deficient mice display an amelogen-
esis imperfecta phenotype. J. Biol. Chem. 277(51), 49598–49604.
Chai, Y., and Slavkin, H. C. (2003). Prospects for tooth regeneration in the 21st century:
A perspective. Microsc. Res. Tech. 60(5), 469–479.
Chai, Y., Jiang, X., Ito, Y., Bringas, P., Jr., Han, J., Rowitch, D. H., Soriano, P., McMahon,
A. P., and Sucov, H. M. (2000). Fate of the mammalian cranial neural crest during tooth and
mandibular morphogenesis. Development 127(8), 1671–1679.
Chapple, I. L. (1993). Hypophosphatasia: Dental aspects and mode of inheritance. J. Clin.
Periodontol. 20(9), 615–622.
Chen, E., Yuan, Z. A., Wright, J. T., Hong, S. P., Li, Y., Collier, P. M., Hall, B., D’Angelo, M.,
Decker, S., Piddington, R., Abrams, W. R., Kulkarni, A. B., et al. (2003). The small bovine
amelogeninLRAP fails to rescue the amelogeninnull phenotype.Calcif. Tissue Int.73(5), 487–495.
Chen, D., Zhao, M., and Mundy, G. R. (2004). Bone morphogenetic proteins. Growth Factors
22(4), 233–241.
Cho, M. I., and Garant, P. R. (1988). Ultrastructural evidence of directed cell migration during
initial cementoblast diVerentiation in root formation. J. Periodontal. Res. 23(4), 268–276.
Cho, M. I., and Garant, P. R. (2000). Development and general structure of the periodontium.
Periodontol. 2000 24, 9–27.
Couble, M. L., Bleicher, F., Farges, J. C., Peyrol, S., Lucchini, M., Magloire, H., and Staquet,
M. J. (2004). Immunodetection of osteoadherin in murine tooth extracellular matrices.
Histochem. Cell Biol. 121(1), 47–53.
Courtney, J. M., Blackburn, J., and Sharpe, P. T. (2005). The Ectodysplasin and NFkappaB
signalling pathways in odontogenesis. Arch. Oral Biol. 50(2), 159–163.
Cui, C. B., Cooper, L. F., Yang,X.,Karsenty,G., andAukhil, I. (2003). Transcriptional coactivation
of bone‐specific transcription factor Cbfa1 by TAZ.Mol. Cell. Biol. 23(3), 1004–1013.
Daluiski, A., Engstrand, T., Bahamonde, M. E., Gamer, L. W., Agius, E., Stevenson, S. L.,
Cox, K., Rosen, V., and Lyons, K. M. (2001). Bone morphogenetic protein‐3 is a negative
regulator of bone density. Nat. Genet. 27(1), 84–88.
D’Errico, J. A., MacNeil, R. L., Takata, T., Berry, J., Strayhorn, C., and Somerman, M. J.
(1997). Expression of bone associated markers by tooth root lining cells, in situ and in vitro.
Bone 20(2), 117–126.
D’Errico, J. A., Berry, J. E., Ouyang, H., Strayhorn, C. L., Windle, J. J., and Somerman, M. J.
(2000). Employing a transgenic animal model to obtain cementoblasts in vitro. J. Periodontol.
71(1), 63–72.
Deutsch, D., Palmon, A., Dafni, L., Mao, Z., Leytin, V., Young, M., and Fisher, L. W. (1998).
Tuftelin–aspects of protein and gene structure. Eur. J. Oral Sci. 106(Suppl. 1), 315–323.
![Page 61: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/61.jpg)
3. Regeneration of the Periodontium 107
Deutsch, D., Leiser, Y., Shay, B., Fermon, E., Taylor, A., Rosenfeld, E., Dafni, L., Charuvi, K.,
Cohen, Y., Haze, A., Fuks, A., and Mao, Z. (2002). The human tuftelin gene
and the expression of tuftelin in mineralizing and nonmineralizing tissues. Connect. Tissue
Res. 43(2–3), 425–434.
Diekwisch, T.G. (2001).The developmental biology of cementum. Int. J.Dev. Biol. 45(5–6), 695–706.
Diekwisch, T. G. (2002). Pathways and fate of migratory cells during late tooth organogenesis.
Connect. Tissue Res. 43(2–3), 245–256.
Doi, T., Ohno, S., Tanimoto, K., Honda, K., Tanaka, N., Ohno‐Nakahara, M., Yoneno, K.,
Suzuki, A., Nakatani, Y., Ueki, M., and Tanne, K. (2003). Mechanical stimuli enhances the
expression of RGD‐CAP/betaig‐h3 in the periodontal ligament.Arch. Oral Biol. 48(8), 573–579.
Drogemuller, C., Distl, O., and Leeb, T. (2001). Partial deletion of the bovine ED1 gene causes
anhidrotic ectodermal dysplasia in cattle. Genome Res. 11(10), 1699–1705.
D’Souza, R. N., Aberg, T., Gaikwad, J., Cavender, A., Owen, M., Karsenty, G., and ThesleV, I.
(1999). Cbfa1 is required for epithelial‐mesenchymal interactions regulating tooth
development in mice. Development 126(13), 2911–2920.
Duailibi, M. T., Duailibi, S. E., Young, C. S., Bartlett, J. D., Vacanti, J. P., and Yelick, P. C.
(2004). Bioengineered teeth from cultured rat tooth bud cells. J. Dent. Res. 83(7), 523–528.
Ducy, P., Zhang, R., GeoVroy, V., Ridall, A. L., and Karsenty, G. (1997). Osf2/Cbfa1:
A transcriptional activator of osteoblast diVerentiation. Cell 89(5), 747–754.
Ebendal, T. (1992). Function and evolution in the NGF family and its receptors. J. Neurosci.
Res. 32(4), 461–470.
Embery, G., Hall, R., Waddington, R., Septier, D., and Goldberg, M. (2001). Proteoglycans in
dentinogenesis. Crit. Rev. Oral Biol. Med. 12(4), 331–349.
Esposito, M., Grusovin, M. G., Coulthard, P., and Worthington, H. V. (2005). Enamel matrix
derivative (Emdogain) for periodontal tissue regeneration in intrabony defects. Cochrane
Database Syst. Rev. (4): CD003875.
Fanchon, S., Bourd, K., Septier, D., Everts, V., Beertsen, W., Menashi, S., and Goldberg, M.
(2004). Involvement of matrix metalloproteinases in the onset of dentin mineralization. Eur.
J. Oral Sci. 112(2), 171–176.
Fedarko, N. S., Jain, A., Karadag, A., and Fisher, L. W. (2004). Three small integrin binding
ligand N‐linked glycoproteins (SIBLINGs) bind and activate specific matrix metalloprotei-
nases. FASEB J. 18(6), 734–736.
Fedde, K. N., Blair, L., Silverstein, J., Coburn, S. P., Ryan, L. M., Weinstein, R. S., Waymire,
K., Narisawa, S., Millan, J. L., MacGregor, G. R., and Whyte, M. P. (1999). Alkaline
phosphatase knock‐out mice recapitulate the metabolic and skeletal defects of infantile
hypophosphatasia. J. Bone Miner. Res. 14(12), 2015–2620.
Feng, J.Q.,Huang,H., Lu,Y.,Ye, L.,Xie,Y., Tsutsui, T.W.,Kunieda, T., Castranio,T., Scott,G.,
Bonewald, L. B., and Mishina, Y. (2003). The Dentin matrix protein 1 (Dmp1) is specifically
expressed inmineralized, but not soft, tissues during development. J. Dent. Res. 82(10), 776–780.
Ferguson, C. M., Miclau, T., Hu, D., Alpern, E., and Helms, J. A. (1998). Common molecular
pathways in skeletal morphogenesis and repair. Ann. N. Y. Acad. Sci. 857, 33–42.
Fisher, L.W., andFedarko,N. S. (2003). Six genes expressed in bones and teeth encode the current
members of the SIBLING family of proteins. Connect. Tissue Res. 44(Suppl. 1), 33–40.
Fong, C. D., and Hammarstrom, L. (2000). Expression of amelin and amelogenin in epithelial
root sheath remnants of fully formed rat molars. Oral Surg. Oral Med. Oral Pathol. Oral
Radiol. Endod. 90(2), 218–223.
Fong, H. K., Foster, B. L., Popowics, T. E., and Somerman, M. J. (2005). The crowning
achievement: Getting to the root of the problem. J. Dent. Educ. 69(5), 555–570.
Foster, B. L., Nociti, F. H., Jr., Swanson, E. C., Matsa‐Dunn, D., Berry, J. E., Cupp, C. J.,
Zhang, P., and Somerman, M. J. (2006). Regulation of cementoblast gene expression by
inorganic phosphate, in vitro. Calcif. Tissue Int. 78, 103–112.
![Page 62: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/62.jpg)
108 Foster et al.
Franceschi, R. T. (2005). Biological approaches to bone regeneration by gene therapy. J. Dent.
Res. 84(12), 1093–1103.
Franceschi, R. T., and Xiao, G. (2003). Regulation of the osteoblast‐specific transcription factor,
Runx2:Responsiveness tomultiple signal transduction pathways. J. Cell Biochem. 88(3), 446–454.
Franceschi, R. T., Xiao, G., Jiang, D., Gopalakrishnan, R., Yang, S., and Reith, E. (2003).
Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate
osteoblast diVerentiation. Connect. Tissue Res. 44(Suppl. 1), 109–116.
Friedrichsen, S., Heuer,H., Christ, S.,Winckler,M., Brauer,D., Bauer, K., andRaivich,G. (2003).
CTGF expression during mouse embryonic development. Cell Tissue Res. 312(2), 175–188.
Friedrichsen, S., Heuer, H., Christ, S., Cuthill, D., Bauer, K., and Raivich, G. (2005). Gene
expression of connective tissue growth factor in adult mouse. Growth Factors 23(1), 43–53.
Fuchs, E., and Segre, J. A. (2000). Stem cells: A new lease on life. Cell 100(1), 143–155.
Fujiwara, N., Tabata, M. J., Endoh, M., Ishizeki, K., and Nawa, T. (2005). Insulin‐like growthfactor‐I stimulates cell proliferation in the outer layer of Hertwig’s epithelial root sheath and
elongation of the tooth root in mouse molars in vitro. Cell Tissue Res. 320(1), 69–75.
Fukae, M., Tanabe, T., Uchida, T., Lee, S. K., Ryu, O. H., Murakami, C., Wakida, K.,
Simmer, J. P., Yamada, Y., and Bartlett, J. D. (1998). Enamelysin (matrix metalloproteinase‐20): Localization in the developing tooth and eVects of pH and calcium on amelogenin
hydrolysis. J. Dent. Res. 77(8), 1580–1588.
Fukae, M., Tanabe, T., Yamakoshi, Y., Yamada, M., Ujiie, Y., and Oida, S. (2001).
Immunoblot detection and expression of enamel proteins at the apical portion of the forming
root in porcine permanent incisor tooth germs. J. Bone Miner. Metab. 19(4), 236–243.
Fukumoto, S., Kiba, T., Hall, B., Iehara, N., Nakamura, T., Longenecker, G., Krebsbach, P. H.,
Nanci, A., Kulkarni, A. B., and Yamada, Y. (2004). Ameloblastin is a cell adhesion molecule
required for maintaining the diVerentiation state of ameloblasts. J. Cell Biol. 167(5), 973–983.
Fukumoto, S., Yamada, A., Nonaka, K., and Yamada, Y. (2005). Essential roles of ameloblastin
in maintaining ameloblast diVerentiation and enamel formation. Cells Tissues Organs 181
(3–4), 189–195.
Gagari, E., Gamer, L. W., and Rosen, V. (2006). BMP‐3 overexpression results in dentin and
periodontal ligament defects. ADEA/AADR/CADR Meeting & Exhibition, Orlando.
Gaikwad, J. S., Cavender, A., and D’Souza, R. N. (2001). Identification of tooth‐specificdownstream targets of Runx2. Gene 279(1), 91–97.
Gamer, L. W., Nove, J., Levin, M., and Rosen, V. (2005). BMP‐3 is a novel inhibitor of both
activin and BMP‐4 signaling in Xenopus embryos. Dev. Biol. 285(1), 156–168.
Ganss, B., Kim, R. H., and Sodek, J. (1999). Bone sialoprotein. Crit. Rev. Oral Biol. Med. 10,
79–98.
Gaur, T., Lengner, C. J., Hovhannisyan, H., Bhat, R. A., Bodine, P. V., Komm, B. S., Javed, A.,
van Wijnen, A. J., Stein, J. L., Stein, G. S., and Lian, J. B. (2005). Canonical WNT signaling
promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 280(39),
33132–33140.
Gazzerro, E., Pereira, R. C., Jorgetti, V., Olson, S., Economides, A. N., and Canalis, E. (2005).
Skeletal overexpression of gremlin impairs bone formation and causes osteopenia.
Endocrinology 146(2), 655–665.
Gericke, A., Qin, C., Spevak, L., Fujimoto, Y., Butler, W. T., Sorensen, E. S., and Boskey,
A. L. (2005). Importance of phosphorylation for osteopontin regulation of biomineraliza-
tion. Calcif. Tissue Int. 77(1), 45–54.
Gestrelius, S., Andersson, C., Lidstrom, D., Hammarstrom, L., and Somerman, M. (1997).
In vitro studies on periodontal ligament cells and enamelmatrix derivative. J. Clin. Periodontol.
24(9 Pt. 2), 685–692.
Gestrelius, S., Lyngstadaas, S. P., and Hammarstrom, L. (2000). Emdogain–periodontal
regeneration based on biomimicry. Clin. Oral Investig. 4(2), 120–125.
![Page 63: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/63.jpg)
3. Regeneration of the Periodontium 109
Giannobile, W. V., and Somerman, M. J. (2003). Growth and amelogenin‐like factors in
periodontal wound healing. A systematic review. Ann. Periodontol. 8(1), 193–204.
Gibson, C. W., Yuan, Z. A., Hall, B., Longenecker, G., Chen, E., Thyagarajan, T., Sreenath, T.,
Wright, J. T., Decker, S., Piddington, R., Harrison, G., and Kulkarni, A. B. (2001a).
Amelogenin deficient mice display an amelogenesis imperfecta phenotype. J. Biol. Chem. 276,
31871–31875.
Gibson, C. W., Yuan, Z. A., Hall, B., Longenecker, G., Chen, E., Thyagarajan, T., Sreenath, T.,
Wright, J. T., and Kulkarni, A. B. (2001b). Targeted disruption of the amelogenin gene results
in abnormal enamel. J. Dent. Res. 80, 143.
Gibson, C. W., Kulkarni, A. B., and Wright, J. T. (2005). The use of animal models to explore
amelogenin variants in amelogenesis imperfecta. Cells Tissues Organs 181(3–4), 196–201.
Gillan, L., Matei, D., Fishman, D. A., Gerbin, C. S., Karlan, B. Y., and Chang, D. D. (2002).
Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and
alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 62(18), 5358–5364.
Glasstone, S. (1965). The development of tooth germs in tissue culture. In ‘‘Cells and Tissues in
Culture, Methods, Biology and Physiology’’ (E. N. Willmer, Ed.), pp. 273–283. Academic
Press, London.
Goding, J. W., Terkeltaub, R., Maurice, M., Deterre, P., Sali, A., and Belli, S. I. (1998). Ecto‐phosphodiesterase/pyrophosphatase of lymphocytes and non‐lymphoid cells: Structure and
function of the PC‐1 family. Immunol. Rev. 161, 11–26.
Goldberg, M., and Smith, A. (2004). Cells and extracellular matrices of dentin and pulp:
A biological basis for repair and tissue engineering. Crit. Rev. Oral Biol. Med. 15(1), 13–27.
Goldberg, M., Septier, D., Rapoport, O., Young, M., and Ameye, L. (2002). Biglycan is a
repressor of amelogenin expression and enamel formation: An emerging hypothesis. J. Dent.
Res. 81(8), 520–524.
Goldberg, M., Septier, D., Bourd, K., Hall, R., George, A., Goldberg, H., and Menashi, S.
(2003). Immunohistochemical localization of MMP‐2, MMP‐9, TIMP‐1, and TIMP‐2 in the
forming rat incisor. Connect. Tissue Res. 44(3–4), 143–153.
Goldberg, M., Septier, D., Rapoport, O., Iozzo, R. V., Young, M. F., and Ameye, L. G. (2005).
Targeted disruption of two small leucine‐rich proteoglycans, biglycan and decorin, excerpts
divergent eVects on enamel and dentin formation. Calcif. Tissue Int. 77(5), 297–310.
Gould, T. R., Melcher, A. H., and Brunette, D. M. (1980). Migration and division of
progenitor cell populations in periodontal ligament after wounding. J. Periodontal. Res.
15(1), 20–42.
Gronostajski, R. M. (2000). Roles of the NFI/CTF gene family in transcription and
development. Gene 249(1–2), 31–45.
Gronthos, S., Mankani, M., Brahim, J., Robey, P. G., and Shi, S. (2000). Postnatal human
dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 97(25),
13625–13630.
Grzesik, W. J., and Narayanan, A. S. (2002). Cementum and periodontal wound healing and
regeneration. Crit. Rev. Oral Biol. Med. 13(6), 474–484.
Grzesik, W. J., Kuzentsov, S. A., Uzawa, K., Mankani, M., Robey, P. G., and Yamauchi, M.
(1998). Normal human cementum‐derived cells: Isolation, clonal expansion, and in vitro and
in vivo characterization. J. Bone Miner. Res. 13(10), 1547–1554.
Guo, R., Rowe, P. S., Liu, S., Simpson, L. G., Xiao, Z. S., and Darryl Quarles, L. D. (2002).
Inhibition of MEPE cleavage by Phex. Biochem. Biophys. Res. Commun. 297(1), 38–45.
Habelitz, S., Kullar, A., Marshall, S. J., DenBesten, P. K., Balooch, M., Marshall, G. W., and
Li, W. (2004). Amelogenin‐guided crystal growth on fluoroapatite glass‐ceramics. J. Dent.
Res. 83(9), 698–702.
Hakki, S. S., Berry, J. E., and Somerman, M. J. (2001). The eVect of enamel matrix protein
derivative on follicle cells in vitro. J. Periodontol. 72(5), 679–687.
![Page 64: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/64.jpg)
110 Foster et al.
Hakkinen, L., Oksala, O., Salo, T., Rahemtulla, F., and Larjava, H. (1993). Immunohisto-
chemical localization of proteoglycans in human periodontium. J. Histochem. Cytochem.
41(11), 1689–1699.
Hakkinen, L., Strassburger, S., Kahari, V. M., Scott, P. G., Eichstetter, I., Lozzo, R. V., and
Larjava, H. (2000). A role for decorin in the structural organization of periodontal ligament.
Lab. Invest. 80(12), 1869–1880.
Hale, J. E., Fraser, J. D., and Price, P. A. (1988). The identification of matrix Gla protein in
cartilage. J. Biol. Chem. 263(12), 5820–5824.
Hamamoto, Y., Nakajima, T., Ozawa, H., and Uchida, T. (1996). Production of amelogenin by
enamel epithelium of Hertwig’s root sheath. Oral Surg. Oral Med. Oral Pathol. Oral Radiol.
Endod. 81(6), 703–709.
Hammarstrom, L. (1997). The role of enamel matrix proteins in the development of cementum
and periodontal tissues. Ciba Found. Symp. 205, 246–255.
Hammarstrom, L., Alatli, I., and Fong, C. D. (1996). Origins of cementum. Oral Dis. 2(1),
63–69.
Hao, J., He, G., Narayanan, K., Zou, B., Lin, L., Muni, T., Ramachandran, A., and George, A.
(2005). Identification of diVerentially expressed cDNA transcripts from a rat odontoblast cell
line. Bone 37(4), 578–588.
Harada, H., and Ohshima, H. (2004). New perspectives on tooth development and the dental
stem cell niche. Arch. Histol. Cytol. 67(1), 1–11.
Harada, H., Kettunen, P., Jung, H. S., Mustonen, T., Wang, Y. A., and ThesleV, I. (1999).
Localization of putative stem cells in dental epithelium and their association with Notch and
FGF signaling. J. Cell Biol. 147(1), 105–120.
Harada, H., Mitsuyasu, T., Toyono, T., and Toyoshima, K. (2002a). Epithelial stem cells in
teeth. Odontology 90(1), 1–6.
Harada, H., Toyono, T., Toyoshima, K., and Ohuchi, H. (2002b). FGF10 maintains stem cell
population during mouse incisor development. Connect. Tissue Res. 43(2–3), 201–204.
Harmey, D., Hessle, L., Narisawa, S., Johnson, K. A., Terkeltaub, R., and Millan, J. L. (2004).
Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank:
An integrated model of the pathogenesis of mineralization disorders. Am. J. Pathol. 164(4),
1199–1209.
Hart, P. S., Hart, T. C., Michalec, M. D., Ryu, O. H., Simmons, D., Hong, S., and Wright, J. T.
(2004). Mutation in kallikrein 4 causes autosomal recessive hypomaturation amelogenesis
imperfecta. J. Med. Genet. 41(7), 545–549.
Hasegawa, N., Kawaguchi, H., Ogawa, T., Uchida, T., and Kurihara, H. (2003).
Immunohistochemical characteristics of epithelial cell rests of Malassez during cementum
repair. J. Periodontal. Res. 38(1), 51–56.
Hashimoto, F., Kobayashi, Y., Kobayashi, E. T., Sakai, E., Kobayashi, K., Shibata,M., Kato, Y.,
and Sakai, H. (2001). Expression and localization of MGP in rat tooth cementum. Arch. Oral
Biol. 46(7), 585–592.
Hatakeyama, J., Sreenath, T., Hatakeyama, Y., Thyagarajan, T., Shum, L., Gibson, C. W.,
Wright, J. T., and Kulkarni, A. B. (2003). The receptor activator of nuclear factor‐kappa B
ligand‐mediated osteoclastogenic pathway is elevated in amelogenin‐null mice. J. Biol. Chem.
278(37), 35743–35748.
Hatakeyama, J., Philp, D., Hatakeyama, Y., Haruyama, N., Shum, L., Aragon,M. A., Yuan, Z.,
Gibson, C. W., Sreenath, T., Kleinman, H. K., and Kulkarni, A. B. (2006). Amelogenin‐mediated regulation of osteoclastogenesis, and periodontal cell proliferation and migration.
J. Dent. Res. 85(2), 144–149.
He, G., Gajjeraman, S., Schultz, D., Cookson, D., Qin, C., Butler,W. T., Hao, J., andGeorge, A.
(2005a). Spatially and temporally controlled biomineralization is facilitated by interaction
between self‐assembled dentin matrix protein 1 and calcium phosphate nuclei in solution.
Biochemistry 44(49), 16140–16148.
![Page 65: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/65.jpg)
3. Regeneration of the Periodontium 111
He, G., Ramachandran, A., Dahl, T., George, S., Schultz, D., Cookson, D., Veis, A., and
George, A. (2005b). Phosphorylation of phosphophoryn is crucial for its function as a
mediator of biomineralization. J. Biol. Chem. 280(39), 33109–33114.
Heden, G., and Wennstrom, J. L. (2006). Five‐year follow‐up of regenerative periodon-
tal therapy with enamel matrix derivative at sites with angular bone defects. J. Periodontol.
77(2), 295–301.
Ho, A. M., Johnson, M. D., and Kingsley, D. M. (2000). Role of the mouse ank gene in control
of tissue calcification and arthritis. Science 289(5477), 265–270.
Hong, J. H., and Yaffe, M. B. (2006). TAZ: A beta‐catenin‐like molecule that regulates
mesenchymal stem cell differentiation. Cell Cycle 5(2), 176–179.
Hong, J. H., Hwang, E. S., McManus, M. T., Amsterdam, A., Tian, Y., Kalmukova, R.,
Mueller, E., Benjamin, T., Spiegelman, B. M., Sharp, P. A., Hopkins, N., and YaVe, M. B.
(2005). TAZ a transcriptional modulator of mesenchymal stem cell diVerentiation. Science
309(5737), 1074–1078.
Horiuchi, K., Amizuka, N., Takeshita, S., Takamatsu, H., Katsuura, M., Ozawa, H., Toyama, Y.,
Bonewald, L. F., and Kudo, A. (1999). Identification and characterization of a novel protein,
periostin, with restricted expression to periosteum and periodontal ligament and increased
expression by transforming growth factor beta. J. Bone Miner. Res. 14(7), 1239–1249.
Hu, H., Hilton, M. J., Tu, X., Yu, K., Ornitz, D. M., and Long, F. (2005). Sequential roles of
Hedgehog and Wnt signaling in osteoblast development. Development 132(1), 49–60.
Hu, J. C., and Yamakoshi, Y. (2003). Enamelin and autosomal‐dominant amelogenesis
imperfecta. Crit. Rev. Oral Biol. Med. 14(6), 387–398.
Hu, J. C., Sun, X., Zhang, C., and Simmer, J. P. (2001). A comparison of enamelin and
amelogenin expression in developing mouse molars. Eur. J. Oral Sci. 109(2), 125–132.
Hu, J. C., Sun, X., Zhang, C., Liu, S., Bartlett, J. D., and Simmer, J. P. (2002). Enamelysin and
kallikrein‐4 mRNA expression in developing mouse molars. Eur. J. Oral Sci. 110(4), 307–315.
Huggins, C. B., McCarroll, H. R., and Dahlberg, A. A. (1934). Transplantation of tooth germ
elements and the experimental heterotopic formation of dentin and enamel. J. Exp. Med. 60,
199–210.
Huq, N. L., Cross, K. J., Ung, M., and Reynolds, E. C. (2005). A review of protein structure and
gene organisation for proteins associated with mineralised tissue and calcium phosphate
stabilisation encoded on human chromosome 4. Arch. Oral Biol. 50(7), 599–609.
Iijima, M., Moriwaki, Y., Wen, H. B., Fincham, A. G., and Moradian‐Oldak, J. (2002).
Elongated growth of octacalcium phosphate crystals in recombinant amelogenin gels under
controlled ionic flow. J. Dent. Res. 81(1), 69–73.
Imbeni, V., Kruzic, J. J., Marshall, G. W., Marshall, S. J., and Ritchie, R. O. (2005). The
dentin‐enamel junction and the fracture of human teeth. Nat. Mater. 4(3), 229–232.
Iozzo, R. V. (1998). Matrix proteoglycans: From molecular design to cellular function. Annu.
Rev. Biochem. 67, 609–652.
Ivkovic, S., Yoon, B. S., PopoV, S. N., Safadi, F. F., Libuda, D. E., Stephenson, R. C., Daluiski,
A., and Lyons, K.M. (2003). Connective tissue growth factor coordinates chondrogenesis and
angiogenesis during skeletal development. Development 130(12), 2779–2791.
Iwasaki, K., Bajenova, E., Somogyi‐Ganss, E., Miller,M., Nguyen, V., Nourkeyhani, H., Gao, Y.,
Wendel, M., and Ganss, B. (2005). Amelotin–a novel secreted, ameloblast‐specific protein.
J. Dent. Res. 84(12), 1127–1132.
Iwata, T., Morotome, Y., Tanabe, T., Fukae, M., Ishikawa, I., and Oida, S. (2002). Noggin
blocks osteoinductive activity of porcine enamel extracts. J. Dent. Res. 81(6), 387–391.
James, M. J., Jarvinen, E., and ThesleV, I. (2004). Bono1: A gene associated with regions of
deposition of bone and dentine. Gene Expr. Patterns 4(5), 595–599.
Janones, D. S., Massa, L. F., and Arana‐Chavez, V. E. (2005). Immunocytochemical
examination of the presence of amelogenin during the root development of rat molars.
Arch. Oral Biol. 50(5), 527–532.
![Page 66: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/66.jpg)
112 Foster et al.
Jayawardena, C. K., Takahashi, N., Watanabae, E., and Takano, Y. (2002). On the origin of
intrinsic matrix of acellular extrinsic fiber cementum: Studies on growing cementum pearls of
normal and bisphosphonate‐aVected guinea pig molars. Eur. J. Oral Sci. 110(3), 261–269.
Jernvall, J., and ThesleV, I. (2000). Reiterative signaling and patterning during mammalian
tooth morphogenesis. Mech. Dev. 92(1), 19–29.
Jin, Q. M., Zhao, M., Webb, S. A., Berry, J. E., Somerman, M. J., and Giannobile, W. V.
(2003). Cementum engineering with three‐dimensional polymer scaVolds. J. Biomed. Mater.
Res. 67A(1), 54–60.
Johnson, K., Goding, J., Van Etten, D., Sali, A., Hu, S. I., Farley, D., Krug, H., Hessle, L.,
Millan, J. L., and Terkeltaub, R. (2003). Linked deficiencies in extracellular PP(i) and
osteopontin mediate pathologic calcification associated with defective PC‐1 and ANK
expression. J. Bone Miner. Res. 18(6), 994–1004.
Kaartinen, M. T., Sun, W., Kaipatur, N., and McKee, M. D. (2005). Transglutaminase
Crosslinking of SIBLING Proteins in Teeth. J. Dent. Res. 84(7), 607–612.
Kalajzic, I., Braut, A., Guo, D., Jiang, X., Kronenberg, M. S., Mina, M., Harris, M. A., Harris,
S. E., and Rowe, D. W. (2004). Dentin matrix protein 1 expression during osteoblastic
differentiation, generation of an osteocyte GFP‐transgene. Bone 35(1), 74–82.
Kaneda, T., Miyauchi, M., Takekoshi, T., Kitagawa, S., Kitagawa,M., Shiba, H., Kurihara, H.,
and Takata, T. (2006). Characteristics of periodontal ligament subpopulations obtained by
sequential enzymatic digestion of rat molar periodontal ligament. Bone 38(3), 420–426.
Kaneko,H.,Hashimoto, S., Enokiya,Y., Ogiuchi,H., and Shimono,M. (1999).Cell proliferation
and death of Hertwig’s epithelial root sheath in the rat. Cell Tissue Res. 298(1), 95–103.
Kaneko, S., Ohashi, K., Soma, K., and Yanagishita, M. (2001). Occlusal hypofunction
causes changes of proteoglycan content in the rat periodontal ligament. J. Periodontal. Res.
36(1), 9–17.
Kangas, A. T., Evans, A. R., ThesleV, I., and Jernvall, J. (2004). Nonindependence of
mammalian dental characters. Nature 432(7014), 211–214.
Kassai, Y., Munne, P., Hotta, Y., Penttila, E., Kavanagh, K., Ohbayashi, N., Takada, S.,
ThesleV, I., Jernvall, J., and Itoh, N. (2005). Regulation of mammalian tooth cusp patterning
by ectodin. Science 309(5743), 2067–2070.
Katagiri, T., and Takahashi, N. (2002). Regulatory mechanisms of osteoblast and osteoclast
differentiation. Oral Dis. 8(3), 147–159.
Kato, M., Patel, M. S., Levasseur, R., Lobov, I., Chang, B. H., Glass, D. A., 2nd, Hartmann,
C., Li, L., Hwang, T. H., Brayton, C. F., Lang, R. A., Karsenty, G., et al. (2002). Cbfa1‐independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye
vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 157(2), 303–314.
Kawase, T., Okuda, K., Momose, M., Kato, Y., Yoshie, H., and Burns, D. M. (2001). Enamel
matrix derivative (EMDOGAIN) rapidly stimulates phosphorylation of the MAP kinase
family and nuclear accumulation of smad2 in both oral epithelial and fibroblastic human
cells. J. Periodontal. Res. 36(6), 367–376.
Kawase, T., Okuda, K., Yoshie, H., and Burns, D. M. (2002). Anti‐TGF‐beta antibody blocks
enamel matrix derivative‐induced upregulation of p21WAF1/cip1 and prevents its inhibition
of human oral epithelial cell proliferation. J. Periodontal. Res. 37(4), 255–262.
Kern, B., Shen, J., Starbuck, M., and Karsenty, G. (2001). Cbfa1 contributes to the osteoblast‐specific expression of type I collagen genes. J. Biol. Chem. 276(10), 7101–7107.
Kii, I., Amizuka, N., Minqi, L., Kitajima, S., Saga, Y., and Kudo, A. (2006). Periostin is an
extracellular matrix protein required for eruption of incisors in mice. Biochem. Biophys. Res.
Commun. 342(3), 766–772.
Kim, J. W., Seymen, F., Lin, B. P., Kiziltan, B., Gencay, K., Simmer, J. P., and Hu, J. C.
(2005). ENAM mutations in autosomal‐dominant amelogenesis imperfecta. J. Dent. Res. 84
(3), 278–282.
![Page 67: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/67.jpg)
3. Regeneration of the Periodontium 113
Kitagawa, M., Kitagawa, S., Kudo, Y., Ogawa, I., Miyauchi, M., Tahara, H., Ide, T., and
Takata, T. (2005). Establishment of cementoblast cell lines from rat cementum lining cells by
transfection with temperature‐sensitive simian virus‐40 T‐antigen gene. Bone 37(2), 220–226.
Koga, T., Matsui, Y., Asagiri, M., Kodama, T., de Crombrugghe, B., Nakashima, K., and
Takayanagi, H. (2005). NFAT and Osterix cooperatively regulate bone formation. Nat.
Med. 11(8), 880–885.
Komori, T., Yagi, H., Nomura, S., Yamaguchi, A., Sasaki, K., Deguchi, K., Shimizu, Y.,
Bronson, R. T., Gao, Y. H., Inada, M., Sato, M., Okamoto, R., et al. (1997). Targeted
disruption of Cbfal results in a complete lack of bone formation owing to maturational
arrest of osteoblasts [see comments]. Cell 89(5), 755–764.
Kong, Y. Y., Yoshida, H., Sarosi, I., Tan, H. L., Timms, E., Capparelli, C., Morony, S.,
Oliveria‐dos‐ Santos, A. J., Van, G., Itie, A., Khoo, W., Wakeham, A., et al. (1999). OPGL is
a key regulator of osteoclastogenesis, lymphocyte development and lymph‐node organogen-esis. Nature 397(6717), 315–3123.
Koike, H., Uzawa, K., Grzesik, W. J., Seki, N., Endo, Y., Kasamatsu, A., Yamauchi, M., and
Tanzawa, H. (2005). GLUT1 is highly expressed in cementoblasts but not in osteoblasts.
Connect. Tissue Res. 46(3), 117–124.
Kollar, E. J., and Baird, G. R. (1969). The influence of the dental papilla on the development of
tooth shape in embryonic mouse tooth germs. J. Embryol. Exp. Morphol. 21(1), 131–148.
Kollar, E. J., and Baird, G. R. (1970a). Tissue interactions in embryonic mouse tooth germs. II.
The inductive role of the dental papilla. J. Embryol. Exp. Morphol. 24(1), 173–186.
Kollar, E. J., and Baird, G. R. (1970b). Tissue interactions in embryonic mouse tooth germs. I.
Reorganization of the dental epithelium during tooth‐germ reconstruction. J. Embryol. Exp.
Morphol. 24(1), 159–171.
Kollar, E. J., and Fisher, C. (1980). Tooth induction in chick epithelium: Expression of
quiescent genes for enamel synthesis. Science 207(4434), 993–995.
Komori, T., Yagi, H., Nomura, S., Yamaguchi, A., Sasaki, K., Deguchi, K., Shimizu, Y.,
Bronson, R. T., Gao, Y. H., Inada, M., Sato, M., Okamoto, R., et al. (1997). Targeted
disruption of Cbfal results in a complete lack of bone formation owing to maturational
arrest of osteoblasts [see comments]. Cell 89(5), 755–764.
Kong, Y. Y., Yoshida, H., Sarosi, I., Tan, H. L., Timms, E., Capparelli, C., Morony, S.,
Oliveria‐dos‐Santos, A. J., Van, G., Itie, A., Khoo, W., Wakeham, A., et al. (1999). OPGL is
a key regulator of osteoclastogenesis, lymphocyte development and lymph‐node organo-
genesis. Nature 397(6717), 315–323.
Kratochwil, K., Dull, M., Farinas, I., Galceran, J., and Grosschedl, R. (1996). Lef1 expression
is activated by BMP‐4 and regulates inductive tissue interactions in tooth and hair
development. Genes Dev. 10(11), 1382–1394.
Kruzynska‐Frejtag, A., Wang, J., Maeda, M., Rogers, R., Krug, E., Hoffman, S., Markwald,
R. R., and Conway, S. J. (2004). Periostin is expressed within the developing teeth at the sites
of epithelial‐mesenchymal interaction. Dev. Dyn. 229(4), 857–868.
Lagerstrom‐Fermer, M., and Landegren, U. (1995). Understanding enamel formation from
mutations causing X‐linked amelogenesis imperfecta. Connect. Tissue Res. 32(1–4), 241–246.
Laino,G.,Graziano,A., d’Aquino,R., Pirozzi,G., Lanza,V., Valiante, S.,DeRosa,A.,Naro, F.,
Vivarelli, E., and Papaccio, G. (2006).An approachable human adult stem cell source for hard‐tissue engineering. J. Cell. Physiol. 206(3), 693–701.
Lallier, T. E., Spencer, A., and Fowler, M. M. (2005). Transcript profiling of periodontal
fibroblasts and osteoblasts. J. Periodontol. 76(7), 1044–1055.
Laurikkala, J., Kassai, Y., Pakkasjarvi, L., ThesleV, I., and Itoh, N. (2003). Identification of a
secreted BMP antagonist, ectodin, integrating BMP FGF and SHH signals from the tooth
enamel knot. Dev. Biol. 264(1), 91–105.
Lezot, F., Davideau, J. L., Thomas, B., Sharpe, P., Forest, N., and Berdal, A. (2000). Epithelial
Dlx‐2 homeogene expression and cementogenesis. J. Histochem. Cytochem. 48(2), 277–284.
![Page 68: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/68.jpg)
114 Foster et al.
Li, G., Oparil, S., Sanders, J. M., Zhang, L., Dai, M., Chen, L. B., Conway, S. J., McNamara,
C. A., and Sarembock, I. J. (2005a). Phosphatidylinositol‐3‐kinase signaling mediates
vascular smooth muscle cell expression of periostin in vivo and in vitro. Atherosclerosis, Epub
ahead of print.
Li, X., Liu, P., Liu, W., Maye, P., Zhang, J., Zhang, Y., Hurley, M., Guo, C., Boskey, A., Sun,
L., Harris, S. E., Rowe, D. W., et al. (2005b). Dkk2 has a role in terminal osteoblast
differentiation and mineralized matrix formation. Nat. Genet. 37(9), 945–952.
Lin, C. G., Leu, S. J., Chen, N., Tebeau, C. M., Lin, S. X., Yeung, C. Y., and Lau, L. F. (2003).
CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family. J. Biol. Chem.
278(26), 24200–24208.
Lin, C. G., Chen, C. C., Leu, S. J., Grzeszkiewicz, T. M., and Lau, L. F. (2005). Integrin‐dependent functions of the angiogenic inducer NOV (CCN3): Implication in wound healing.
J. Biol. Chem. 280(9), 8229–8237.
Ling, Y., Rios, H. F., Myers, E. R., Lu, Y., Feng, J. Q., and Boskey, A. L. (2005). DMP1
depletion decreases bone mineralization in vivo: An FTIR imaging analysis. J. Bone Miner.
Res. 20(12), 2169–2177.
Liu, H., Li, W., Gao, C., Kumagai, Y., Blacher, R. W., and DenBesten, P. K. (2004). Dentonin,
a fragment of MEPE enhanced dental pulp stem cell proliferation. J. Dent. Res. 83(6),
496–499.
Liu, D., Yao, S., Pan, F., and Wise, G. E. (2005a). Chronology and regulation of gene
expression of RANKL in the rat dental follicle. Eur. J. Oral Sci. 113(5), 404–409.
Liu, H., Li, W., Shi, S., Habelitz, S., Gao, C., and Denbesten, P. (2005b). MEPE is
downregulated as dental pulp stem cells diVerentiate. Arch. Oral Biol. 50(11), 923–928.
Liu, S., Brown, T. A., Zhou, J., Xiao, Z. S., Awad, H., Guilak, F., and Quarles, L. D. (2005c).
Role of matrix extracellular phosphoglycoprotein in the pathogenesis of X‐linkedhypophosphatemia. J. Am. Soc. Nephrol. 16(6), 1645–1653.
Lu, C., Huang, S., Miclau, T., Helms, J. A., and Colnot, C. (2004). Mepe is expressed during
skeletal development and regeneration. Histochem. Cell Biol. 121(6), 493–499.
Luan, X., Ito, Y., and Diekwisch, T. G. (2006). Evolution and development of Hertwig’s
epithelial root sheath. Dev. Dyn. 235(5), 1167–1180.
Luo, G., D’Souza, R., Hogue, D., and Karsenty, G. (1995). The matrix Gla protein gene is
a marker of the chondrogenesis cell lineage during mouse development. J. Bone Miner. Res.
10(2), 325–334.
Luo, G., Ducy, P., McKee, M. D., Pinero, G. J., Loyer, E., Behringer, R. R., and Karsenty, G.
(1997). Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA
protein. Nature 386(6620), 78–81.
Luo, W., Slavkin, H. C., and Snead, M. L. (1991). Cells from Hertwig’s epithelial root sheath
do not transcribe amelogenin. J. Periodontal. Res. 26(1), 42–47.
Luo, W., Wen, X., Wang, H. J., MacDougall, M., Snead, M. L., and Paine, M. L. (2004). In vivo
overexpression of tuftelin in the enamel organic matrix. Cells Tissues Organs 177(4), 212–220.
Lyngstadaas, S. P., Lundberg, E., Ekdahl, H., Andersson, C., and Gestrelius, S. (2001).
Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix
derivative. J. Clin. Periodontol. 28(2), 181–188.
Maas, R., and Bei, M. (1997). The genetic control of early tooth development. Crit. Rev. Oral
Biol. Med. 8(1), 4–39.
MacDougall, M., Simmons, D., Gu, T. T., and Dong, J. (2002). MEPE/OF45, a new dentin/
bone matrix protein and candidate gene for dentin diseases mapping to chromosome 4q21.
Connect. Tissue Res. 43(2–3), 320–330.
MacNeil, R. L., and Somerman, M. J. (1999). Development and regeneration of the
periodontium: Parallels and contrasts. Periodontol. 2000 19, 8–20.
MacNeil, R. L., and Thomas, H. F. (1993). Development of the murine periodontium. II.
Role of the epithelial root sheath in formation of the periodontal attachment. J. Periodontol.
64(4), 285–291.
![Page 69: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/69.jpg)
3. Regeneration of the Periodontium 115
MacNeil, R. L., Sheng, N., Strayhorn, C. L., Fisher, L. W., and Somerman, M. J. (1994). Bone
sialoprotein is localized to the root surface during cementogenesis. J. Bone Miner. Res. 9(10),
1597–1606.
MacNeil, R. L., D’Errico, J. A., Ouyang, H., Berry, J., Strayhorn, C., and Somerman, M. J.
(1998). Isolation of murine cementoblasts: Unique cells or uniquely‐positioned osteoblasts?
Eur. J. Oral Sci. 106(Suppl. 1), 350–356.
Marshall, G. W., Jr., Balooch, M., Gallagher, R. R., Gansky, S. A., and Marshall, S. J. (2001).
Mechanical properties of the dentinoenamel junction: AFM studies of nanohardness, elastic
modulus, and fracture. J. Biomed. Mater. Res. 54(1), 87–95.
Maruya, Y., Sasano, Y., Takahashi, I., Kagayama, M., and Mayanagi, H. (2003). Expression of
extracellularmatrixmolecules,MMPsandTIMPs in alveolarbone, cementumandperiodontal
ligaments during rat tooth eruption. J. Electron. Microsc. (Tokyo) 52(6), 593–604.
Massa, L. F., Ramachandran, A., George, A., and Arana‐Chavez, V. E. (2005). Developmental
appearance of dentin matrix protein 1 during the early dentinogenesis in rat molars
as identified by high‐resolution immunocytochemistry. Histochem. Cell Biol. 124(3–4),
197–205.
Masuya, H., Shimizu, K., Sezutsu, H., Sakuraba, Y., Nagano, J., Shimizu, A., Fujimoto, N.,
Kawai, A., Miura, I., Kaneda, H., Kobayashi, K., Ishijima, K., et al. (2005). Enamelin
(Enam) is essential for amelogenesis: ENU‐induced mouse mutants as models for
diVerent clinical subtypes of human amelogenesis imperfecta (AI). Hum. Mol. Genet.
14(5), 575–583.
Matias, M. A., Li, H., Young, W. G., and Bartold, P. M. (2003). Immunohistochemical
localization of fibromodulin in the periodontium during cementogenesis and root formation
in the rat molar. J. Periodontal. Res. 38(5), 502–507.
Maycock, J., Wood, S. R., Brookes, S. J., Shore, R. C., Robinson, C., and Kirkham, J. (2002).
Characterization of a porcine amelogenin preparation, EMDOGAIN a biological treatment
for periodontal disease. Connect. Tissue Res. 43(2–3), 472–476.
McCulloch, C. A. (1985). Progenitor cell populations in the periodontal ligament of mice. Anat.
Rec. 211(3), 258–262.
McCulloch, C. A. (1995). Origins and functions of cells essential for periodontal repair: The role
of fibroblasts in tissue homeostasis. Oral Dis. 1(4), 271–278.
Melcher, A. H. (1985). Cells of periodontium: Their role in the healing of wounds. Ann. R. Coll.
Surg. Engl. 67(2), 130–131.
Miao, D., Bai, X., Panda, D. K., Karaplis, A. C., Goltzman, D., and McKee, M. D. (2004).
Cartilage abnormalities are associated with abnormal Phex expression and with altered
matrix protein and MMP‐9 localization in Hyp mice. Bone 34(4), 638–647.
Mina, M., and Kollar, E. J. (1987). The induction of odontogenesis in non‐dentalmesenchyme combined with early murine mandibular arch epithelium. Arch. Oral Biol. 32(2),
123–127.
Miura, M., Gronthos, S., Zhao, M., Lu, B., Fisher, L. W., Robey, P. G., and Shi, S. (2003).
SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA
100(10), 5807–5812.
MoVatt, P., Salois, P., Gaumond, M. H., St‐Amant, N., Godin, E., and Lanctot, C. (2002).
Engineered viruses to select genes encoding secreted and membrane‐bound proteins in
mammalian cells. Nucleic Acids Res. 30(19), 4285–4294.
Moradian‐Oldak, J., Simmer, J. P., Lau, E. C., Diekwisch, T., Slavkin, H. C., and Fincham,
A. G. (1995). A review of the aggregation properties of a recombinant amelogenin. Connect.
Tissue Res. 32(1–4), 125–130.
Moradian‐Oldak, J., Paine, M. L., Lei, Y. P., Fincham, A. G., and Snead, M. L. (2000). Self‐assembly properties of recombinant engineered amelogenin proteins analyzed by dynamic
light scattering and atomic force microscopy. J. Struct. Biol. 131(1), 27–37.
Mori, K., Shioi, A., Jono, S., Nishizawa, Y., and Morii, H. (1998). Expression of matrix Gla
protein (MGP) in an in vitro model of vascular calcification. FEBS Lett. 433(1–2), 19–22.
![Page 70: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/70.jpg)
116 Foster et al.
Morotomi, T.,Kawano, S., Toyono, T.,Kitamura,C., Terashita,M.,Uchida, T., Toyoshima,K.,
and Harada, H. (2005). In vitro diVerentiation of dental epithelial progenitor cells through
epithelial‐mesenchymal interactions. Arch. Oral Biol. 50(8), 695–705.
Morsczeck, C., Gotz, W., Schierholz, J., Zeilhofer, F., Kuhn, U., Mohl, C., Sippel, C., and
HoVmann, K. H. (2005). Isolation of precursor cells (PCs) from human dental follicle of
wisdom teeth. Matrix Biol. 24(2), 155–165.
Mouri, Y., Shiba, H., Mizuno, N., Noguchi, T., Ogawa, T., and Kurihara, H. (2003).
DiVerential gene expression of bone‐related proteins in epithelial and fibroblastic cells
derived from human periodontal ligament. Cell Biol. Int. 27(7), 519–524.
Murshed, M., Harmey, D., Millan, J. L., McKee, M. D., and Karsenty, G. (2005). Unique
coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of
ECM mineralization to bone. Genes Dev. 19(9), 1093–1104.
Nadiri, A., Kuchler‐Bopp, S., Haikel, Y., and Lesot, H. (2004). Immunolocalization of BMP‐2/‐4,FGF‐4, andWNT10b in the developingmouse first lowermolar. J. Histochem. Cytochem. 52(1),
103–112.
Nagano, T., Oida, S., Ando, H., Gomi, K., Arai, T., and Fukae, M. (2003). Relative levels of
mRNA encoding enamel proteins in enamel organ epithelia and odontoblasts. J. Dent. Res.
82(12), 982–986.
Nakahara, T. (2006). A review of new developments in tissue engineering therapy for
periodontitis. Dent. Clin. North Am. 50(2), 265–276, ix–x.
Nakamura, S., Terashima, T., Yoshida, T., Iseki, S., Takano, Y., Ishikawa, I., and Shinomura, T.
(2005). Identificationof genespreferentially expressed inperiodontal ligament:Specific expression
of a novel secreted protein, FDC‐SP. Biochem. Biophys. Res. Commun. 338(2), 1197–1203.
Nakanishi, T., Takahashi, K., Aoki, C., Nishikawa, K., Hattori, T., and Taniguchi, S. (1994).
Expression of nerve growth factor family neurotrophins in a mouse osteoblastic cell line.
Biochem. Biophys. Res. Commun. 198(3), 891–897.
Nakanishi, T.,Yamaai, T.,Asano,M.,Nawachi,K., Suzuki,M., Sugimoto, T., andTakigawa,M.
(2001). Overexpression of connective tissue growth factor/hypertrophic chondrocyte‐specificgene product 24 decreases bone density in adult mice and induces dwarfism.Biochem. Biophys.
Res. Commun. 281(3), 678–681.
Nakashima, K., Zhou, X., Kunkel, G., Zhang, Z., Deng, J. M., Behringer, R. R., and de
Crombrugghe, B. (2002). The novel zinc finger‐containing transcription factor osterix is
required for osteoblast differentiation and bone formation. Cell 108(1), 17–29.
Nakatomi, M., Morita, I., Eto, K., and Ota, M. S. (2006). Sonic Hedgehog Signaling is
Important in Tooth Root Development. J. Dent. Res. 85(5), 427–431.
Nampei, A., Hashimoto, J., Hayashida, K., Tsuboi, H., Shi, K., Tsuji, I., Miyashita, H.,
Yamada, T., Matsukawa, N., Matsumoto, M., Morimoto, S., Ogihara, T., et al. (2004).
Matrix extracellular phosphoglycoprotein (MEPE) is highly expressed in osteocytes in
human bone. J. Bone Miner. Metab. 22(3), 176–184.
Nanci, A. (2003). Enamel: Composition, formation, and structure. In ‘‘Ten Cate’s Oral
Histology: Development, Structure, and Function’’ (A. Nanci, Ed.), p. 445. Mosby, St.
Louis.
Nanci, A., and Somerman, M. J. (2003). Periodontium. In ‘‘Ten Cate’s Oral Histology:
Development, Structure, and Function’’ (A. Nanci, Ed.), p. 445. Mosby, St. Louis.
Nanci, A., and Bosshardt, D. D. (2006). Structure of periodontal tissues in health and disease.
Periodontol 2000 40, 11–28.
Narayanan, A. S., Ikezawa, K., Wu, D., and Pitaru, S. (1995). Cementum specific components
which influence periodontal connective tissue cells. Connect. Tissue Res. 33(1–3), 19–21.
Narayanan,K., Ramachandran, A., Hao, J., He, G., Park, K.W., Cho,M., andGeorge, A. (2003).
Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene
transcription by activation of intracellular Ca2þ store. J. Biol. Chem. 278(19), 17500–17508.
![Page 71: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/71.jpg)
3. Regeneration of the Periodontium 117
Nebgen, D. R., Inoue, H., Sabsay, B., Wei, K., Ho, C. S., and Veis, A. (1999). Identification of
the chondrogenic‐inducing activity from bovine dentin (bCIA) as a low‐molecular‐mass
amelogenin polypeptide. J. Dent. Res. 78(9), 1484–1494.
Nieminen, P., Pekkanen, M., Aberg, T., and ThesleV, I. (1998). A graphical WWW‐database ongene expression in tooth. Eur. J. Oral Sci. 106(Suppl. 1), 7–11.
Nifuji, A., and Noda, M. (1999). Coordinated expression of noggin and bone morphogenetic
proteins (BMPs) during early skeletogenesis and induction of noggin expression by BMP‐7.J. Bone Miner. Res. 14(12), 2057–2066.
Nociti, F. H., Jr., Berry, J. E., Foster, B. L., Gurley, K. A., Kingsley, D. M., Takata, T.,
Miyauchi, M., and Somerman, M. J. (2002). Cementum: A phosphate‐sensitive tissue.
J. Dent. Res. 81(12), 817–821.
Nociti, F. H., Jr., Foster, B. L., Barros, S. P., Darveau, R. P., and Somerman, M. J. (2004).
Cementoblast gene expression is regulated by Porphyromonas gingivalis lipopolysaccharide
partially via toll‐like receptor‐4/MD‐2. J. Dent. Res. 83(8), 602–607.
Nosrat, C. A., Fried, K., Ebendal, T., and Olson, L. (1998). N, G. F., BD, N. F., NT3, NT4 and
GDNF in tooth development. Eur. J. Oral Sci. 106(Suppl. 1), 94–99.
Nurnberg, P., Thiele, H., Chandler, D., Hohne, W., Cunningham,M. L., Ritter, H., Leschik, G.,
Uhlmann, K., Mischung, C., Harrop, K., Goldblatt, J., Borochowitz, Z. U., et al. (2001).
Heterozygous mutations in ANKH the human ortholog of the mouse progressive ankylosis
gene, result in craniometaphyseal dysplasia. Nat. Genet. 28(1), 37–41.
Nusse, R. (2005). Wnt signaling in disease and in development. Cell Res. 15(1), 28–32.
Ogawa,T.,Onishi,T.,Hayashibara,T., Sakashita, S.,Okawa,R., andOoshima,T. (2006).Dentinal
defects in Hyp mice not caused by hypophosphatemia alone. Arch. Oral Biol. 51(1), 58–63.
Ohazama, A., Courtney, J. M., and Sharpe, P. T. (2004a). Opg, Rank, and Rankl in tooth
development: Co‐ordination of odontogenesis and osteogenesis. J. Dent. Res. 83(3), 241–244.
Ohazama, A., Modino, S. A., Miletich, I., and Sharpe, P. T. (2004b). Stem‐cell‐based tissue
engineering of murine teeth. J. Dent. Res. 83(7), 518–522.
Ohno, S., Doi, T., Fujimoto, K., Ijuin, C., Tanaka, N., Tanimoto, K., Honda, K., Nakahara,M.,
Kato, Y., and Tanne, K. (2002). RGD‐CAP (betaig‐h3) exerts a negative regulatory functionon mineralization in the human periodontal ligament. J. Dent. Res. 81(12), 822–825.
Ohno, S., Tanaka, N., Ueki, M., Honda, K., Tanimoto, K., Yoneno, K., Ohno‐Nakahara, M.,
Fujimoto, K., Kato, Y., and Tanne, K. (2005).Mechanical regulation of terminal chondrocyte
diVerentiation via RGD‐CAP/beta ig‐h3 induced by TGF‐beta. Connect. Tissue Res. 46(4–5),227–234.
Ohshima, H., Nakasone, N., Hashimoto, E., Sakai, H., Nakakura‐Ohshima, K., and Harada, H.
(2005). The eternal tooth germ is formed at the apical end of continuously growing teeth.Arch.
Oral Biol. 50(2), 153–157.
Oida, S., Nagano, T., Yamakoshi, Y., Ando, H., Yamada, M., and Fukae, M. (2002).
Amelogenin gene expression in porcine odontoblasts. J. Dent. Res. 81(2), 103–108.
Okawa, A., Nakamura, I., Goto, S., Moriya, H., Nakamura, Y., and Ikegawa, S. (1998).
Mutation in Npps in a mouse model of ossification of the posterior longitudinal ligament of
the spine. Nat. Genet. 19(3), 271–273.
Otto, F., Thornell, A. P., Crompton, T., Denzel, A., Gilmour, K. C., Rosewell, I. R., Stamp,
G. W., Beddington, R. S., Mundlos, S., Olsen, B. R., Selby, P. B., and Owen, M. J. (1997).
Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast
differentiation and bone development. Cell 89(5), 765–771.
Ozdemir, D., Hart, P. S., Ryu, O. H., Choi, S. J., Ozdemir‐Karatas, M., Firatli, E., Piesco, N.,
and Hart, T. C. (2005). MMP20 active‐site mutation in hypomaturation amelogenesis
imperfecta. J. Dent. Res. 84(11), 1031–1035.
Paine, M. L., and Snead, M. L. (2005). Tooth developmental biology: Disruptions to enamel‐matrix assembly and its impact on biomineralization. Orthod. Craniofac. Res. 8(4), 239–251.
![Page 72: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/72.jpg)
118 Foster et al.
Paine, C. T., Paine, M. L., and Snead, M. L. (1998). Identification of tuftelin‐ and amelogenin‐interacting proteins using the yeast two‐hybrid system. Connect. Tissue Res. 38(1–4),
257–267; discussion 295–303.
Paine, M. L., Deutsch, D., and Snead, M. L. (1996). Carboxyl‐region of tuftelin mediates self‐assembly. Connect. Tissue Res. 35(1–4), 157–161.
Paine, M. L., White, S. N., Luo, W., Fong, H., Sarikaya, M., and Snead, M. L. (2001).
Regulated gene expression dictates enamel structure and tooth function. Matrix Biol. 20
(5–6), 273–292.
Paine, M. L., Wang, H. J., Luo, W., Krebsbach, P. H., and Snead, M. L. (2003). A transgenic
animal model resembling amelogenesis imperfecta related to ameloblastin overexpression.
J. Biol. Chem. 278(21), 19447–19452.
Paine, M. L., Zhu, D. H., Luo, W., and Snead, M. L. (2004). Overexpression of TRAP in the
enamel matrix does not alter the enamel structural hierarchy. Cells Tissues Organs 176(1–3),
7–16.
Papagerakis, P., MacDougall, M., Hotton, D., Bailleul‐Forestier, I., Oboeuf, M., and Berdal, A.
(2003). Expression of amelogenin in odontoblasts. Bone 32(3), 228–240.
Paynter, K. J., and Pudy, G. (1958). A study of the structure, chemical nature, and development
of cementum in the rat. Anat. Rec. 131(2), 233–251.
Pispa, J., and ThesleV, I. (2003). Mechanisms of ectodermal organogenesis. Dev. Biol. 262(2),
195–205.
Pispa, J., Mustonen, T., Mikkola, M. L., Kangas, A. T., Koppinen, P., Lukinmaa, P. L.,
Jernvall, J., and ThesleV, I. (2004). Tooth patterning and enamel formation can be
manipulated by misexpression of TNF receptor Edar. Dev. Dyn. 231(2), 432–440.
Pitaru, S., Narayanan, S. A., Olson, S., Savion, N., Hekmati, H., Alt, I., and Metzger, Z.
(1995). Specific cementum attachment protein enhances selectively the attachment and
migration of periodontal cells to root surfaces. J. Periodontal. Res. 30(5), 360–368.
Pitaru, S., Pritzki, A., Bar‐Kana, I., Grosskopf, A., Savion, N., and Narayanan, A. S. (2002).
Bone morphogenetic protein 2 induces the expression of cementum attachment protein in
human periodontal ligament clones. Connect. Tissue Res. 43(2–3), 257–264.
Plikus, M. V., Zeichner‐David, M., Mayer, J. A., Reyna, J., Bringas, P., Thewissen, J. G.,
Snead, M. L., Chai, Y., and Chuong, C. M. (2005). Morphoregulation of teeth: Modulating
the number, size, shape and diVerentiation by tuning Bmp activity. Evol. Dev. 7(5), 440–457.
Popowics, T. E., Foster, B. L., Swanson, E. C., Fong, H., and Somerman, M. J. (2005).
Defining the roots of cementum formation. Cells Tissues Organs 181, 248–257.
Price, J. A., Bowden, D. W., Wright, J. T., Pettenati, M. J., and Hart, T. C. (1998).
Identification of a mutation in DLX3 associated with tricho‐dento‐osseous (TDO)
syndrome. Hum. Mol. Genet. 7(3), 563–569.
Price, P. A., Urist, M. R., and Otawara, Y. (1983). Matrix Gla protein, a new gamma‐carboxyglutamic acid‐containing protein which is associated with the organic matrix of
bone. Biochem. Biophys. Res. Commun. 117(3), 765–771.
Qin, C., Brunn, J. C., Cadena, E., Ridall, A., Tsujigiwa, H., Nagatsuka, H., Nagai, N., and
Butler, W. T. (2002). The expression of dentin sialophosphoprotein gene in bone. J. Dent.
Res. 81(6), 392–394.
Qin, C., Brunn, J. C., Cadena, E., Ridall, A., and Butler, W. T. (2003). Dentin sialoprotein
in bone and dentin sialophosphoprotein gene expressed by osteoblasts. Connect. Tissue Res.
44(Suppl. 1), 179–183.
Qin, C., Baba, O., and Butler,W. T. (2004). Post‐translationalmodifications of sibling proteins and
their roles in osteogenesis and dentinogenesis. Crit. Rev. Oral Biol. Med. 15(3), 126–136.
Quarles, L. D. (2003). FGF23, PHEX and MEPE regulation of phosphate homeostasis and
skeletal mineralization. Am. J. Physiol. Endocrinol. Metab. 285(1), E1–E9.
Rajpar, M. H., Harley, K., Laing, C., Davies, R. M., and Dixon, M. J. (2001). Mutation of the
gene encoding the enamel‐specific protein, enamelin, causes autosomal‐dominant amelogen-
esis imperfecta. Hum. Mol. Genet. 10(16), 1673–1677.
![Page 73: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/73.jpg)
3. Regeneration of the Periodontium 119
Randall, L. E., and Hall, R. C. (2002). Temperospatial expression of matrix metalloproteinases
1, 2, 3, and 9 during early tooth development. Connect. Tissue Res. 43(2–3), 205–211.
Rani, C. S., and MacDougall, M. (2000). Dental cells express factors that regulate bone
resorption. Mol. Cell. Biol. Res. Commun. 3(3), 145–152.
Razzaque, M. S., Sitara, D., Taguchi, T., St‐Arnaud, R., and Lanske, B. (2006). Premature
aging‐like phenotype in fibroblast growth factor 23 null mice is a vitamin D‐mediated
process. FASEB J. 20(6), 720–722.
Reichenberg, E., Redlich, M., Cancemi, P., Zaks, B., Pitaru, S., Fontana, S., Pucci‐Minafra, I.,
and Palmon, A. (2005). Proteomic Analysis of Protein Components in Periodontal Ligament
Fibroblasts. J. Periodontol. 76(10), 1645–1653.
Reichenberger, E., Tiziani, V.,Watanabe, S., Park,L.,Ueki,Y., Santanna,C., Baur, S. T., Shiang,
R., Grange, D. K., Beighton, P., Gardner, J., Hamersma, H., et al. (2001). Autosomal
dominant craniometaphyseal dysplasia is caused by mutations in the transmembrane protein
ANK. Am. J. Hum. Genet. 68(6), 1321–1326.
Rincon, J. C., Xiao, Y., Young, W. G., and Bartold, P. M. (2005). Production of osteopontin
by cultured porcine epithelial cell rests of Malassez. J. Periodontal. Res. 40(5), 417–426.
Rios, H., Koushik, S. V., Wang, H., Wang, J., Zhou, H. M., Lindsley, A., Rogers, R., Chen, Z.,
Maeda, M., Kruzynska‐Frejtag, A., Feng, J. Q., and Conway, S. J. (2005). Periostin null
mice exhibit dwarfism, incisor enamel defects, and an early‐onset periodontal disease‐likephenotype. Mol. Cell. Biol. 25(24), 11131–11144.
Ripamonti, U. (2005). Bone induction by recombinant human osteogenic protein‐1 (hOP‐1,BMP‐7) in the primate Papio ursinus with expression of mRNA of gene products of the
TGF‐beta superfamily. J. Cell Mol. Med. 9(4), 911–928.
Ripamonti, U., and Reddi, A. H. (1997). Tissue engineering, morphogenesis and regeneration of
the periodontal tissues by bonemorphogenetic proteins.Crit. Rev. Oral Biol. Med. 8, 154–163.
Risbud, M. V., and Shapiro, I. M. (2005). Stem cells in craniofacial and dental tissue
engineering. Orthod. Craniofac. Res. 8(2), 54–59.
Risnes, S., Peterkova, R., and Lesot, H. (2005). Distribution and structure of dental enamel in
incisors of Tabby mice. Arch. Oral Biol. 50(2), 181–184.
Robey, P. G. (2000). Stem cells near the century mark. J. Clin. Invest. 105(11), 1489–1491.
Robinson, C., Briggs, H. D., and Atkinson, P. J. (1981). Histology of enamel organ and
chemical composition of adjacent enamel in rat incisors. Calcif. Tissue Int. 33(5), 513–520.
Rowe, P. S. (2004). The wrickkened pathways of FGF23, MEPE and PHEX. Crit. Rev. Oral
Biol. Med. 15(5), 264–281.
Rowe, P. S., de Zoysa, P. A., Dong, R., Wang, H. R., White, K. E., Econs, M. J., and Oudet,
C. L. (2000). MEPE a new gene expressed in bone marrow and tumors causing osteomalacia.
Genomics 67(1), 54–68.
Rowe, P. S., Kumagai, Y., Gutierrez, G., Garrett, I. R., Blacher, R., Rosen, D., Cundy, J.,
Navvab, S., Chen, D., Drezner, M. K., Quarles, L. D., and Mundy, G. R. (2004). MEPE has
the properties of an osteoblastic phosphatonin and minhibin. Bone 34(2), 303–319.
Rutsch, F., and Terkeltaub, R. (2005). Deficiencies of physiologic calcification inhibitors and
low‐grade inflammation in arterial calcification: Lessons for cartilage calcification. Joint
Bone Spine 72(2), 110–118.
Rutsch, F., Schauerte, P., Kalhoff, H., Petrarulo, M., August, C., and Diekmann, L. (2000).
Low levels of urinary inorganic pyrophosphate indicating systemic pyrophosphate deficiency
in a boy with idiopathic infantile arterial calcification. Acta Paediatr. 89(10), 1265–1269.
Rutsch, F., Vaingankar, S., Johnson, K., Goldfine, I., Maddux, B., Schauerte, P., KalhoV, H.,
Sano, K., Boisvert, W. A., Superti‐Furga, A., and Terkeltaub, R. (2001). PC‐1 nucleoside
triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification.
Am. J. Pathol. 158(2), 543–554.
Ryu, O. H., Fincham, A. G., Hu, C. C., Zhang, C., Qian, Q., Bartlett, J. D., and Simmer, J. P.
(1999). Characterization of recombinant pig enamelysin activity and cleavage of recombi-
nant pig and mouse amelogenins. J. Dent. Res. 78(3), 743–750.
![Page 74: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/74.jpg)
120 Foster et al.
Sah, V. P., Attardi, L. D., Mulligan, G. J., Williams, B. O., Bronson, R. T., and Jacks, T.
(1995). A subset of p53‐deficient embryos exhibit exencephaly. Nat. Genet. 10(2), 175–180.
Saito,M., Iwase,M.,Maslan, S., Nozaki, N., Yamauchi,M., Handa, K., Takahashi, O., Sato, S.,
Kawase, T., Teranaka, T., and Narayanan, A. S. (2001). Expression of cementum‐derivedattachment protein in bovine tooth germ during cementogenesis. Bone 29(3), 242–248.
Saito,M.,Handa,K.,Kiyono, T., Hattori, S., Yokoi, T., Tsubakimoto, T., Harada,H.,Noguchi,
T., Toyoda,M., Sato, S., andTeranaka,T. (2005). Immortalizationof cementoblast progenitor
cells with Bmi‐1 and TERT. J. Bone Miner. Res. 20(1), 50–57.
Saito, Y., Yoshizawa, T., Takizawa, F., Ikegame, M., Ishibashi, O., Okuda, K., Hara, K.,
Ishibashi, K., Obinata, M., and Kawashima, H. (2002). A cell line with characteristics of the
periodontal ligament fibroblasts is negatively regulated for mineralization and Runx2/Cbfa1/
Osf2 activity, part of which can be overcome by bone morphogenetic protein‐2. J. Cell Sci.115(Pt. 21), 4191–4200.
Sakata,M.,Shiba,H.,Komatsuzawa,H.,Fujita,T.,Ohta,K.,Sugai,M.,Suginaka,H., andKurihara,
H. (1999).Expressionofosteoprotegerin (osteoclastogenesis inhibitory factor) inculturesofhuman
dental mesenchymal cells and epithelial cells. J. BoneMiner. Res. 14(9), 1486–1492.
Satokata, I., Ma, L., Ohshima, H., Bei, M., Woo, I., Nishizawa, K., Maeda, T., Takano, Y.,
Uchiyama, M., Heaney, S., Peters, H., Tang, Z., et al. (2000). Msx2 deficiency in mice causes
pleiotropic defects in bone growth and ectodermal organ formation.Nat. Genet. 24(4), 391–395.
Saygin, N. E., Giannobile, W. V., and Somerman, M. J. (2000). Molecular and cell biology of
cementum. Periodontology 2000 24, 73–98.
Seo, B. M., Miura, M., Gronthos, S., Bartold, P. M., Batouli, S., Brahim, J., Young, M.,
Robey, P. G., Wang, C. Y., and Shi, S. (2004). Investigation of multipotent postnatal stem
cells from human periodontal ligament. Lancet 364(9429), 149–155.
Seo, B. M., Miura, M., Sonoyama, W., Coppe, C., Stanyon, R., and Shi, S. (2005). Recovery of
stem cells from cryopreserved periodontal ligament. J. Dent. Res. 84(10), 907–912.
Sharpe, P. T. (2001). Fish scale development: Hair today, teeth and scales yesterday? Curr. Biol.
11(18), R751–R752.
Shaw, W. J., Campbell, A. A., Paine, M. L., and Snead, M. L. (2004). The COOH terminus of
the amelogenin, LRAP is oriented next to the hydroxyapatite surface. J. Biol. Chem. 279(39),
40263–40266.
Shi, S., Robey, P. G., and Gronthos, S. (2001). Comparison of human dental pulp and bone
marrow stromal stem cells by cDNA microarray analysis. Bone 29(6), 532–539.
Shi, S., Bartold, P. M., Miura, M., Seo, B. M., Robey, P. G., and Gronthos, S. (2005). The
eYcacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod.
Craniofac. Res. 8(3), 191–199.
Shigeyama, Y., Grove, T. K., Strayhorn, C., and Somerman, M. J. (1996). Expression of
adhesion molecules during tooth resorption in feline teeth: A model system for aggressive
osteoclastic activity. J. Dent. Res. 75(9), 1650–1657.
Shimizu, E., Saito, R., Nakayama, Y., Nakajima, Y., Kato, N., Takai, H., Kim, D. S., Arai, M.,
Simmer, J., and Ogata, Y. (2005). Amelogenin stimulates bone sialoprotein (BSP) expression
through fibroblast growth factor 2 response element and transforming growth factor‐betalactivation element in the promoter of the BSP gene. J. Periodontol. 76(9), 1482–1489.
Shimo, T., Wu, C., Billings, P. C., Piddington, R., Rosenbloom, J., Pacifici, M., and Koyama, E.
(2002).Expression, gene regulation, and roles ofFisp12/CTGF indeveloping tooth germs.Dev.
Dyn. 224(3), 267–278.
Simmer, J. P., and Fincham, A. G. (1995). Molecular machanisms of dental enamel formation.
Crit. Rev. Oral Biol. Med. 6(2), 84–108.
Simmer, J. P., and Hu, J. C. (2002). Expression, structure, and function of enamel proteinases.
Connect. Tissue Res. 43(2–3), 441–449.
Simmer, J. P., Fukae, M., Tanabe, T., Yamakoshi, Y., Uchida, T., Xue, J., Margolis, H. C.,
Shimizu,M.,DeHart,B.C.,Hu,C.C., andBartlett, J.D. (1998). Purification, characterization,
and cloning of enamel matrix serine proteinase 1. J. Dent. Res. 77(2), 377–386.
![Page 75: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/75.jpg)
3. Regeneration of the Periodontium 121
Sitara, D., Razzaque, M. S., Hesse, M., Yoganathan, S., Taguchi, T., Erben, R. G., Juppner, H.,
and Lanske, B. (2004). Homozygous ablation of fibroblast growth factor‐23 results in
hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex‐deficient mice.Matrix Biol. 23(7), 421–432.
Slavkin, H. C. (1976). Towards a cellular and molecular understanding of periodontics.
Cementogenesis revisited. J. Periodontol. 47(5), 249–255.
Slavkin, H. C., and Boyde, A. (1975). Cementum: An epithelial secretory product? J. Dent. Res.
53, 157.
Slavkin, H. C., Bessem, C., Fincham, A. G., Bringas, P., Jr., Santos, V., Snead, M. L., and
Zeichner‐David, M. (1989a). Human and mouse cementum proteins immunologically related
to enamel proteins. Biochim. Biophys. Acta 991(1), 12–18.
Slavkin, H. C., Bringas, P., Jr., Bessem, C., Santos, V., Nakamura, M., Hsu, M. Y., Snead,
M. L., Zeichner‐David, M., and Fincham, A. G. (1989b). Hertwig’s epithelial root sheath
diVerentiation and initial cementum and bone formation during long‐term organ culture of
mouse mandibular first molars using serumless, chemically‐defined medium. J. Periodontal.
Res. 24(1), 28–40.
Sodek, J., and McKee, M. D. (2000). Molecular and cellular biology of alveolar bone.
Periodontol. 2000 24, 99–126.
Sodek, J., Ganss, B., and McKee, M. D. (2000). Osteopontin. Crit. Rev. Oral Biol. Med. 11(3),
279–303.
Sorsa, T., Tjaderhane, L., and Salo, T. (2004). Matrix metalloproteinases (MMPs) in oral
diseases. Oral Dis. 10(6), 311–318.
Speer, M. Y., McKee, M. D., Guldberg, R. E., Liaw, L., Yang, H. Y., Tung, E., Karsenty, G.,
and Giachelli, C. M. (2002). Inactivation of the osteopontin gene enhances vascular
calcification of matrix Gla protein‐deficient mice: Evidence for osteopontin as an inducible
inhibitor of vascular calcification in vivo. J. Exp. Med. 196(8), 1047–1055.
Sreenath, T., Thyagarajan, T., Hall, B., Longenecker, G., D’Souza, R., Hong, S., Wright, J. T.,
MacDougall, M., Sauk, J., and Kulkarni, A. B. (2003). Dentin sialophosphoprotein
knockout mouse teeth display widened predentin zone and develop defective dentin
mineralization similar to human dentinogenesis imperfecta type III. J. Biol. Chem. 278(27),
24874–24880.
Steele‐Perkins, G., Butz, K. G., Lyons, G. E., Zeichner‐David, M., Kim, H. J., Cho, M. I., and
Gronostajski, R. M. (2003). Essential role for NFI‐C/CTF transcription‐replication factor in
tooth root development. Mol. Cell. Biol. 23(3), 1075–1084.
Steele‐Perkins, G., Plachez, C., Butz, K. G., Yang, G., Bachurski, C. J., Kinsman, S. L., Litwack,
E. D., Richards, L. J., and Gronostajski, R. M. (2005). The transcription factor gene Nfib is
essential for both lung maturation and brain development. Mol. Cell. Biol. 25(2), 685–698.
St‐Jacques, B., Hammerschmidt, M., and McMahon, A. P. (1999). Indian hedgehog signaling
regulates proliferation and differentiation of chondrocytes and is essential for bone
formation. Genes Dev. 13(16), 2072–2086.
Stock, D. W., Ellies, D. L., Zhao, Z., Ekker, M., Ruddle, F. H., and Weiss, K. M. (1996).
The evolution of the vertebrate Dlx gene family. Proc. Natl. Acad. Sci. USA 93(20),
10858–10863.
Suzuki, H., Amizuka, N., Kii, I., Kawano, Y., Nozawa‐Inoue, K., Suzuki, A., Yoshie, H.,
Kudo, A., and Maeda, T. (2004). Immunohistochemical localization of periostin in tooth
and its surrounding tissues in mouse mandibles during development. Anat. Rec. A Discov.
Mol. Cell Evol. Biol. 281(2), 1264–1275.
Suzuki, S., Nagano, T., Yamakoshi, Y., Gomi, K., Arai, T., Fukae,M., Katagiri, T., andOida, S.
(2005). Enamel matrix derivative gel stimulates signal transduction of BMP and TGF‐{beta}.J. Dent. Res. 84(6), 510–514.
Swanson, E. C., Fong, H. K., Foster, B. L., Paine, M. L., Gibson, C. W., Snead, M. L., and
Somerman, M. J. (2006). Amelogenins regulate expression of genes associated with
cementoblasts in vitro. Eur. J. Oral Sci. 114(Suppl. 1), 1–6.
![Page 76: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/76.jpg)
122 Foster et al.
Taba, M., Jr., Jin, Q., Sugai, J. V., and Giannobile, W. V. (2005). Current concepts in
periodontal bioengineering. Orthod. Craniofac. Res. 8(4), 292–302.
Tai, G., Polak, J. M., Bishop, A. E., Christodoulou, I., and Buttery, L. D. (2004).
DiVerentiation of osteoblasts from murine embryonic stem cells by overexpression of the
transcriptional factor osterix. Tissue Eng. 10(9–10), 1456–1466.
Tai, G., Christodoulou, I., Bishop, A. E., and Polak, J. M. (2005). Use of green fluorescent
fusion protein to track activation of the transcription factor osterix during early osteoblast
diVerentiation. Biochem. Biophys. Res. Commun. 333(4), 1116–1122.
Takahashi, H., and Ikeda, T. (1996). Transcripts for two members of the transforming growth
factor‐beta superfamily BMP‐3 and BMP‐7 are expressed in developing rat embryos. Dev.
Dyn. 207(4), 439–449.
Takahashi, I., Nishimura, M., Onodera, K., Bae, J. W., Mitani, H., Okazaki, M., and Sasano, Y.
(2003). Expression of MMP‐8 and MMP‐13 genes in the periodontal ligament during tooth
movement in rats. J. Dent. Res. 82(8), 646–651.
Takahashi, N., Udagawa, N., and Suda, T. (1999). A new member of tumor necrosis factor
ligand family, ODF/OPGL/TRANCE/RANKL regulates osteoclast diVerentiation and
function. Biochem. Biophys. Res. Commun. 256(3), 449–455.
Takano, Y., Sakai, H., Watanabe, E., Ideguchi‐Ohma, N., Jayawardena, C. K., Arai, K.,
Asawa, Y., Nakano, Y., Shuda, Y., Sakamoto, Y., and Terashima, T. (2003). Possible role of
dentin matrix in region‐specific deposition of cellular and acellular extrinsic fibre cementum.
J. Electron. Microsc. (Tokyo) 52(6), 573–580.
Takayama, T., Suzuki, N., Narukawa, M., Tokunaga, T., Otsuka, K., and Ito, K. (2005).
Enamel matrix derivative stimulates core binding factor alpha1/Runt‐related transcription
factor‐2 expression via activation of Smad1 in C2C12 cells. J. Periodontol. 76(2), 244–249.
Takeda, K., Shiba, H., Mizuno, N., Hasegawa, N., Mouri, Y., Hirachi, A., Yoshino, H.,
Kawaguchi, H., and Kurihara, H. (2005). Brain‐derived neurotrophic factor enhances
periodontal tissue regeneration. Tissue Eng. 11(9–10), 1618–1629.
Tartaix, P. H., Doulaverakis, M., George, A., Fisher, L. W., Butler, W. T., Qin, C., Salih, E.,
Tan, M., Fujimoto, Y., Spevak, L., and Boskey, A. L. (2004). In vitro eVects of dentin matrix
protein‐1 on hydroxyapatite formation provide insights into in vivo functions. J. Biol. Chem.
279(18), 18115–18120.
Ten Cate, A. R. (1997). The development of the periodontium — a largely ectomesenchymally
derived unit. Periodontol. 2000 13, 9–19.
Terkeltaub, R. A. (2001). Inorganic pyrophosphate generation and disposition in pathophysi-
ology. Am. J. Physiol. Cell Physiol. 281(1), C1–C11.
ThesleV, I. (2003). Epithelial‐mesenchymal signalling regulating tooth morphogenesis. J. Cell
Sci. 116(Pt. 9), 1647–1648.
ThesleV, I., andAberg, T. (1999).Molecular regulation of tooth development.Bone 25(1), 123–125.
ThesleV, I., and Mikkola, M. (2002). The role of growth factors in tooth development. Int. Rev.
Cytol. 217, 93–135.
ThesleV, I., and Tummers, M. (2003). Stem cells and tissue engineering: Prospects for
regenerating tissues in dental practice. Med. Princ. Pract. 12(Suppl. 1), 43–50.
ThesleV, I., Vaahtokari, A., and Partanen, A. M. (1995). Regulation of organogenesis.
Common molecular mechanisms regulating the development of teeth and other organs. Int.
J. Dev. Biol. 39(1), 35–50.
Thesleff, I., Keranen, S., and Jernvall, J. (2001). Enamel knots as signaling centers linking tooth
morphogenesis and odontoblast differentiation. Adv. Dent. Res. 15, 14–18.
Thomas, B. L., Tucker, A. S., Qui, M., Ferguson, C. A., Hardcastle, Z., Rubenstein, J. L., and
Sharpe, P. T. (1997). Role of Dlx‐1 and Dlx‐2 genes in patterning of the murine dentition.
Development 124(23), 4811–4818.
![Page 77: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/77.jpg)
3. Regeneration of the Periodontium 123
Thomas, G., MoVatt, P., Salois, P., Gaumond, M. H., Gingras, R., Godin, E., Miao, D.,
Goltzman, D., and Lanctot, C. (2003). Osteocrin, a novel bone‐specific secreted protein that
modulates the osteoblast phenotype. J. Biol. Chem. 278(50), 50563–50571.
Thomas, H. F. (1995). Root formation. Int. J. Dev. Biol. 39(1), 231–237.
Tokiyasu, Y., Takata, T., Saygin, E., and Somerman, M. J. (2000). Enamel factors regulate
expression of genes associated with cementoblasts. J. Periodontol. 71(12), 1829–1839.
Tompkins, K., and Veis, A. (2002). Polypeptides translated from alternatively spliced
transcripts of the amelogenin gene, devoid of the exon 6a, b, c region, have specific eVects
on tooth germ development in culture. Connect. Tissue Res. 43(2–3), 224–231.
Tompkins, K., Alvares, K., George, A., and Veis, A. (2005). Two related low molecular mass
polypeptide isoforms of amelogenin have distinct activities in mouse tooth germ
diVerentiation in vitro. J. Bone Miner. Res. 20(2), 341–349.
Tompkins, K., George, A., and Veis, A. (2006). Characterization of a mouse amelogenin [A‐4]/M59 cell surface receptor. Bone 38(2), 172–180.
Tsubota, M., Sasano, Y., Takahashi, I., Kagayama, M., and Shimauchi, H. (2002). Expression
of MMP‐8 and MMP‐13 mRNAs in rat periodontium during tooth eruption. J. Dent. Res.
81(10), 673–678.
Tsuda, E., Goto, M., Mochizuki, S., Yano, K., Kobayashi, F., Morinaga, T., and Higashio, K.
(1997). Isolation of a novel cytokine from human fibroblasts that specifically inhibits
osteoclastogenesis. Biochem. Biophys. Res. Commun. 234(1), 137–142.
Tucker, A., and Sharpe, P. (2004). The cutting‐edge of mammalian development; how the
embryo makes teeth. Nat. Rev. Genet. 5(7), 499–508.
Tucker, A. S., Headon, D. J., Schneider, P., Ferguson, B. M., Overbeek, P., Tschopp, J., and
Sharpe, P. T. (2000). Edar/Eda interactions regulate enamel knot formation in tooth
morphogenesis. Development 127(21), 4691–4700.
Tummers, M., and ThesleV, I. (2003). Root or crown: A developmental choice orchestrated by
the diVerential regulation of the epithelial stem cell niche in the tooth of two rodent species.
Development 130(6), 1049–1057.
Vaes, B. L., Dechering, K. J., van Someren, E. P., Hendriks, J. M., van de Ven, C. J., Feijen, A.,
Mummery, C. L., Reinders, M. J., Olijve, W., van Zoelen, E. J., and Steegenga, W. T. (2005).
Microarray analysis reveals expression regulation of Wnt antagonists in differentiating
osteoblasts. Bone 36(5), 803–811.
van denBos, T., Handoko,G., Niehof, A., Ryan,L.M., Coburn, S. P.,Whyte,M. P., andBeertsen,
W. (2005). Cementum and dentin in hypophosphatasia. J. Dent. Res. 84(11), 1021–1025.
Veis, A. (2003). Amelogenin gene splice products: Potential signaling molecules. Cell. Mol. Life
Sci. 60(1), 38–55.
Veis, A., Tompkins, K., Alvares, K., Wei, K., Wang, L., Wang, X. S., Brownell, A. G., Jengh,
S. M., and Healy, K. E. (2000). Specific amelogenin gene splice products have signaling
eVects on cells in culture and in implants in vivo. J. Biol. Chem. 275(52), 41263–41272.
Venezia, E., Goldstein, M., Boyan, B. D., and Schwartz, Z. (2004). The use of enamel matrix
derivative in the treatment of periodontal defects: A literature review and meta‐analysis.Crit. Rev. Oral Biol. Med. 15(6), 382–402.
Viswanathan, H. L., Berry, J. E., Foster, B. L., Gibson, C. W., Li, Y., Kulkarni, A. B., Snead,
M. L., and Somerman, M. J. (2003). Amelogenin: A potential regulator of cementum
associated genes. J. Periodontol. 74, 1423–1431.
Wang, H., Tannukit, S., Zhu, D., Snead, M. L., and Paine, M. L. (2005a). Enamel matrix
protein interactions. J. Bone Miner. Res. 20(6), 1032–1040.
Wang,H. L.,Greenwell,H., Fiorellini, J., Giannobile,W., OVenbacher, S., Salkin, L., Townsend,
C., Sheridan, P., and Genco, R. J. (2005b). Periodontal regeneration. J. Periodontol. 76(9),
1601–1622.
![Page 78: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/78.jpg)
124 Foster et al.
Wang, X., Kua, H. Y., Hu, Y., Guo, K., Zeng, Q., Wu, Q., Ng, H. H., Karsenty, G., de
Crombrugghe, B., Yeh, J., and Li, B. (2006). p53 functions as a negative regulator of
osteoblastogenesis, osteoblast‐dependent osteoclastogenesis, and bone remodeling. J. Cell
Biol. 172(1), 115–125.
Wang, X. P., Aberg, T., James, M. J., Levanon, D., Groner, Y., and ThesleV, I. (2005c). Runx2
(Cbfa1) inhibits Shh signaling in the lower but not upper molars of mouse embryos and
prevents the budding of putative successional teeth. J. Dent. Res. 84(2), 138–143.
Wentz, F. M., Weinmann, J. P., and Schour, I. (1950). Prevalence, distribution and
morphological changes of the epithelial remnants in the molar region of the rat. J. Dent.
Res. 29, 639–646.
Westendorf, J. J., Kahler, R. A., and Schroeder, T. M. (2004). Wnt signaling in osteoblasts and
bone diseases. Gene 341, 19–39.
White, K. E., Larsson, T. E., and Econs, M. J. (2006). The roles of specific genes implicated as
circulating factors involved in normal and disordered phosphate homeostasis: Frizzled
related protein‐4, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23.
Endocr. Rev. 27(3), 221–241.
Whyte, M. P. (2002). Hypophosphatasia Nature’s window to alkaline phosphatase in man.
In ‘‘Principles of Bone Biology’’ (Bilezikian, Raisz, and Rodan, Eds.), pp. 1229–1248.
Academic Press, San Diego, CA.
Whyte, M. P., Landt, M., Ryan, L. M., Mulivor, R. A., Henthorn, P. S., Fedde, K. N.,
Mahuren, J. D., and Coburn, S. P. (1995). Alkaline phosphatase: Placental and tissue‐nonspecific isoenzymes hydrolyze phosphoethanolamine, inorganic pyrophosphate, and
pyridoxal 50‐phosphate. Substrate accumulation in carriers of hypophosphatasia corrects
during pregnancy. J. Clin. Invest. 95(4), 1440–1445.
Wilde, J., Yokozeki, M., Terai, K., Kudo, A., and Moriyama, K. (2003). The divergent
expression of periostin mRNA in the periodontal ligament during experimental tooth
movement. Cell Tissue Res. 312(3), 345–351.
Wise, G. E., Frazier‐Bowers, S., and D’Souza, R. N. (2002). Cellular, molecular, and genetic
determinants of tooth eruption. Crit. Rev. Oral Biol. Med. 13(4), 323–334.
Wise, G. E., Yao, S., Odgren, P. R., and Pan, F. (2005). CSF‐1 regulation of osteoclastogenesis
for tooth eruption. J. Dent. Res. 84(9), 837–841.
Wright, J. T., Hart, P. S., Aldred, M. J., Seow, K., Crawford, P. J., Hong, S. P., Gibson, C. W.,
and Hart, T. C. (2003). Relationship of phenotype and genotype in X‐linked amelogenesis
imperfecta. Connect. Tissue Res. 44(Suppl. 1), 72–78.
Wu, D., Ikezawa, K., Parker, T., Saito, M., and Narayanan, A. S. (1996). Characterization of a
collagenous cementum‐derived attachment protein. J. Bone Miner. Res. 11(5), 686–692.
Xiao,G., Jiang,D.,Ge, C., Zhao, Z., Lai, Y., Boules,H., Phimphilai,M., Yang,X., Karsenty,G.,
and Franceschi, R. T. (2005). Cooperative interactions between activating transcription factor
4 and Runx2/Cbfa1 stimulate osteoblast‐specific osteocalcin gene expression. J. Biol. Chem.
280(35), 30689–30696.
Xu, H. H., Smith, D. T., Jahanmir, S., Romberg, E., Kelly, J. R., Thompson, V. P., and Rekow,
E. D. (1998). Indentation damage and mechanical properties of human enamel and dentin.
J. Dent. Res. 77(3), 472–480.
Yamaai, T., Nakanishi, T., Asano, M., Nawachi, K., Yoshimichi, G., Ohyama, K., Komori, T.,
Sugimoto, T., and Takigawa, M. (2005). Gene expression of connective tissue growth factor
(CTGF/CCN2) in calcifying tissues of normal and cbfa1‐null mutant mice in late stage of
embryonic development. J. Bone Miner. Metab. 23(4), 280–288.
Yamakoshi, Y., Hu, J. C., Fukae, M., Yamakoshi, F., and Simmer, J. P. (2006). How do
enamelysin and kallikrein 4 process the 32‐kDa enamelin? Eur. J. Oral Sci. 114(Suppl. 1),
45–51.
![Page 79: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/79.jpg)
3. Regeneration of the Periodontium 125
Yamamoto, H., Cho, S. W., Kim, E. J., Kim, J. Y., Fujiwara, N., and Jung, H. S. (2004a).
Developmental properties of the Hertwig’s epithelial root sheath in mice. J. Dent. Res. 83(9),
688–692.
Yamamoto, T., Domon, T., Takahashi, S., Arambawatta, A. K., and Wakita, M. (2004b).
Immunolocation of proteoglycans and bone‐related noncollagenous glycoproteins in
developing acellular cementum of rat molars. Cell Tissue Res. 317(3), 299–312.
Yamashiro, T., Fukunaga, T., Yamashita, K., Kobashi, N., and Takano‐Yamamoto, T. (2001).
Gene and protein expression of brain‐derived neurotrophic factor and TrkB in bone and
cartilage. Bone 28(4), 404–409.
Yamashiro, T., Tummers, M., and ThesleV, I. (2003). Expression of bone morphogenetic
proteins and Msx genes during root formation. J. Dent. Res. 82(3), 172–176.
Yamashiro, T., Wang, X. P., Li, Z., Oya, S., Aberg, T., Fukunaga, T., Kamioka, H., Speck,
N. A., Takano‐Yamamoto, T., and ThesleV, I. (2004). Possible roles of runx1 and sox9 in
incipient intramembranous ossification. J. Bone Miner. Res. 19(10), 1671–1677.
Yanagita, M. (2005). BMP antagonists: Their roles in development and involvement in
pathophysiology. Cytokine Growth Factor Rev. 16(3), 309–317.
Yang, X., Matsuda, K., Bialek, P., Jacquot, S., Masuoka, H. C., Schinke, T., Li, L.,
Brancorsini, S., Sassone‐Corsi, P., Townes, T. M., Hanauer, A., and Karsenty, G. (2004).
ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for
CoYn‐Lowry Syndrome. Cell 117(3), 387–398.
Yao, S., Norton, J., and Wise, G. E. (2004). Stability of cultured dental follicle cells. Cell Prolif.
37(3), 247–254.
Yao, S., Ring, S., Henk, W. G., and Wise, G. E. (2004). In vivo expression of RANKL in the rat
dental follicle as determined by laser capture microdissection. Arch. Oral Biol. 49(6),
451–456.
Yasuda, H., Shima, N., Nakagawa, N., Mochizuki, S. I., Yano, K., Fujise, N., Sato, Y., Goto,
M., Yamaguchi, K., Kuriyama, M., Kanno, T., Murakami, A., et al. (1998). Identity of
osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): A mechanism by
which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139(3), 1329–1337.
Ye, L., MacDougall, M., Zhang, S., Xie, Y., Zhang, J., Li, Z., Lu, Y., Mishina, Y., and Feng,
J. Q. (2004). Deletion of dentin matrix protein‐1 leads to a partial failure of maturation of
predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal
during postnatal tooth development. J. Biol. Chem. 279(18), 19141–19148.
Ye, L., Mishina, Y., Chen, D., Huang, H., Dallas, S. L., Dallas, M. R., Sivakumar, P.,
Kunieda, T., Tsutsui, T. W., Boskey, A., Bonewald, L. F., and Feng, J. Q. (2005). Dmp1‐deficient mice display severe defects in cartilage formation responsible for a chondrodys-
plasia‐like phenotype. J. Biol. Chem. 280(7), 6197–6203.
Yokohama‐Tamaki, T., Ohshima, H., Fujiwara, N., Takada, Y., Ichimori, Y., Wakisaka, S.,
Ohuchi, H., and Harada, H. (2006). Cessation of Fgf10 signaling, resulting in a defective
dental epithelial stem cell compartment, leads to the transition from crown to root
formation. Development 133(7), 1359–1366.
Yonemura,K.,Narayanan,A. S.,Miki,Y., Page,R.C., andOkada,H. (1992). Isolation andpartial
characterization of a growth factor from human cementum. Bone Miner. 18(3), 187–198.
Yonemura, K., Raines, E. W., Ahn, N. G., and Narayanan, A. S. (1993). Mitogenic signaling
mechanismsof human cementum‐derivedgrowth factors.J. Biol.Chem. 268(35), 26120–26126.
Young, C. S., Terada, S., Vacanti, J. P., Honda, M., Bartlett, J. D., and Yelick, P. C. (2002).
Tissue engineering of complex tooth structures on biodegradable polymer scaVolds. J. Dent.
Res. 81(10), 695–700.
Yu, X., and White, K. E. (2005). FGF23 and disorders of phosphate homeostasis. Cytokine
Growth Factor Rev. 16(2), 221–232.
![Page 80: Adv in Cementum Devt[1]](https://reader034.fdocuments.net/reader034/viewer/2022051113/55cf99ce550346d0339f453c/html5/thumbnails/80.jpg)
126 Foster et al.
Zeichner‐David, M. (2001). Is there more to enamel matrix proteins than biomineralization?
Matrix Biol. 20(5–6), 307–316.
Zeichner‐David, M. (2006). Regeneration of periodontal tissues: Cementogenesis revisited.
Periodontol. 2000 41, 196–217.
Zeichner‐David, M., Vo, H., Tan, H., Diekwisch, T., Berman, B., Thiemann, F., Alcocer,
M. D., Hsu, P., Wang, T., Eyna, J., Caton, J., Slavkin, H. C., et al. (1997). Timing of the
expression of enamel gene products during mouse tooth development. Int. J. Dev. Biol. 41(1),
27–38.
Zeichner‐David, M., Oishi, K., Su, Z., Zakartchenko, V., Chen, L. S., Arzate, H., and Bringas,
P., Jr. (2003). Role of Hertwig’s epithelial root sheath cells in tooth root development. Dev.
Dyn. 228(4), 651–663.
Zhang, Y., Zhang, Z., Zhao, X., Yu, X., Hu, Y., Geronimo, B., Fromm, S. H., and Chen, Y. P.
(2000). A new function of BMP4: Dual role for BMP4 in regulation of Sonic hedgehog
expression in the mouse tooth germ. Development 127(7), 1431–1443.
Zhang, Y. D., Chen, Z., Song, Y. Q., Liu, C., and Chen, Y. P. (2005). Making a tooth: Growth
factors, transcription factors, and stem cells. Cell Res. 15(5), 301–316.
Zhao, G. Q. (2003). Consequences of knocking out BMP signaling in the mouse. Genesis 35(1),
43–56.
Zhao, M., Reddi, A., Jin, Q., Berry, J. E., and Somerman, M. J. (2001). BMP‐2 induces
diVerentiation of dental follicle cells. J. Dent. Res. 80, 786.
Zohar, R., and Tenenbaum, H. C. (2005). How predictable are periodontal regenerative
procedures? J. Can. Dent. Assoc. 71(9), 675–680.