Acute Pediatric Ulcerative Colitis: Lessons from the OSCI Trial
description
Transcript of Acute Pediatric Ulcerative Colitis: Lessons from the OSCI Trial

Acute Pediatric Ulcerative Colitis: Lessons from the OSCI Trial
James Markowitz, MD
Professor of PediatricsHofstra – North Shore LIJ School of Medicine
Department of PediatricsCohen Children’s Medical Center of NY
New Hyde Park, NY

Disclosures
I have the following financial relationships :Janssen Biotech: research support,
consultantUCB: consultantAbbvie: consultant

What is acute severe colitis?

Acute Severe Colitis in Adults(European Crohn’s and Colitis Organization, 2008)
At least six bloody stools per day and
at least one of the following:
Tachycardia ( > 90 b.p.m.)Temperature > 37.8 ° CAnemia (hemoglobin < 10.5 g/ dl)Elevated ESR ( > 30 mm/ h)
Travis SPL, et al. J Crohn Colitis 2008;2 :24-62

Pediatric UC Activity Index (PUCAI)ITEM POINTS4. Number of stools per 24 hours0-23-56-8>8
05
1015
5. Nocturnal stools (any episode causing wakening)
NoYes
010
6. Activity levelNo limitation of activityOccasional limitation of activitySevere restricted activity
05
10
SUM OF PUCAI (0-85)
Turner D, et al. Gastroenterology 2007;133:423-32.
ITEM POINTS1. Abdominal pain:
No painPain can be ignoredPain cannot be ignored
05
10
2. Rectal bleedingNoneSmall amount only, in <50% of
stoolsSmall amount with most stoolsLarge amount (>50% of the
stool content)
010
20
30
3. Stool consistency of most stoolsFormedPartially formedCompletely unformed
05
10

PUCAI cutoffs (n=205)
None Mild Moderate Severe
Physician global assessment
0
20
40
60
80
100
PUC
AI s
core
170
13853
20
142
None ModerateMild Severe
PUCA
I sco
re
None: <10
Mild: 10-30
Mod: 35-60
Severe: >65
0.99 (0.99-1)
0.98 (0.97-0.99)
0.97 (0.95-0.99)
0.97 (0.95-0.99)
DefinitionDefinition AUC of AUC of ROCROC
Sens/Sens/SpecSpec
95%/99%
89%/94%
96%/91%
96%/91%
n=81
n=42
n=57
n=25
r=0.91P<0.001
Turner D et al; Gastroenterology 2007;133:423-432

How common is acute severe colitis?

Acute Severe Colitis: FrequencyAdults: 18-25% of patients over 10 years
Edwards FC, Truelove SC. Gut. 1963 Dec;4:299-315.Dinesen LC, et al. J Crohn Colitis. 2010 Oct;4(4):431-7. Epub 2010 Feb 19.
Children: Retrospective review of admissions to Hospital for
Sick Children in Toronto (1991-1996)“Population based” sample estimate (only children with
addresses from the Greater Toronto area)55/196 (28%) of children with UC <15 yrs of age
admitted for IV corticosteroidsTurner D, et al. Gut. 2008 Mar;57(3):331-8.

Natural History of UC:Looking back the picture was not prettyAuthor Year Study cohort Outcome
Hardy1 1933 95 adults 75% mortality at 1 year
Truelove/Witts2 1955 Adult UC treated with HC
6% HC and 15% control pts died within 6 months
Goel3 1973 25 hospitalized children with UC
20% died (post-colectomy), 19 had chronic disease
Michener4 1979 336 children 35% colectomy, 5% died (cancer); 69% chronically ill
Langholz5 1994 1161 adults 30% colectomy by 15 years
1BMJ 1933;2:812; 2BMJ 1955;4947:1041; 3Arch Dis Child 1973;48:337; 4J Clin Gastroenterol 1979;1:301; 5Gastroenterology 1994;107:3

Cumulative Probability of Colectomy In Children with UC (1975-1995)
Mild vs moderate/severe, P<0.03
Hyams JS et al. J Pediatr. 1996;129:81.
Colectomy Rate(N=171)
1 Yr 5% (2%,9%)
5 Yr 19% (12%, 25%)

Cumulative Probability of Colectomy in Children with UC (1975-1995)
Mild vs moderate/severe, P<0.03
Hyams JS et al. J Pediatr. 1996;129:81.
0.4
0.3
0.2
0.1
0.0 0 1 2 3 4 5 6 7
Years From Diagnosis
Cum
ulat
ive
Prob
abili
ty
Moderate/Severe (n=98)
Mild (n=73)26%
9%Colectomy Rate
(N=171)
1 Yr 5% (2%,9%)
5 Yr 19% (12%, 25%)

How might we change the outcome of children with acute
severe colitis?

Severe/Fulminant Ulcerative Colitis
16 year old presents with10 bloody stools per day,Hb 9, unresponsive to oral corticosteroids

OSCI = Outcome of Steroid therapy in Colitis Individuals
What is the OSCI Trial?

How effective are intravenous corticosteroids
for acute severe UC?

IV Corticosteroids for Acute Severe UCIV Corticosteroids for Acute Severe UC
Study populationStudy populationN=99N=99
IV Steroid SuccessIV Steroid SuccessN=53 (53%)N=53 (53%)
IV Steroid FailureIV Steroid FailureN=46 (46%)N=46 (46%)
Toronto 1991-2000 (retrospective)Toronto 1991-2000 (retrospective)
Turner D et al. Gut 2008; 57:331-338Turner D et al. Gut 2008; 57:331-338
Short term: 53% avoid colectomy &/or rescue therapy1 Year:18% steroid free without colectomy &/or rescue therapy
Turner D et al Gastro 2010;138(7):2282-91 Turner D et al Gastro 2010;138(7):2282-91
Study populationStudy populationN=128N=128
IV Steroid SuccessIV Steroid SuccessN=91 (71%)N=91 (71%)
IV Steroid FailureIV Steroid FailureN=37 (29%)N=37 (29%)
OSCI 2008-2009 (prospective)OSCI 2008-2009 (prospective)
Short term: 71% avoid colectomy &/or rescue therapy1 Year:59% steroid free without colectomy &/or rescue therapy

What factors predict likely response to intravenous corticosteroids (IVCS)?

Predictors of IV Corticosteroid Response on Day 0: OSCI Trial
IV Steroid Success (n=91)
IV Steroid Failure (n=37)
Age at Rx (yrs)* 12.2 ± 4.1 14.4 ± 3.2% male 44% 54%+ Fam Hx IBD 12% 12%First UC attack* 56% 30%Months from Dx 12.4 (5-38) 11 (4-19.6)PUCAI score on admission
71 ± 12 74 ± 11
Turner D et al Gastro 2010;138(7):2282-91

How long should we wait for intravenous corticosteroids
to be effective?

2003 Peds GI Symposium Audience Response

Duration of Initial Therapy• Recommendations vary, depends upon absence of
absolute indications for surgery
• If you wait too long complications will occur– 7 days1,2
– 10 to 14 days3,4
– >14 days is acceptable5
• Treatment for 15 to 36 days• 1 patient to surgery, perforation, 11 units packed
RBC
1Truelove SC et al. Lancet. 1974;1:1067.2Goligher JC et al. Br Med J. 1970 Dec 19;4(737):703-6.3Werlin SL et al. Gastroenterology. 1977;73:828.
4Meyers S. J Clin Gastroenterol. 1990 Aug;12(4):479-80 5Gold et al. Am J Gastroenterol. 1995 May;90(5):732-5.

Predicting IV Corticosteroid Failure and Need for Rescue Therapy
Day 3: PUCAI >45 (moderate) – start planning Day 5: PUCAI >70 (severe) – time for 2nd line Rx
Turner D et al Gastro 2010;138(7):2282-91
Day 3 Day 5Sensitivity (95% CI) 92% (72 - 98) 35% (23 - 36)Specificity 50% (44 - 52) 100% (94 - 100)NPV 94% (84 - 98) 79% (68 - 97)PPV 43% (37 - 45) 100% (65 - 100)Odds ratio 11.1 (3 - 49);
P<0.000164 (5 - >1000);
P<0.0001

Time to 2nd Line Therapy Stratified by PUCAI on Day 3 and Day 5
Turner D et al Gastro 2010;138(7):2282-91

What therapy is best when iv corticosteroids fail?
Calcineurin inhibitors (cyclosporine, tacrolimus) vs
Anti-TNF (infliximab)

Tacrolimus in Peds UC
UCLAUCLA 18 patients18 patients Retrospective, open-labelRetrospective, open-label
9 steroid resistant UC9 steroid resistant UC
Acute response: Acute response: 8/9 (89%)8/9 (89%)
Colectomy by 1 yr: Colectomy by 1 yr: 6/9 (67%)6/9 (67%)
MulticenterMulticenter 14 patients14 patients Prospective, open-labelProspective, open-label
10 UC, 2 CD, 2 IC10 UC, 2 CD, 2 IC Minimum 7 days of Minimum 7 days of
severe colitis despite CSsevere colitis despite CS Acute response:Acute response:
13/14 (93%) 13/14 (93%) Colectomy by 1 yr:Colectomy by 1 yr:
8/13 (62%)8/13 (62%)
Ziring at al. JPGN 2007;45(3):306-311.Ziring at al. JPGN 2007;45(3):306-311. Bousvaros A et al. Bousvaros A et al. J PediatrJ Pediatr. 2000;137:794. 2000;137:794

Tacrolimus
Pediatric Experience (Boston Children’s)Pediatric Experience (Boston Children’s) 46 children, retrospective, open-label46 children, retrospective, open-label
All steroid refractory UCAll steroid refractory UC 43/46 (93%) acute response43/46 (93%) acute response 60% colectomy at 26 months60% colectomy at 26 months
Watson S et al. Inflamm Bowel Dis. 2011 Jan;17(1):22-9. Epub 2010 Aug 18

Infliximab Induction in Chronic Active UCOpen label induction with 5 mg/kg infliximab at 0, 2, 6 weeks
Treatment outcome assessed at 8 weeks
10%
36%40%33%
67%73%
0%
10%
20%
30%
40%
50%
60%
70%
80%
T72 ACT 1/2 ACT 1/2 placebo
RemissionResponse
Adult trialsPediatric trialHyams J et al. Clin Gastroenterol Hepatol 2012;10(4):391-9 Rutgeerts P, at al. N Engl J Med 2005;353:2462-76

Infliximab in Acute Severe ColitisSwedish double-blind placebo controlled Swedish double-blind placebo controlled
adult UC trialadult UC trial24 infliximab, 21 placebo24 infliximab, 21 placebo3 month colectomy rates3 month colectomy rates
Infliximab: 7/24 (29%)Infliximab: 7/24 (29%)Placebo: 14/21 (67%)Placebo: 14/21 (67%)
(P = .017; odds ratio, 4.9; 95% confidence interval, 1.4-17) (P = .017; odds ratio, 4.9; 95% confidence interval, 1.4-17)
Jarnerot G, et al. Gastroenterol. 2005 Jun;128(7):1805-11Jarnerot G, et al. Gastroenterol. 2005 Jun;128(7):1805-11

2nd Line Rx for Acute Severe UC:Infliximab
Turner D et al Gastro 2010;138(7):2282-91Turner D et al Gastro 2010;138(7):2282-91
Study populationStudy populationN=128N=128
IV Steroid SuccessIV Steroid SuccessN=91 (71%)N=91 (71%)
IV Steroid FailureIV Steroid FailureN=37 (29%)N=37 (29%)
InfliximabInfliximabN=33N=33
ColectomyColectomyN=3N=3
CyclosporineCyclosporineN=1N=1
OSCI 2008-2009 (prospective)OSCI 2008-2009 (prospective)Short Term Response to Infliximab: 25/33 + 5/7 = 30/40 (75%) avoid imminent colectomyResponse at 1 Year 18/33 + 5/7 = 23/40 (58%) avoid colectomy
Acute OutcomeAcute OutcomeResponse N = 25Response N = 25Colectomy N = 8Colectomy N = 8
Outcome at 1 YearOutcome at 1 YearColectomy N = 7Colectomy N = 7
Infliximab N=7Infliximab N=7Colectomy N=3Colectomy N=3
Outcome at 1 YearOutcome at 1 YearResponse N = 5Response N = 5Colectomy N = 2Colectomy N = 2

Colectomy Following Rescue Therapy for IVCS Resistant Acute Severe Colitis
0%10%20%30%40%50%60%70%80%90%
100%
Baseline Atdischarge
1 Yr 2+Yrs
Col
ecto
my
free
sur
viva
l (%
)
IFX (OSCI) n=33
Turner D et al Turner D et al Gastro 2010;138(7):2282-91Gastro 2010;138(7):2282-91
Turner D et al.Turner D et al.Gut 2008; 57:331-338Gut 2008; 57:331-338
Ziring at al. Ziring at al. JPGN 2007;45(3):306-311JPGN 2007;45(3):306-311
Bousvaros A et al. Bousvaros A et al. J PediatrJ Pediatr. 2000;137:794. 2000;137:794
Watson S et al. Watson S et al. IBD 2011;17(1):22-9IBD 2011;17(1):22-9

Colectomy Following Rescue Therapy for IVCS Resistant Acute Severe Colitis
0%10%20%30%40%50%60%70%80%90%
100%
Baseline Atdischarge
1 Yr 2+Yrs
Col
ecto
my
free
sur
viva
l (%
)
IFX (OSCI) n=33CsA/Tacro (Toronto) n=6Tacro (UCLA) n=9Tacro (Multicenter) n=14Tacro (Boston) n=46
Turner D et al Turner D et al Gastro 2010;138(7):2282-91Gastro 2010;138(7):2282-91
Turner D et al.Turner D et al.Gut 2008; 57:331-338Gut 2008; 57:331-338
Ziring at al. Ziring at al. JPGN 2007;45(3):306-311JPGN 2007;45(3):306-311
Bousvaros A et al. Bousvaros A et al. J PediatrJ Pediatr. 2000;137:794. 2000;137:794
Watson S et al. Watson S et al. IBD 2011;17(1):22-9IBD 2011;17(1):22-9

Cyclosporine vs Infliximab in Acute Severe UC: A parallel, open-label
randomised controlled adult trial
Patients (N=115) Severe, acute UC IV steroid resistant
Treatments IV CsA (2 mg/kg/d x 1 week,
then po x 98 days) IFX (5mg/kg at 0-2-6 wks) In patients with a clinical
response at week 7, azathioprine initiated
and steroids decreased
CsAN=58
IFXN=57
Treatment failure 60% 54%
Day 7 response 84% 86%
Day 98 colectomy (n) 10 13
SAE (n) 10 16
Laharie D et al. Lancet 2012; 380: 1909–15

What else can we do to decrease colectomy rates further?
Why are today’s outcomes following iv steroids better than in the past?
Remember to look for: C. Difficile
CMV

C difficile Complicating IBDC diff prevalence in hospitalized children
IBD (24.7%) vs non-IBD controls (8.9%)OR 3.3 (95%CI 1.5 to 7.6)
Pascarella F, et al. J Pediatr. 2009 Jun;154(6):854-8
Compared to adults with either condition
alone, those with both C diff and IBD havelengthier hospitalizationsfourfold increased mortality
Ananthakrishnan AN, McGinley EL, Binion DG. Gut. 2008 Feb;57(2):205-10

C difficile and IBDDiagnosis
Immunoassays or ELISAs for toxin A and toxin BCytotoxicity assayPCR
Diagnostic accuracyOne toxin assay fails to identify most infections
Toxin A assay missed 41.5% of infectionsToxin B assay missed 34.9% of infectionsMarkowitz JE, et al. Am J Gastroenterol. 2001 Sep;96(9):2688-90

C difficile Can be Difficult to Diagnose in IBD Despite Assay for Both Toxin A and B
54%
75% 78%
92%
0%10%20%30%40%50%60%70%80%90%
100%
1 2 3 4Number of Stool Samples
C d
iff p
ositi
ve s
ubje
cts
(%)
Issa M, et al. Clin Gastroenterol Hepatol. 2007 Mar;5(3):345-51

CMV Complicating UCCMV Complicating UC CMV disease = CMV in tissueCMV disease = CMV in tissue
Sigmoidoscopy and BxSigmoidoscopy and Bx Immunohistochemistry better than Immunohistochemistry better than
light microlight micro Patient characteristicsPatient characteristics
Seropositive; ImmunosuppressedSeropositive; Immunosuppressed Effects: steroid resistanceEffects: steroid resistance
5-36% of CS resistant UC vs 0-10% 5-36% of CS resistant UC vs 0-10% with CS sensitive UC with CS sensitive UC
Rx: Gangcyclovir, ?d/c immune Rx: Gangcyclovir, ?d/c immune suppressionsuppression
rihes.cmu.ac.thrihes.cmu.ac.th
markwickmd.commarkwickmd.com
Dommenech E, et al. Dommenech E, et al. Inflamm Bowel Dis Inflamm Bowel Dis 2008;14:1373–13792008;14:1373–1379


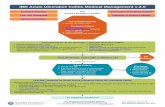
Lessons of the OSCI StudyAcute severe colitis continues to present
significant clinical challengesColectomy: 9% acutely, 19% at 1 year
Use PUCAI to judge severity of disease and response to therapyPUCAI >45 on Day 3
→ plan rescue Rx …(flex sig for CMV) PUCAI ≥70 on Day 5
→ time for 2nd line treatment

Both infliximab and calcineurin inhibitors Can induce remission in ~75% of children with
steroid resistant severe acute colitisCan avoid colectomy long term in 40-60%

Fulminant UC is still a very difficult group to treat
Surgery will never be an attractive alternative for most patients, but for some there is no choice
We need more data on how to treat our patients