ACIDS & BASES EQ: Why are some aqueous solutions acidic, others basic, and some neutral? What makes...
-
Upload
josephine-singleton -
Category
Documents
-
view
219 -
download
1
Transcript of ACIDS & BASES EQ: Why are some aqueous solutions acidic, others basic, and some neutral? What makes...

ACIDS & BASESACIDS & BASES
EQ: Why are some aqueous solutions EQ: Why are some aqueous solutions acidic, others basic, and some acidic, others basic, and some
neutral? What makes them that way?neutral? What makes them that way?
GPS: GPS: SC7. Students will characterize the properties that SC7. Students will characterize the properties that describe solutions and the nature of acids and describe solutions and the nature of acids and
bases.bases.b. Compare, contrast, and evaluate the nature of acids and b. Compare, contrast, and evaluate the nature of acids and
bases: bases: • • Arrhenius, Bronsted-Lowry Acid/Bases Arrhenius, Bronsted-Lowry Acid/Bases • • Strong vs. weak acids/bases in terms of percent dissociation Strong vs. weak acids/bases in terms of percent dissociation • • Hydronium ion concentration Hydronium ion concentration • • pH pH • • Acid-Base neutralizationAcid-Base neutralization

The Theory of IonizationThe Theory of Ionization
Many substances form ions when dissolved in
water.

AcidsAcids(Arrhenius’ Definition)(Arrhenius’ Definition)
Acids form hydrogen ions
(H+)when dissolved in water

AcidsAcids(Arrhenius’ Modified)(Arrhenius’ Modified)
Acids form hydronium ions
(H3O+) when dissolved in
water

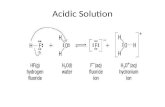
HCl +HHCl +H22O O
HH22SOSO44+2H+2H22O O
HH33OO++ + Cl + Cl--
2H2H33OO+++ SO+ SO44-2-2

Common AcidsCommon Acids
HCl HydrochloricHCl Hydrochloric HH22SOSO44 Sulfuric Sulfuric
HNOHNO33 Nitric Nitric
HH33POPO44 Phosphoric Phosphoric
HH22COCO33 Carbonic Carbonic

ACIDSACIDS
ACID: a chemical that produces ACID: a chemical that produces hydronium ions (Hhydronium ions (H33OO+1+1) when dissolved ) when dissolved in waterin water
Acids are ionic compounds ( a Acids are ionic compounds ( a compound with a positive or negative compound with a positive or negative charge) that break apart in water to charge) that break apart in water to form a hydrogen ion (H+).form a hydrogen ion (H+).
HH33OO+1+1 is just a hydrogen ion joined to a is just a hydrogen ion joined to a water molecule.water molecule.
Hydrogen is always the first element in Hydrogen is always the first element in the acid formulathe acid formula
HCl + HHCl + H22O O H H+1+1 + Cl + Cl-1-1 + H + H22O O H H33OO+1+1 + + ClCl-1-1
(H(H+1+1 is an acidic hydrogen or proton) is an acidic hydrogen or proton) HH+1+1 is used to mean the same as H is used to mean the same as H33OO+1+1
HH+1+1= = HH33OO+1+1

ACIDSACIDS
Properties:Properties: Sour taste (vinegar—acetic acid, Sour taste (vinegar—acetic acid,
lemons—citric acid)lemons—citric acid) Are electrolytes (compounds that Are electrolytes (compounds that
conduct electricity)conduct electricity) React w/ metals to form HReact w/ metals to form H2 2 gas; React gas; React
w/most metals to corrode them w/most metals to corrode them (Strong (Strong acids are dangerous and can burn your skin).acids are dangerous and can burn your skin).
Change indicator colors (blue to red)Change indicator colors (blue to red) React w/ carbonates (COReact w/ carbonates (CO33
-2-2) to form ) to form COCO22..
React w/or neutralize bases to form React w/or neutralize bases to form salt & watersalt & water
Produces HProduces H+ + ions in waterions in water pH<7pH<7

Bases Bases (Arrhenius’ Definition)(Arrhenius’ Definition)
Bases form hydroxide ions
(OH-)when dissolved in water

NaOH +HNaOH +H22O O
NaNa++ + OH + OH -- + H + H22O O
Mg(OH)Mg(OH)22 +H +H22O O
MgMg+2+2 + 2OH + 2OH -- + H + H22O O

Common BasesCommon Bases
NaOH Sodium HydroxideNaOH Sodium Hydroxide NHNH44OH Ammonium Hyd.OH Ammonium Hyd.
Ca(OH)Ca(OH)22 Calcium Hydrox. Calcium Hydrox.

BASES- BASES- AKA: AKA: alkalisalkalis
Base: chemical that produces hydroxide ions Base: chemical that produces hydroxide ions (OH (OH-1-1) when dissolved in water.) when dissolved in water.
Bases are ionic compounds that break apart Bases are ionic compounds that break apart to form a negatively charged hydroxide ion to form a negatively charged hydroxide ion (OH-) in water.(OH-) in water.
The strength of a base is determined by the The strength of a base is determined by the concentration of Hydroxide ions (OH-). The concentration of Hydroxide ions (OH-). The greater the concentration of OH- ions the greater the concentration of OH- ions the stronger the base.stronger the base.
Two kinds of bases:Two kinds of bases: 1. Ionic bases (Metal hydroxides: alkali & 1. Ionic bases (Metal hydroxides: alkali &
alkaline-earth metals)alkaline-earth metals) NaOHNaOH Na Na+1+1 + OH + OH-1-1
2. Covalent bases that accept H2. Covalent bases that accept H+1 +1 from waterfrom water
(NH(NH33 = ammonia) = ammonia) NHNH33 + H + H22O O NH NH44
+1+1 + OH + OH-1-1

BASES: propertiesBASES: properties
Bitter tasteBitter taste Feel slipperyFeel slippery Are electrolytesAre electrolytes Do not react with most metalsDo not react with most metals Change indicator colors (red to blue)Change indicator colors (red to blue) React w/ or neutralize acids to React w/ or neutralize acids to
produce salt & waterproduce salt & water Produce OHProduce OH- - in waterin water To name: name the metal & To name: name the metal &
hydroxidehydroxide pH>7pH>7 Strong bases are very dangerous and Strong bases are very dangerous and
can burn your skincan burn your skin

Acid-Base IndicatorsAcid-Base Indicators
Dye that changes color in the Dye that changes color in the presence of an acid or basepresence of an acid or base
Example: Litmus paper (red Example: Litmus paper (red when an acid is present; blue when an acid is present; blue when a base is present)when a base is present)
Example: Red cabbage juice Example: Red cabbage juice (pink when an acid is present; (pink when an acid is present; purple/blue when a base is purple/blue when a base is presentpresent

Acids and BasesAcids and Bases(Bronsted-Lowry Def’n)(Bronsted-Lowry Def’n)
Acid Acid -proton -proton (H+) donor(H+) donor
BaseBase
-proton (H+) -proton (H+) acceptoracceptor

Naming AcidsNaming Acids
Binary acids (2 ions) – begin with the prefix Binary acids (2 ions) – begin with the prefix hydro, hydro, the root name of the element, and the the root name of the element, and the suffix -suffix -icic
To name: Look at ending:To name: Look at ending: -ate polyatomic ions-ate polyatomic ions -ic acids -ic acids -ite polyatomic ions -ite polyatomic ions -ous acids -ous acids -ide monatomic ions-ide monatomic ions hydro –ic acid hydro –ic acid
Monoprotic acid: Has one acidic hydrogen, like Monoprotic acid: Has one acidic hydrogen, like HClHCl
Diprotic acid: Has two acidic hydrogens, like Diprotic acid: Has two acidic hydrogens, like HH22SOSO44
Triprotic acid: Has three acidic hydrogens, like Triprotic acid: Has three acidic hydrogens, like HH33POPO44
Acids containing Acids containing moremore oxygen than the common oxygen than the common form are named by adding the prefixform are named by adding the prefix per per to the to the common name. common name.
acid containing fewer oxygen atoms is given acid containing fewer oxygen atoms is given the prefix the prefix hypohypo and the suffix and the suffix -ous -ous

Naming AcidsNaming Acids
PerPer – ROOT – – ROOT – icic More oxygen More oxygen
ROOT – ROOT – icic Most common Most common
ROOT – ROOT – ousous Less oxygenLess oxygen
Hypo – Hypo – ROOT – ROOT – ousous
Still less oxygenStill less oxygen

Common Bases/ Common Bases/ NamesNames
NaOH Sodium HydroxideNaOH Sodium Hydroxide NHNH44OH Ammonium Hyd.OH Ammonium Hyd.
Ca(OH)Ca(OH)22 Calcium Hydrox. Calcium Hydrox.
Naming Bases:Naming Bases: Name the cation; name the anionName the cation; name the anion

Ionization of Acids and BasesIonization of Acids and Bases
When in water, solid acid and base When in water, solid acid and base compounds dissolve and ionize (or dissociate) compounds dissolve and ionize (or dissociate) IonizeIonize: Break up into ions that float freely in : Break up into ions that float freely in
solution; can then conduct electricity.solution; can then conduct electricity. Strong acids & bases ionize/dissociates Strong acids & bases ionize/dissociates
completely, every molecule separates into completely, every molecule separates into ions.ions.
Strong acid: Strong acid: HCl + HHCl + H22O O H H+1+1 + Cl + Cl-1 -1 + H+ H22O O H H33OO+1+1 + +
ClCl-1-1 Strong base: NaOH Strong base: NaOH Na Na+1+1 + OH + OH-1-1
Weak acids & bases do not ionize completely Weak acids & bases do not ionize completely when dissolved in waterwhen dissolved in water

Common strong acids & basesCommon strong acids & bases
Common strong Common strong acids acids
Common strong Common strong basesbases
Perchloric acid, Perchloric acid, Lithium hydroxide, Lithium hydroxide, LiOHLiOH
Sulfuric acid, HSulfuric acid, H22SOSO44 Sodium hydroxide, Sodium hydroxide, NaOHNaOH
Hydroiodic acid, HIHydroiodic acid, HI Potassium Potassium hydroxide, KOHhydroxide, KOH
Hydrobromic acid, Hydrobromic acid, HBrHBr
Calcium hydroxide, Calcium hydroxide, Ca(OH)Ca(OH)22
Hydrochloric acid, Hydrochloric acid, HClHCl
Barium hydroxide, Barium hydroxide, Ba(OH)Ba(OH)22
Nitric acid, HNONitric acid, HNO33 Magnesium Magnesium hydroxide, Mg(OH)hydroxide, Mg(OH)22

The pH ScaleThe pH Scale
pH = - log [H3O+]
0 7 14
acid neutral base

pHpH
Scale used to express the acidity of a Scale used to express the acidity of a solution.solution.
The pH scale is a measure of the hydrogen The pH scale is a measure of the hydrogen ion (H+) concentration.ion (H+) concentration.
pH actually measures the hydronium ion pH actually measures the hydronium ion concentration ([Hconcentration ([H33OO+1+1]) of a solution.]) of a solution.
We use acid-base indicators to measure the We use acid-base indicators to measure the pH of a solution.pH of a solution.
Scale runs from 0 to 14.Scale runs from 0 to 14. Bases have pH>7; [HBases have pH>7; [H33OO+1+1]<1 x 10]<1 x 10-7-7MM Acids have pH<7; [HAcids have pH<7; [H33OO+1+1] > 1 x 10] > 1 x 10-7-7MM Neutral solns:pH=7; [HNeutral solns:pH=7; [H33OO+1+1]=1 x10]=1 x10--
77MM 0-----------> 7 -----------> 140-----------> 7 -----------> 14

pHpH


The pH Scale: Some Examples pH Value H+ Concentration
Relative to Pure WaterExample
0 10 000 000 battery acid
1 1 000 000 sulfuric acid
2 100 000 lemon juice, vinegar
3 10 000 orange juice, soda
4 1 000 tomato juice, acid rain
5 100 black coffee, bananas
6 10 urine, milk
7 1 pure water
8 0.1 sea water, eggs
9 0.01 baking soda
10 0.001 Great Salt Lake, milk of magnesia
11 0.000 1 ammonia solution
12 0.000 01 soapy water
13 0.000 001 bleach, oven cleaner
14 0.000 000 1 liquid drain cleaner

Self ionization of water:Self ionization of water:
HH22O + HO + H22O O H H33OO++ + OH + OH--
Hydronium ion: HHydronium ion: H33OO++ Hydroxide ion: OHHydroxide ion: OH--
Only 1 x 10 Only 1 x 10 –14–14 M of water M of water molecules do this so molecules do this so
[H[H33OO++][OH][OH--] = 1 x 10 ] = 1 x 10 –14–14 M M If [HIf [H33OO++]=[OH]=[OH--] neutral ] neutral
solutionsolution If [HIf [H33OO++]>[OH]>[OH--] acidic solution] acidic solution If [HIf [H33OO++]<[OH]<[OH--] basic or ] basic or
alkaline solution alkaline solution

Formulas:Formulas:we can calculate the pH of a we can calculate the pH of a solution if we know [Hsolution if we know [H33OO+1+1]:]:
((recall: Hrecall: H33OO+1 +1 same as Hsame as H+1+1)) pH=-log [HpH=-log [H33OO+1+1] or =-log [H] or =-log [H+1+1] ]
Use to find pH given [HUse to find pH given [H++]] 1. Find the pH if the hydronium ion 1. Find the pH if the hydronium ion
concentration is 2.1 X 10concentration is 2.1 X 10-5-5M.M. Is this solution Is this solution of baking soda acidic or basic?of baking soda acidic or basic?
(on calculator enter 2.1, EE, -5, log, +/-)(on calculator enter 2.1, EE, -5, log, +/-)
2. What is the pH of a solution of baking 2. What is the pH of a solution of baking soda which has an [Hsoda which has an [H33OO+1+1] of 3.98 x 10] of 3.98 x 10-9 -9 M?M?
Is this solution of baking soda acidic or Is this solution of baking soda acidic or basic?basic?
3. Find the pH of a 0.0025 M HCl solution. 3. Find the pH of a 0.0025 M HCl solution. The HCl is a strong acid and is 100% ionized The HCl is a strong acid and is 100% ionized in water. The hydronium ion concentration in water. The hydronium ion concentration is 0.0025 M. is 0.0025 M.
HCl › H+ + Cl-HCl › H+ + Cl- 0.0025 M 0.0025 M0.0025 M 0.0025 M

Formulas:Formulas:we can calculate the pOH of a we can calculate the pOH of a
solution if we know [OHsolution if we know [OH-1-1]:]:
pOH= -log [OHpOH= -log [OH--]] Use to find pOH given [OHUse to find pOH given [OH--]]
1.Find the pOH if the hydroxide 1.Find the pOH if the hydroxide ion concentration is 4.1 X 10ion concentration is 4.1 X 10--
55M.M. (on calculator enter 4.1, EE, -5, log, (on calculator enter 4.1, EE, -5, log,
+/-)+/-)
2. What is the pOH of a solution 2. What is the pOH of a solution that has a hydroxide ion that has a hydroxide ion concentration of 4.82 x 10concentration of 4.82 x 10-5-5 M? M?

FormulasFormulaswe can also calculate the hydronium ion we can also calculate the hydronium ion
concentration ([H3Oconcentration ([H3O+1+1]) if we know the hydroxide ion ]) if we know the hydroxide ion concentration ([OHconcentration ([OH-1-1]) or vice versa]) or vice versa
[H[H33OO++][OH][OH--]= 1 X 10 ]= 1 X 10 –14–14 M M oror [H [H++][OH][OH--]= 1 X 10 ]= 1 X 10 –14–14 M M
Use to find one [ ] given the other [ ]Use to find one [ ] given the other [ ]1. What is the [H3O1. What is the [H3O+1+1] of a solution if the ] of a solution if the
[OH[OH-1-1] is equal to 1 x 10] is equal to 1 x 10-5-5M ?M ? [H3O[H3O+1+1] x [OH] x [OH-1-1] = 1 x 10] = 1 x 10-14-14
[H3O[H3O+1+1] x [1 x 10] x [1 x 10-5-5M] = 1 x 10M] = 1 x 10-14-14
[H3O[H3O+1+1] = [] = [1 x 101 x 10-14-14] ] =1 x 10 =1 x 10-9-9MM [1 x 10[1 x 10-5-5M]M]
2.Find the [OH2.Find the [OH--] ion concentration if the ] ion concentration if the hydronium ion concentration is 1 x 10hydronium ion concentration is 1 x 10-5-5M. Is M. Is this acidic, basic or neutral?this acidic, basic or neutral?
3. If the hydroxide concentration is 3. If the hydroxide concentration is 1 x 101 x 10–4 –4 M, what is the hydronium ionM, what is the hydronium ion concentration?concentration?

pHpH
pH: 1-6 7 8-14pH: 1-6 7 8-14 Acid neutral baseAcid neutral base
pOHpOH: : 14-8 7 6-114-8 7 6-1
Each one unit change in the pH represents a ten-fold Each one unit change in the pH represents a ten-fold change in the [H3O+]. If you increase the acid or base change in the [H3O+]. If you increase the acid or base strength by a concentration factor of 10, the pH will strength by a concentration factor of 10, the pH will change by 1.change by 1.
As the H3O+ concentration increases, the pH will decrease As the H3O+ concentration increases, the pH will decrease (becomes more acidic and less basic).(becomes more acidic and less basic).
If H3O+ is 1.0 x 10-7 and is changed to 1.0 x 10-5 (by the If H3O+ is 1.0 x 10-7 and is changed to 1.0 x 10-5 (by the addition of more acid). Then the concentration of H3O+ addition of more acid). Then the concentration of H3O+ has increased so the pH goes from 7 to 5 has increased so the pH goes from 7 to 5 (decreases).(decreases).
As the H3O+ ion concentration decreases, the pH will As the H3O+ ion concentration decreases, the pH will increase (becomes more basic and less acidic).The pH increase (becomes more basic and less acidic).The pH value represents the relationship between the H3O+ value represents the relationship between the H3O+ concentration and the OH- concentration.concentration and the OH- concentration.
[H3O+] + [OH-] = 1 x 10[H3O+] + [OH-] = 1 x 10-14-14
Since the pH tells you the concentration of the H3O+, then Since the pH tells you the concentration of the H3O+, then you can use the pH to calculate the concentration of OH- you can use the pH to calculate the concentration of OH- in a solution.in a solution.

pH/ pOHpH/ pOH
pH + pOH = 14pH + pOH = 14 Use to find one given the other.Use to find one given the other. 1. Find the pOH if the pH is 8.2.1. Find the pOH if the pH is 8.2.
pOH= 14 - 8.2= 5.8pOH= 14 - 8.2= 5.8
[H[H++]= 10]= 10-pH-pH or [H+] = antilog (- pH) or [H+] = antilog (- pH)
Use to find [HUse to find [H++] given to pH] given to pH 2. Find the [H2. Find the [H++] given a pH of 11.5.] given a pH of 11.5. [on calculator enter 11.5, +/-, 2nd, log (10x)][on calculator enter 11.5, +/-, 2nd, log (10x)] 3. Calculate the [H+] for a solution with a pH of 8.32.3. Calculate the [H+] for a solution with a pH of 8.32.
[OH[OH--]= 10]= 10-pOH-pOH or or antilog (- pOH)antilog (- pOH)
Use to find [OHUse to find [OH--] given to pOH] given to pOH 4. Find the [OH4. Find the [OH--] given a pOH of 7.5] given a pOH of 7.5 [on calculator enter 7.5, +/-, 2nd, log (10x)][on calculator enter 7.5, +/-, 2nd, log (10x)]
What is the pH? What is the pH? Is the solution acidic, basic or neutral?Is the solution acidic, basic or neutral?
5. What is the hydroxide ion concentration in a solution that has a pOH of 5. What is the hydroxide ion concentration in a solution that has a pOH of 5.70? 5.70?
What is the pH? What is the pH? Is the solution acidic, basic or neutral?Is the solution acidic, basic or neutral?