9.3 Acidic Environment Booklet
-
Upload
col-harrison -
Category
Documents
-
view
89 -
download
0
description
Transcript of 9.3 Acidic Environment Booklet

Unit 9.3
HSC Chemistry The Acidic Environment P a g e | 1

HSC Chemistry
Unit 2: 9.3 The Acidic Environment
ContentsIndicators..............................................................................................................5
Question Set 1....................................................................................................5
Common Indicators...............................................................................................6
Question Set 2....................................................................................................6
A Natural Indicator...............................................................................................7
A Range of Indicators............................................................................................8
Everyday uses of Indicators..................................................................................9
Question Set 3....................................................................................................9
Classifying Household Substances......................................................................10
Non-Metal Oxides................................................................................................11
Question Set 4..................................................................................................11
Mapping the oxides on the Periodic Table..........................................................12
Question Set 5..................................................................................................12
Le Chatelier’s Principle.......................................................................................13
Question Set 6..................................................................................................13
Reversible Reactions...........................................................................................14
Question Set 7..................................................................................................14
Solubility of CO2 in Water...................................................................................16
Question Set 8..................................................................................................16
De-carbonating Soft Drinks.................................................................................18
Nitrogen Dioxide Fact Sheet...............................................................................19
Question Set 9..................................................................................................20
Sulfur Dioxide Fact Sheet...................................................................................22
Question Set 10................................................................................................23
SOx and NOx.......................................................................................................24
Question Set 11................................................................................................24
Changing Atmospheric Levels of SOx and NOx..................................................26
Question Set 12................................................................................................27
Calculating Gas Volumes....................................................................................28
Acid Rain.............................................................................................................29
Question Set 13................................................................................................29
HSC Chemistry The Acidic Environment P a g e | 2

Common Acids....................................................................................................31
Question Set 14................................................................................................31
Measuring pH.....................................................................................................33
Some Definitions of Acids...................................................................................34
Question Set 15................................................................................................34
Using the pH scale..............................................................................................36
pH........................................................................................................................ 38
Question Set 16................................................................................................38
Ionisation of Acids...............................................................................................40
Question Set 17................................................................................................40
Relative Strength of Acids..................................................................................42
Commonly occurring acids and bases.................................................................43
Acid Base equilibrium.........................................................................................45
Question Set 18................................................................................................45
Classifying Acids.................................................................................................46
Question Set 19................................................................................................46
Brönsted-Lowry Acids and Bases........................................................................48
Question Set 20................................................................................................49
Conjugate Pairs...................................................................................................50
Question Set 21................................................................................................51
Salts.................................................................................................................... 52
Question Set 22................................................................................................53
pH of Salt Solutions............................................................................................54
Neutralisation.....................................................................................................55
Volumetric Analysis.............................................................................................56
Question Set 23................................................................................................57
Preparing a Standard Solution............................................................................58
Question Set 24................................................................................................59
Identifying End Points of Titrations....................................................................60
Question Set 25................................................................................................60
Titrations – Exploring Indicators........................................................................62
Titrations – Finding the Concentration of an Unknown Solution........................63
Buffer Solutions...................................................................................................65
Comparing Concentrations using Different Methods.........................................66
Chemical Spills and Neutralisation.....................................................................67
Question Set 26................................................................................................67
HSC Chemistry The Acidic Environment P a g e | 3

Alkanols and Alkanoic Acids...............................................................................69
Question Set 27................................................................................................69
Esterification.......................................................................................................70
Question Set 28................................................................................................70
Physical properties of alkanols and alkanoic acids.............................................73
Question Set 29................................................................................................73
Esterification using Reflux..................................................................................74
Refluxing.............................................................................................................75
Question Set 30................................................................................................75
Esters.................................................................................................................. 76
Question Set 31................................................................................................76
HSC Chemistry The Acidic Environment P a g e | 4

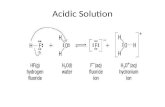
I can classify common substances as acidic, basic or neutral.Indicators
Indicators are dyes which use specific pH values. Most acid-base indicators are weak acids whose corresponding (conjugate) base is a different colour.
1. Litmus: Unionised acid litmus is dominant at pH < 5.5 and so the solution is red. At pH > 8, the conjugate base is dominant and the solution turns blue. Through the transition range the indicator changes through shades of purple as the equilibrium shifts. Litmus can be used as a solution, or soaked paper.
2. Universal: Universal indicator is a very useful indicator as it shows a range of colour changes over a range of acidic or basic concentrations (pH values). Universal indicator is a mixture of other indicators.
Question Set 11. What is an indicator?
...................................……………………………………………………………
……………………...................................………………………………………
……………………………………………………………………………………………………………
2. Name three places or situations where pH is an important factor.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
3. Name 3 indicators with which you are familiar?
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 5

I can identify that indicators such as litmus, phenolphthalein, methyl orange and bromothymol blue can be used to determine the acidic or basic nature of a material over a range, and that the range is identified by a change in colour.
Common Indicators
There are a number of common indicators that we use in Chemistry. The table below shows some of the colour change ranges.
Question Set 21. If a substance turns yellow in bromothymol blue, how
would you classify it?
…………………………………..………………………………………………
2. What other result would you need to conclude that the substance had a pH of around 5?
……………………………………………………………………..
……………………………………………………………………..
3. Which type of indicator might have produced the results shown to the right? Justify your answer.
……………………………………………………………………..
………………………………………………………………………
………………………………………………………………………..
HSC Chemistry The Acidic Environment P a g e | 6

I can perform a first-hand investigation to prepare and test a natural indicator.
A Natural Indicator
Prepare and test an indicator made from red cabbage. Use water, hydrochloric acid, vinegar, sodium hydroxide and ammonia.
Method:
…………………………………………………………………………………
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
Results:
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
…………………………………………………………………………………………………………..
HSC Chemistry The Acidic Environment P a g e | 7

I can identify data and choose resources to gather information about the colour changes of a range of indicators.
A Range of Indicators
Test the following indicators on each the these substances: water, hydrochloric acid, vinegar, sodium hydroxide and ammonia. Indicators: universal indicator, red and blue litmus, phenolphthalein, methyl orange and bromothymol blue.
Method:
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
Results:
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
HSC Chemistry The Acidic Environment P a g e | 8

I can identify and describe some everyday uses of indicators including the testing of soil acidity/basicity.Everyday uses of Indicators
There are a number of situations where indicators are used to maintain key conditions for living things.
In swimming pools, pH must be kept within a narrow range to ensure the safety of swimmers and prevent growth of algae.
In natural water systems, plants use CO2, causing a rise in pH, animals produce CO2 causing a drop in pH.
pH affects body chemistry. Protein digestion starts in the stomach. An enzyme called pepsin catalyses the reaction. This enzyme works optimally at very low pH values. Blood and body cells require a neutral pH. This is maintained by a buffer consisting of H2CO3 and HCO3
- as well as amino acids.
Soil pH can affect the colour and growth of plants.
As foods ripen, there are changes in acidity. Low pH values preserve foods against pathogens.
pH is also important in the cosmetics industry, to ensure against skin irritations.
Question Set 31. Explain the process for testing soil pH. Carry out this
process in a section of the school garden.
.................................................................................................................
.................................................................................................................
.................................................................................................................
......................................................................................................................
.................................
......................................................................................................................
.................................
......................................................................................................................
.................................2. What is a buffer? Why are buffers important in natural systems, such as
the bloodstream?..............................................................................................................................................................................................................................................................................................................
HSC Chemistry The Acidic Environment P a g e | 9

I can solve problems by applying information about the colour changes of indicators to classify some household substances as acidic, neutral or basic.
......................................................................................................................
.................................
......................................................................................................................
.................................
......................................................................................................................
.................................
Classifying Household Substances
Apply information about the colour changes of indicators to classify some household substances as acidic, basic or neutral.
Method:
…………………………………………………………………………………
…………………………………………………………………………………
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
Results:
HSC Chemistry The Acidic Environment P a g e | 10

I can identify oxides of non-metals which act as acids and describe the conditions under which they act as acids.Non-Metal Oxides
Elements to the right in the Periodic Table (not including the Noble gases) can be reacted with oxygen to form non-metal oxides. Examples include carbon dioxide, nitrogen dioxide or sulfur dioxide.
When these no-metal oxides are dissolved in water, they can produce acidic solutions. The strength of the acid depends on the particular non-metal.
Eg. SO2(g) + H2O(l) H2SO3(aq)
The sulfur dioxide is an acidic oxide as it produces sulfurous acid when diossolved in water. It will increase the concentration of hydrogen ions.
Question Set 41. Write down balanced chemical reactions for two other
non-metallic oxides to show their acidic nature.
…………………………………………………………………………………
…………………………………………………………………………………
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
HSC Chemistry The Acidic Environment P a g e | 11

I can analyse the position of these non-metal in the Periodic Table and outline the relationship between position of elements in the Periodic Table and acidity/basicity of oxides.
Mapping the oxides on the Periodic Table
Now we have an idea about non-metallic oxides, it should be a small stretch to realise there are metallic oxides and these produce basic solutions. There are also substances, such as aluminium which are amphoteric. Their oxides can act as either weak acids or weak bases.
Question Set 51. Outline the relationship between the position of
elements in the Periodic Table and the acidity/basicity of oxides.
…………………………………………………………………………………
…………………………………………………………………………………
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
2. Describe the conditions under which non-metallic oxides act as acids and metallic oxides act as bases.
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
HSC Chemistry The Acidic Environment P a g e | 12

I can define Le Chatelier’s Principle.
Le Chatelier’s Principle
So far we have considered reactions as proceeding to completion, however many products of a reaction may themselves react and reform the original substances; this is called a reversible reaction.
Both a forward reaction and a reverse reaction may be occurring at the same time. When the rate of the forward reaction equals the rate of the reverse reaction, we say the system has reached equilibrium.
There are three important features of equilibrium systems.
Equilibrium is achieved when the system is closed. (Matter will neither be lost or gained.)
Constant macroscopic properties are an indicator of dynamic equilibrium. (includes colour, pressure, volume, electrical conductivity.)
Chemical equilibria are dynamic. (the rate of the forward reaction = the rate of the reverse direction).
Le Chatelier’s Principle applies to systems which are in equilibrium.
When a system in equilibrium is subjected to a change in concentration, temperature, external pressure, or some other factor which upsets the equilibrium, the system reacts in such a way as to counteract that change.
Question Set 61. What is Le Chatelier’s Principle?
………………………………………………………………………………
………………………………………………………………………………………
……………………………………………………………………………………………………………
2. What are the three important features of equilibrium systems?
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 13

I can identify factors which can affect the equilibrium in a reversible reaction.Reversible Reactions
There are many examples of reversible reactions which are important in natural systems. Various factors affect both the position of equilibrium and how the system can be changed. Factors which affect a system in equilibrium include:
Temperature Gas pressure (if one or more substances exist in their gaseous form) Concentration
Note while both the presence of a catalyst and the surface area of one or more reactants can change the rate of the reaction, neither can shift the position at equilibrium.
One example of a natural system in equilibrium which we have previously mentioned is human blood.
Oxygen exchange and blood acidity levels.
A. HHb+ + O2(aq) HbO2 + H+(aq)
haemoglobin oxyhaemoglobin
B. H+(aq) + HCO3
-(aq) H2CO3(aq) H2O + CO2(aq)
In the lungs, high oxygen pressure shifts A to the right to form oxyhaemoglobin. This molecule is moved in the blood to an oxygen poor cell where the equilibrium of A shifts to the left releasing the oxygen for the cell.
A shift in the equilibrium position of A increases the [H+] (blood acidity).
This will in turn shift B to the right, reducing blood acidity levels and increasing [CO2], which can escape from the lungs.
The maintenance of blood acidity levels using shifts in the equilibrium position is known as buffering. Buffer solutions are vital in maintaining balanced conditions in the body as well as in many other situations.
Question Set 71. What factors affect the equilibrium in a reversible reaction?
………………………………………………………………………………………
………………………………………………………………………………………..
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 14

2. Explain what is happening to the equilibrium system shown below.
………………………………………………………………………...…………………………………
…………………………………...…………………………………...…………………………………
...…………………………………
…………………………………...
…………………………………...
…………………………………...
…………………………………...
HSC Chemistry The Acidic Environment P a g e | 15

I can describe the solubility of carbon dioxide in water under various conditions as an equilibrium process and explain in terms of Le Chatelier’s Principle.
Solubility of CO2 in Water
Carbon dioxide is slightly soluble in water. When CO2 is bubbled into water, an equilibrium is established. The carbon dioxide is ionised to become a bicarbonate ion (HCO3
-), by accepting an OH- ion from the water. This leaves a H+ ion. Write an equation to demonstrate this equilibrium.
Soft drinks contain dissolved carbon dioxide which bubbles out of the solution when the pressure is reduced according to the following equation.
A. CO2(g) CO2(aq)
B. H2O + CO2(aq) H2CO3(aq) H+(aq) + HCO3
-(aq)
CO2 is pumped into the solution under pressure, driving both equilibrium positions to the right. This gives soft drinks a slightly acidic taste (this is why sugar is a major ingredient in soft drinks). The equilibrium is maintained until the bottle is opened. The system now becomes an open system. CO2 escapes and is replaced by CO2 bubbling out of solution. Both equilibria shift to the left. The soft drink loses its acidity and eventually becomes flat.
Question Set 81. How does the solubility of carbon dioxide change under
different conditions?
………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 16

2. Why does a soft drink eventually go flat even if you keep the lid on it?
………………………………………………………………………
………………………………………………………………………
………………………………………………………………………
………………………………………………………………………
………………………………………………………………………
………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 17

I can identify data, plan and perform a first-hand investigation to decarbonate soft drink and gather data to measure the mass changes involved and calculate the volume of gas released at 25oC and 100kPa.
De-carbonating Soft Drinks
Decarbonate a soft drink and gather data to measure the mass changes involved. Calculate the volume of gas released at SLC.
Method:
…………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
Results:
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 18

I can identify natural and industrial sources of oxides of nitrogen.
I can analyse information from secondary sources to summarise the industrial origins of oxides of nitrogen and evaluate reasons for concern about their release into the environment.
Nitrogen Dioxide Fact Sheet
What is nitrogen dioxide?Nitrogen dioxide is a nasty-smelling gas that is acidic enough to corrode metal, stone and other materials. Some nitrogen dioxide is formed naturally in the atmosphere by lightning and by natural changes (oxidation) to ammonia found in plants, soil and water. However, only about 1% of the total amount of nitrogen dioxide found in our cities' air is formed this way.
An important problem with nitrogen dioxide is that it forms photochemical smog, which is extremely dangerous for human health.
The major source of nitrogen dioxide in Australia's atmosphere is from burning our main fossil fuels: coal, oil and gas. Most of the nitrogen dioxide in cities comes from motor vehicle exhaust (about 80%). Other sources of nitrogen dioxide are petrol and metal refining, electricity generation from coal-fired power stations, other manufacturing industries and food processing and manufacturing.
Unflued gas heaters and cookers are the major source of nitrogen dioxide in Australian homes.
How does nitrogen dioxide affect your health?The main effect of breathing in nitrogen dioxide is that you would be more likely to suffer from respiratory problems. Nitrogen dioxide inflames the lining of the lungs, and it can reduce immunity to lung infections. This can cause problems such as wheezing, coughing, colds, flu and bronchitis.
Nitrogen dioxide is even more dangerous for asthmatics because it can cause more frequent and more intense attacks.
Children with asthma and older people with heart disease are the most at risk.
How much of a problem is nitrogen dioxide in Australia?The amount of nitrogen dioxide in our atmosphere is generally higher in autumn and winter than at other times of the year. Although in most Australian towns and cities even the highest amounts of nitrogen dioxide have been at a level that is thought to be safe for humans since the early 1990s.
HSC Chemistry The Acidic Environment P a g e | 19

In some of Australia's larger cities, it is possible that the concentration of nitrogen dioxide sometimes increases for a short amount of time to levels that are not safe for people who are the most at risk. Air pollution authorities are monitoring the situation to see if this is the case.
What are we doing to manage nitrogen dioxide?Because of the dangers to our health of high concentrations of nitrogen dioxide, the Australian Government has taken steps to manage and reduce the amount of nitrogen dioxide produced. These include:
reviewing our fuel production methods and implementing national fuel quality standards supporting the implementation of tighter vehicle emission standards developing a diesel NEPM, to improve the in-service performance of diesel vehicles developing and promoting alternative fuels developing pollution forecasting systems for Australia's major cities encouraging community awareness and involvement through initiatives such as Smogbusters.
The Commonwealth, State and Territory Governments have also agreed on a National Environment Protection Measure (NEPM) for Ambient Air Quality. By the year 2008, the NEPM aims to keep the ambient air concentration of nitrogen dioxide to:
less than 0.12°ppm (parts per million) over a one hour period less than 0.03 ppm averaged over a one year period.
These levels have not been exceeded since the early 1990s in any Australian capital city.
(Australian Government Department of the Environment and Heritage)
Question Set 91. Identify natural sources of oxides of nitrogen.
………………………………………………………………………………………
………………………………………………………………………………………
2. Identify industrial sources of oxides of nitrogen.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 20

3. Evaluate reasons for concern about the release of oxides of nitrogen into the atmosphere.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 21

I can identify natural and industrial sources of sulphur dioxide.
I can analyse information from secondary sources to summarise the industrial origins of sulphur dioxide and evaluate reasons for concern about their release into the environment.
Sulfur Dioxide Fact Sheet
What is sulfur dioxide?Sulfur dioxide is a gas that is in our atmosphere. It is invisible, but it has a dreadful, sharp smell, and it reacts easily with other substances to form harmful compounds, such as sulfuric acid, sulfurous acid and sulfate particles. About 99% of the sulfur dioxide in our atmosphere comes from human sources.
The main source of sulfur dioxide in the atmosphere is industrial activity that processes materials that contain sulfur. This includes generating electricity from coal, oil or gas that contains sulfur. Some mineral ores also contain sulfur, and sulfur dioxide is released when they are processed. Industrial activities that burn fossil fuels that contain sulfur can also be important sources of sulfur dioxide.
In Australian towns and cities, pollution from motor vehicle exhaust is an important but not the main source of sulfur dioxide in our atmosphere.
How does sulfur dioxide affect your health?Sulfur dioxide affects your health when you breathe it in. It irritates the nose, throat, and airways to cause coughing, wheezing, shortness of breath, or a tight feeling around the chest. Sulfur dioxide works very quickly, so most people would feel the worst symptoms in 10 or 15 minutes after they had breathed it in.
Asthmatics and other people with similar conditions are most at risk of developing problems if they are exposed to sulfur dioxide.
How much of a problem is sulfur dioxide in Australia?The amount of sulfur dioxide in Australia's atmosphere is well within a safe level in most Australian towns and cities. The highest concentrations of sulfur dioxide in the air are found around petrol refineries, chemical manufacturing industries, mineral ore processing plants and power stations.
The only areas with high amounts of sulfur dioxide in the atmosphere, and then only occasionally, are around Mt Isa and Kalgoorlie.
What are we doing to manage sulfur dioxide?Because of the dangers to our health of high levels of sulfur dioxide, the Commonwealth Government has taken steps to manage and reduce the amount
HSC Chemistry The Acidic Environment P a g e | 22

I can describe, using equations, examples of chemical reactions which release sulphur dioxide and chemical reactions which release oxides of nitrogen.
of sulfur dioxide produced. These include:
reviewing our fuel production methods and implementing national fuel quality standards supporting the implementation of tighter vehicle emission standards promoting alternative fuels developing pollution forecasting systems for Australia's major cities.
The Commonwealth, State and Territory Governments have also agreed on a National Environment Protection Measure (NEPM) for Ambient Air Quality. The NEPM aims to keep the ambient air concentration of sulfur dioxide to:
less than 0.20 ppm (parts per million) averaged over a one hour period less than 0.08 ppm averaged over a 24 hour period less than 0.02 ppm averaged over a one year period.
We expect that most areas in Australia will meet these standards by the year 2008
(Australian Government Department of the Environment and Heritage)
Question Set 101. Identify natural sources of sulfur dioxide.
………………………………………………………………………………………
………………………………………………………………………………………
2. Identify industrial sources of sulfur dioxide.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
3. Evaluate reasons for concern about the release of sulfur dioxide into the atmosphere.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
SOx and NOx
HSC Chemistry The Acidic Environment P a g e | 23

1 mole of gas at 101.3 kPa has a volume of
L at 273 K (0°C) or L at 298 K (25°C).
Sulfur is present in many of the minerals which are processed in factories. This includes coal used in power stations. Write an equation to represent the reaction between sulfur and oxygen.
What important property of SO2 and NO2 has environmental implications?
SO2 in the air can react with water forming sulfurous acid. What is the equation?
Before this happens, most SO2 is oxidised to SO3 which forms a stronger acid, sulfuric acid, with water. What is the equation for this?
Nitrogen is the main atmospheric gas, accounting for around 78% of the total constitution. When fuels are burned in car engines, there is a large amount of notirogen mixed with the oxygen required for combustion. As a result, the nitrogen can combine with oxygen at the high temperatures produced when fuel combusts. Write an equation to represent this process.
NO2 is usually the main NOx in air: 2NO2 + H2O ↔
Question Set 111. Identify the most common sources of sulfur oxides and
nitrogen oxides in the air.
………………………………………………………………………………...………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
2. Why are scientists concerned about their release into the atmosphere?
HSC Chemistry The Acidic Environment P a g e | 24

……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……...……………………………………………………………
……………………………………………………………………
……………………………………………………………………
……………………………………………………………………
……………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 25

I can assess the evidence which indicates increases in atmospheric concentration of oxides of sulphur and nitrogen.
Changing Atmospheric Levels of SOx and NOx
Australia have nationally agreed standards for protection and regulation of air quality.
Through the National Environment Protection Council, the Australian, State and Territory Governments agreed to the National Environment and Protection Measure for Ambient Air Quality on 26 June 1998. These are set out in the table below:
National Air Quality Standards
Pollutant Concentration and averaging period
Carbon monoxide
9.0 ppm (parts per million) measured over an eight hour period
Nitrogen dioxide 0.12 ppm averaged over a one hour period
0.03 ppm averaged over a one year period
Ozone 0.10 ppm of ozone measured over a one hour period
0.08 ppm of ozone measured over a four hour period
Sulfur dioxide 0.20 ppm averaged over a one hour period
0.08 ppm averaged over a 24 hour period
0.02 ppm averaged over a one year period
Lead 0.5 µg/m³ (micrograms per cubic metre) averaged over a one year period
Particles as PM10 50 µg/m³ averaged over a 24-hour period
Particles as PM2.5 Advisory reporting standard: 25 µg/m³ over a one day period; 8 µg/m³ over a one year period
Note: PM10 ‘inhalable particles’ are less than or equal to 10μm in diameter. PM2.5 ‘respirable particles’ are less than or equal to 2.5μm in diameter.
The 2001 report revealed the following changes in concentration of NO2 and SO2 for the period 1991-2001.
HSC Chemistry The Acidic Environment P a g e | 26

I can calculate volumes of gases given masses of some substances in reactions, and calculate masses of substances given gaseous volumes, in reactions involving gases at 0oC and 100kPa or 25oC and 100kPa.
Question Set 121. What has happened to the atmospheric levels of nitrogen
dioxide and sulphur dioxide over the past 10 years?
………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
2. Is this pattern the same for different parts of the world?
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
3. Explain the patterns you have observed.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
Calculating Gas Volumes
HSC Chemistry The Acidic Environment P a g e | 27

I can explain the formation and effects of acid rain.
1. 50 g of sulfur reacts with excess oxygen from the air to produce sulfur dioxide gas. Calculate the mass of oxygen needed for complete combustion. What volume of sulfur dioxide would be produced at STP?
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
2. A student produced 100 mL of carbon dioxide gas at 25oC and 101.3 kPa by decomposing a lump of calcium carbonate. What was the mass of the original sample?
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
3. Sulfur dioxide produced in a furnace reacts with atmospheric oxygen to produce sulfur trioxide. If the original quantity of sulfur dioxide had a mass of 1 kg, what would be the difference in the amount of sulfur trioxide formed at 0oC compared with that formed at 25oC (assume the pressure is constant at 101.3 kPa).
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
Acid Rain
HSC Chemistry The Acidic Environment P a g e | 28

Distilled water in contact with the atmosphere is not neutral. It has a pH of 5.5 to 6, due to absorption of the acidic gas CO2 from the atmosphere. In Australia, unpolluted rainwater has a pH between 5 and 6. If the pH is below 5, an acidic substance, such as SO2 or NO2, has dissolved in the water, which is sometimes called acid rain. In the Northern Hemisphere, pHs as low as 2 have been recorded in acid rain. The source of the SO2 or NO2 could be hundreds or thousands of kilometres from where the acid rain falls.
SO2 sources, such as fossil fuel burning power stations and metal sulfide smelters, are larger but fewer in number than NO2 sources, like internal combustion engines in vehicles. If the quantity of acid rain is greater than the capacity of an environment to neutralise it then the following can occur.
Soil pH can drop, making it difficult for plants to absorb sufficient calcium or potassium.
Soil chemistry can change, leading to the death of important microorganisms and release of normally insoluble aluminium and mercury into soil water.
Protective waxes can be lost from leaves, causing leaf damage.
Buildings made of carbonates, such as concrete, mortar, limestone and marble, can be gradually dissolved away.
Aquatic animals can die as water pH drops below 5.
Smog and acid rain can combine to form killer fog, as happened after the Second World War in London, when many homes burnt sulfur dioxide-releasing coal.
Question Set 131. Explain the formation and effects of acid rain (include at
least one equation):
………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 29

……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 30

I can define acids as proton donors and describe the ionisation of acids in water.
I can identify acids including acetic (ethanoic), citric (2-hydroxypropane-1,2,3-tricarboxylic), hydrochloric and sulphuric acid.
Common Acids
An acid is a proton donor. When an acid molecule is in contact with water it can ionise, donating a proton to a water molecule. A hydrogen atom, H, consists of one proton and one electron. A hydrogen ion, H+ , is formed when a H atom loses its electron, leaving just a proton. A proton and a hydrogen ion are thus the same and can be represented by H+. When an acid molecule is placed in water, it can ionise, releasing a proton and forming a negative ion. The proton, H+, can attach to a water molecule, H2O, forming what is called a hydrated hydrogen ion or hydronium ion, H3O+.
Complete these equations:
HCl + H2O →
HNO3 + H2O →
H2SO4 + H2O →
Sulfuric acid is called a diprotic acid because each molecule can release up to two protons.
H2SO4 + 2H2O →
Phosphoric acid is called a triprotic acid because each molecule can release up to three protons.
H3PO4 + 3H2O →
Question Set 141. To what does the term proton donor refer?
………………………………………………………………………………………
………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 31

2. Draw structural formulae for the following acids:a) Acetic acid (ethanoic acid)
b) Citric acid (2-hydroxypropane-1,2,3-tricarboxylic acid)
3. Draw Lewis structures to represent hydrochloric acid, carbonic acid, nitric acid and sulfuric acid.
HSC Chemistry The Acidic Environment P a g e | 32

I can solve problems and perform a first-hand investigation to use pH meters/probes and indicators to distinguish between acidic, basic and neutral chemicals.
Measuring pH
Previously we looked at indicators as a method for identifying the acidity of a solution. pH meters and/or probes can also be used to idenitfy the acidity or basicity of a solution.
Measure the pH of a weak acid and base, a strong acid and base and a neutral substance using a pH probe or meter.
List the substances you will test in the table below.
Describe the method used to test the substances, then complete the table.
Method:
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
Results:
Substance pH meter Nature of substance
HSC Chemistry The Acidic Environment P a g e | 33

I can describe acids and their solutions with the appropriate use of the terms strong, weak, concentrated and dilute.
I can plan and perform a first-hand investigation to measure the pH of identical concentrations of strong and weak acids.
Some Definitions of Acids
Acids are classified on the basis of their strength and concentration.
The strength of the acid relates to
……………………………………………………………………………………
The difference between strong acids and weak acids is
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
The concentration of an acid refers to
……………………………………………………………………………………………………………
The difference between a concentrated acid and a dilute acid is
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
Question Set 151. Use the pH meter from the previous experiment to record
the pH of 1M solutions of sulfuric acid, hydrochloric acid and acetic acid. Discuss the significance of your results.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 34

……………………………………………………………………………………………………………
2. Start with a 2M hydrochloric acid solution and perform a series of dilutions so you end up with 4 different hydrochloric acid solutions with the following concentrations: 2M, 1M, 0.5M and 0.1M. Test each of these solutions with the pH meter and record your observations. Discuss the significance of your results.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 35

I can describe the use of the pH scale in comparing acids and bases.Using the pH scale
The pH scale is the most convenient and comprehensive tool for identifying the relative acidity or basicity of solutions. The table below shows the colours and their corresponding pH values and some examples of substances which have the corresponding pH. We can use the pH scale not only to compare acids and bases, but also to compare relative strengths of different acids.
What could you say about the acid strength in lemon juice compared to tomato juice?
Why do you think toothpaste has such a high pH?
Water is a weak electrolyte which partly ionises (in itself!) to produce the following equilibria:
H2O ↔
At 25oC, [H+] = [OH-] = 10-7 mol.L-1. This corresponds to a pH of 7.
HSC Chemistry The Acidic Environment P a g e | 36

In water, acids increase [H+]. A proton is equivalent to an H+ ion. Protons are highly reactive and attach to water molecules forming H3O+ (hydronium ions).
Example: 0.005 mol of sulfuric acid is dissolved in sufficient water to make 500 mL of solution. Calculate the concentration of hydroxide and hydrogen ions in this solution.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
In general,
Acid solutions: pH < 7 [H+] > 10-7 mol L-1
Neutral solutions: pH = 7 [H+] = 10-7 mol L-1
Basic solutions: pH > 7 [H+] < 10-7 mol L-1
Examples of naturally occurring acids are:
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
Examples of manufactured acids include
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 37

I can identify pH as –log10[H+] and explain that a change in pH of 1 means a ten-fold change in [H+].
I can process information from secondary sources to calculate pH of strong acids given appropriate hydrogen ion concentrations.
pH
As we saw above, a simpler way to determine the acid, basic or neutral nature of a solution is to use a function called pH. Mathematically,
pH = - log10 [H+] also pH + pOH = 14
[H+] x [OH-] = 10-14
Question Set 16
1. Calculate the pH of a 0.01 M HCl solution.
………………………………………………………………………………………
………………………………………………………………………………………
……………………………………………………………………………………………………………
2. Calculate the pH of a 0.05 M NaOH solution.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
Acidic solutions have [H+] > 10-7 M. The table below shows equivalent pH values for acid solutions.
[H+] M
1.0 0.1 0.01
0.001
0.0001
0.00001
0.000001
pH 0 1 2 3 4 5 6
Neutral solutions have a pH of 7 while basic solutions have a [H+] < 10-7 M.
[OH-] M 1.0
0.1
0.01
0.001
0.0001
0.00001
0.000001
pH 1 13 12 11 10 9 8
HSC Chemistry The Acidic Environment P a g e | 38

4
3. What is the pH of a 0.2 M Ca(OH)2 solution?
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
4. A small quantity of tartaric acid was dissolved in water to give a final hydrogen ion concentration of 1.3 x 10-6 mol.L-1. Calculate the pH of this solution.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 39

I can gather and process information from secondary sources to write ionic equations to represent the ionisation of acids.
I can use available evidence to model the molecular nature of acids and simulate the ionisation of strong and weak acids.
Ionisation of Acids
Acids are classified on the basis of their strength and concentration.
The strength of an acid relates to its degree of ionisation. Strong acids are completely ionised, weak acids are only partly ionised.
The concentration of an acid is equivalent to its molarity.
Do not confuse the terms weak and strong with dilute and concentrated.
Acids may also be classified on the basis of the number of protons they are capable of donating in an acid-base reaction.
• Monoprotic: Donate one proton per molecule eg. HCl
• Diprotic: Donate two protons per molecule eg. H2SO4
• Triprotic: Donate three protons per molecule eg. H3PO4
Question Set 171. Write ionic equations to represent the following acids in
solution:a) Hydrochloric acid
b) Sulfuric acid
c) Acetic acid
d) Carbonic acid
HSC Chemistry The Acidic Environment P a g e | 40

2. Look at the model of a weak acid right. Now look at the model of a strong acid below right.Explain how such models can be used to explain the difference between a weak acid and a strong acid.
.......................................................................
.......................................................................
.......................................................................
.......................................................................
.......................................................................
.......................................................................
.......................................................................
.......................................................................
......................................................................
......................................................................
......................................................................
......................................................................
......................................................................
......................................................................
......................................................................
HSC Chemistry The Acidic Environment P a g e | 41
http://highered.mcgraw-hill.com/sites/dl/free/0072512644/117354/07_Strong_Weak_Nonelectrolytes.swf

......................................................................
......................................................................
......................................................................
......................................................................
......................................................................
HSC Chemistry The Acidic Environment P a g e | 42
http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/acid13.swf

I can compare the relative strengths of equal concentrations of citric, acetic and hydrochloric acids and explain in terms of the degree of ionisation of their molecules.
Relative Strength of Acids
Differences in the degree of ionisation allows us to explain differences in the relative strengths of equal concentrations of different acids.
Record the pH of 1M solutions of citric acid, acetic acid and hydrochloric acid in the table below.
Use your knowledge of ionisation to help explain any differences.
Acid (1M) pH Explanation
Citric acid
Acetic acid
Hydrochloric acid
HSC Chemistry The Acidic Environment P a g e | 43

I can gather and process information from secondary sources to explain the use of acids as food additives.
I can identify data, gather and process information from secondary sources to identify examples of naturally occurring acids and bases and their chemical composition.
Commonly occurring acids and bases
Acids and bases are everywhere. They are important for our swimming pools, fish tanks, gardens as well as our bloodstream and digestive system. There are even acids in the food we eat.
Weak acids are added to foods to inhibit the growth of microorganisms, prevent spoilage, improve shelf life, improve flavour or as a leavening agent.
Find out what sorts of foods contain the following acids and explain why they are used.
Citric acid
Lactic acid
Ascorbic acid
Tartaric acid
Malic acid
Phosphoric acid
For each of the acids listed below, draw the structural formula and identify the natural source.
HSC Chemistry The Acidic Environment P a g e | 44

HSC Chemistry The Acidic Environment P a g e | 45

Acid Structural formula Source
Benzoic acid
Citric acid
Malic acid
Tartaric acid
HSC Chemistry The Acidic Environment P a g e | 46

I can describe the difference between a strong and a weak acid in terms of an equilibrium between the intact molecule and its ions.
Acid Base equilibrium
We now know that many acids are only partially ionised in water. The stronger the acid, the more readily it is ionised. The weaker the acid, the less it is ionised.
An equilibrium exists between the acid molecule and its constituent ions.
HA(aq) H+(aq) + A-
(aq)
The extent to which acids are ionised can be determined using a special equilibrium constant known as the acid ionisation constant, Ka. You do NOT need to remember, nor perform calculations with the acid equilibrium constant, however it may help you understand these key concepts for acid strength.
For weak monoprotic acids which undergo partial ionisation thus:
[H+] [A-]Ka = ------------
[HA]The smaller the value of Ka the weaker the acid.
Question Set 181. Explain the meaning of the following statement: “When a
weak acid dissolves in water an equilibrium is established between the acid molecule and its constituent ions”.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
2. Why is it incorrect to identify a strong acid solution, such as hydrochloric acid in water, as an equilibrium?
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 47

I can outline the historical development of ideas about acids including those of: Lavoisier, Davy and Arrhenius.
Classifying Acids
Antoine Lavoisier (1780) first defined acids as an oxide of a non-metal. He notice that non-metal oxides formed acidic solutions when reacted with water. He concluded that acids must contain oxygen.
Humphry Davy (1815) observed displacement reactions where the hydrogen in the acid was displaced by a metal during a reaction. He noted that metal oxides were basic. He concluded that acids must contain hydrogen.
Svante Arrhenius’ (1884) definition of acids was not based on direct observation, as were the ideas of Lavoisier and Davy. It was theoretical, based on the particulate behaviour of acids and bases in water. He concluded that acids produce H+ ions when dissolved in water and bases produce OH- ions when dissolved in water.
Arrhenius’ definition of acids was based on his observation that hydrogen gas was evolved at the anode when an electric current was passed through an acid. He suggested that acids contain H+ ions in solution and it was these positive ions which gave acids their characteristic properties. (Note: Free H+ ions do not exist in water but become bound by co-ordinate bonds to water molecules forming the hydronium ion (H3O+))
A substance which provides H+ ions in aqueous solutions (or a compound with hydrogen which ionises water producing hydronium ions) is termed an Arrhenius acid. The excess [H+] gives the solution its “acidic” properties.
eg. H2SO4(aq) → 2H+ + SO42-
A substance which produces hydroxide ions in aqueous solutions is called an Arrhenius base. The excess [OH-] gives the solution its “basic” properties.
eg. Ca(OH)2 → Ca2+ + 2OH-
Question Set 191. Contrast the theories of Lavoisier, Davy and Arrhenius
regarding the nature of acids.
........……………………………………………………………………………………
……......……….…………………………………………………………………………………………
…………........
HSC Chemistry The Acidic Environment P a g e | 48

……………………………………………………………………………………………
……………………………………………………………………………………………………………
2. Write a chemical equation to demonstrate an example of the behaviour of an acid according to each theory below:a) Lavoisier definition of an acid
b) Davy definition of an acid
c) Arrhenius definition of an acid
HSC Chemistry The Acidic Environment P a g e | 49

I can outline the Brönsted-Lowry theory of acids and bases.
Brönsted-Lowry Acids and Bases
Despite the fact that the Arrhenius definition is good enough to be used for Junior High School Chemistry, there are some problems with the Arrhenius definition of an acid and base.
Problem 1: ZnCO3(s) + 2H+ → Zn2 + CO2(g) + H2O(l)
Here, the zinc carbonate has neutralised the acid (producing water) and so could be classified as a base, yet the zinc carbonate does not produce hydroxide ions in aqueous solutions.
Problem 2: Na2CO3(s) → 2Na+ + CO32-
CO32- + H2O → HCO3
- + OH-
CO32- + 2H+ → H2O(l) + CO2(g)
The sodium carbonate dissolves in water to produce a solution which contains OH- ions. Solutions turn red litmus blue and neutralise acids liberating CO2 gas.
Hydroxide ions form as a result of the ionising reaction between CO32- and H2O.
Reactions involving water which lead to changes in acidity or basicity are called hydrolysis reactions. In the reactions above, the resulting solutions are said to be alkaline, due to the presence of OH- ions.
Whilst the Arrhenius definition works for many acid base reactions, it does not explain them all.
A better explanation, based on proton transfer, was independently outlined in 1923 by the Johannes Brönsted, and Thomas Lowry.
A Brönsted-Lowry acid is a proton donor.
A Brönsted-Lowry base is a proton acceptor.
An acid-base reaction involves proton transfer from an acid to a base.
So:
A species capable of donating a proton (molecule or ion) is classified as an ACID.
A species capable of accepting a proton is cl;assified as a BASE.
Acid-Base reactions involve the transfer of a proton from an acid species to a base species:
HSC Chemistry The Acidic Environment P a g e | 50

I can gather and process information from secondary sources to trace developments in understanding and describing acid/base reactions.
Eg. HCl(aq) + NH3(aq) → NH4+Cl-.
(A coordinate covalent bond forms - H+ has no electrons!)
Question Set 201. Complete the following statements:
a) A Brönsted-Lowry acid isb) A Brönsted-Lowry base isc) In an Acid-Base reaction a proton is transferred from
2. Complete the timeline below to identify the major developments in our understanding of acids and bases.
HSC Chemistry The Acidic Environment P a g e | 51

I can describe the relationship between an acid and its conjugate base and a base and its conjugate acid.
Conjugate Pairs
Conjugate pairs are species involved in the transfer of protons.
Generally:
Acid 1 + Base 2 → Conjugate Base 1 +Conjugate Acid 2
The equilibrium position of an acid base system is determined by the relative strengths of the bases involved. If the equilibrium lies to the right, acid 1 is a stronger proton donor than conjugate acid 2. (ipso facto for the bases.)
Strong bases have weak conjugate acids and vice versa.
The strength of an acid is a measure of its ability to transfer protons to water molecules.
HCl is a strong acid, almost all molecules form ions, so its conjugate base Cl- is a weak base.
CH3COOH is a weak acid, only a small percentage of the molecules form ions, so its conjugate base CH3COO- is relatively strong.
Acid → H+ + Conjugate Base
HA → H+ + A-
The strength of a base is a measure of its ability to accept protons from water molecules.
NaOH is a strong base, so its conjugate acid H2O is a weak acid.
NH3 is a weak base, only a small percentage of the molecules form ions, so its conjugate base NH4
+ is relatively strong.
Base + H+ → Conjugate Acid
A- + H+ → HA
HSC Chemistry The Acidic Environment P a g e | 52

I can identify conjugate acid/base pairs.
I can identify a range of salts which form acidic, basic or neutral solutions and explain their acidic, neutral or basic nature.
Question Set 21
1. Complete the equations below by identifying the product and one conjugate pair.
HCl → H+ +
HNO3 → H+ +
H3O+ → H+ +
HSO4- → H+ +
HF → H+ +
HCOOH → H+ +
CH3COOH → H+ +
NH4+ → H+ +
H2O → H+ +
2. Identify the conjugate pairs in the following reaction:
HCl + NH3 → NH4+ + Cl-
Salts
HSC Chemistry The Acidic Environment P a g e | 53

I can identify amphiprotic substances and construct equations to describe their behaviour in acidic and basic solutions.
Salt ions formed when weak acids or weak bases dissolve in water can be added to water to reform the acid or base. During these hydrolysis reactions, they release OH- or H+ions which can produce basic or acidic salt solutions.
Salts can be classified on the basis of their reaction with water.
Ammonium salt solutions are acidic, because
NH4+
(aq) + H2O(l) NH3(aq) + H3O+
Sodium chloride solution is neutral, because Na+ and Cl- (ions from the strong base NaOH and the strong acid HCl) do not undergo hydrolysis.
Sodium carbonate solution is basic, because the carbonate ion from the weak acid carbonic acid can hydrolyse.
CO32- + H2O(l) HCO3
- + OH-
Likewise, potassium acetate solution is basic.
CH3COO- + H2O(l) CH3COOH(aq) + OH-
If a salt is made up of two ions that hydrolyse to the same extent, the salt solution could be close to neutral, e.g. ammonium acetate NH4CH3COO.
NH4+ + H2O(l) NH3(aq) + H3O+
CH3COO- + H2O(l) CH3COOH(aq) + OH-
The resulting reaction, H3O+ + OH- 2H2O(l), results in a neutral solution.
There are some species which are capable of behaving as either a base or an acid according to the Bronsted-Lowry definition given certain circumstances. We say that a species is amphiprotic if is capable of donating or accepting a proton
eg. HCO3-, HSO4
- and H2O are good examples.
HCO3- + OH- CO3
2- + H2O(l)
HCO3- + H3O+ H2CO3 + H2O(l)
A polyprotic species is an acid which can donate more than one proton.
eg. H2SO4, H2CO3, H3PO4.
HSC Chemistry The Acidic Environment P a g e | 54

An amphoteric species is a species capable of acting as either an acid or a base. This definition is not dependent on proton transfer, so it can include Arrhenius acids and bases. This becomes clearer when we look at some oxides.
MgO(s) + 2HNO3 (aq) → 2NaNO3(aq) + H2O(l)
basic oxide
SO2(g) + 2NaOH(aq) → Na2SO3(aq) + H2O(l)
acidic oxide
Al2O3(s) + 3H2SO4 (aq) → Al2(SO4)3(aq) + 3H2O(l)
amphoteric oxide
Al2O3(s) + 2KOH(aq) → 2KAlO2(aq) + H2O(l)
amphoteric oxide
Question Set 221. Show that water is also an amphiprotic substance
2. Complete the following sentences:a) Polyprotic acids are acids which b) An example of a diprotic acid is
c) An example of a triprotic acid is
d) An acidic salt is
e) A basic salt is
f) A neutral salt is
HSC Chemistry The Acidic Environment P a g e | 55

I can choose equipment and perform a first-hand investigation to identify the pH of a range of salt solutions.
pH of Salt Solutions
Test the following salts with universal indicator and identify whether they are acidic, neutral or basic: ammonium nitrate, sodium nitrate, aluminium nitrate, magnesium nitrate, sodium chloride, sodium carbonate, sodium sulfate, sodium bicarbonate, sodium iodide.
Method:
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
Results:
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
HSC Chemistry The Acidic Environment P a g e | 56

I can identify neutralisation as a proton transfer reaction which is exothermic.
Neutralisation
Neutralisation has previously been defined as the reaction between an acid and a base to form a salt and water.
The solutions reacted to demonstrate neutralisation are usually of a strong acid, such as hydrochloric acid, and a strong base, such as sodium hydroxide.
acid + base → salt + water
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
OR
H+ + Cl- + Na+ + OH- → Na+ + Cl- + H2O(l)
The net ionic equation for reaction is:
H+ + OH- → H2O(l)
or if you used the hydronium ion instead of hydrogen ions
H3O+ + OH- → 2H2O(l)
The net ionic equation shows that neutralisation is a proton transfer reaction. A proton from the acid transfers to the hydoxide ion of the base.
All neutralisations are exothermic. When the heat of neutralisation is measured for a range of strong acids and strong bases, the amount of heat released is always approximately 57 kJ per mole of water formed.
This is the heat change for the following reaction:
H+ + OH- → H2O(l) = - 57 kJ mol-1
A study of the heat energy released for various (strong) acid-base reactions reveal values which are very close to one another, suggesting that the same type of reaction is occurring each time, ie. the production of a salt and water, or more specifically, the transfer of a proton species from a proton donor to a proton acceptor.
We will be focussing on neutralisation reactions in the next few sections.
To review:
Neutralisation reactions may regarded as a _ _ _ _ _ _ transfer reactions. They are exothermic/endothermic.
HSC Chemistry The Acidic Environment P a g e | 57

I can describe the correct technique for conducting titrations and preparation of standard solutions.
Volumetric Analysis
Volumetric analysis is an important technique in chemistry. It is used to measure the volume of a solution of known concentration needed to react with a solution of unknown concentration. The technique used is called titration.
Titration is the process by which a solution of known concentration reacts with a solution of unknown concentration until the equivalence point is reached. This point is usually identified by the use of an indicator changing colour. Measurement of volumes allows the unknown concentration to be determined (using stoichiometry).
We use the following reaction types in acid-base titration. The reactions need to proceed to completion in order that the calculations will be accurate.
1. Strong acid - strong base. In these reactions, both species are completely dissociated, so the net ionic equation will be H+ + OH- → H2O(l) The mole ratio here is 1:1. This ratio will change if we use a diprotic acid or base or a triprotic acid. It is essential to write out an equation for the reaction so that the mole ratio can be established prior to calculations. The final solution is neutral so the indicator chosen should have a colour change where the end point coincides with the equivalence point. Phenolphthalein fulfils this requirement.
2. Weak acid - strong base. Weak acids only partially ionise, so much of the acid remains in molecular form. Consider acetic acid (CH3COOH). The OH- ions from the base react directly with the molecular acid. The reaction again proceeds to completion. The equation becomes:
CH3COOH(aq) + OH- → CH3COO- + H2O(l)
Again this is a 1:1 ratio, but not all weak acids are monoprotic (eg. H2CO3), so once again write out an equation prior to solving the problem. In this case, the equilibrium mixture is basic at the equivalence point (CH3COO- ions react with water molecules to produce CH3COOH molecules and OH- ions). Once again phenolphthalein is suitable to use as an indicator.
3. Strong acid - weak base. This time much of the base remains in molecular form. The reverse situation from that above occurs. The H+ from the acid reacts directly with the unionised base. The reaction again goes to completion. One example is:
NH3(aq) + H+ → NH4+
HSC Chemistry The Acidic Environment P a g e | 58

The ammonium ion reacts with water to liberate hydronium ions. As a result the equilibrium mixture is slightly acidic at the equivalence point. Methyl orange is a suitable indicator in this case
4. Weak acid – weak base.We cannot titrate a weak acid against a weak base as it will not proceed through to completion. In these titrations the change in pH is so gradual that it is almost impossible to monitor with an indicator, no rapid pH transition occurs at the equivalence point. For such titrations pH meters are more suitable.
Question Set 231. Define the following terms:
a) Volumetric analysisb) Titrationc) Equivalence Point
2. If we were to titrate a solution of acetic acid against sodium hydroxide, what equation would we write? What type of reaction would this be? Which indicator would you choose for this reaction?…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
…………………………………………………………………………………………………
3.
HSC Chemistry The Acidic Environment P a g e | 59

I can describe the correct technique for conducting titrations and preparation of standard solutions.
Preparing a Standard Solution
An important component of a titration is the solution of known concentration. This is called the standard solution. It is prepared by adding a known weight of reagent in a definite volume of solution.
Prior to preparing a standard solution a primary standard must be selected. Not all substances are suitable to be used as standards. NaOH is hygroscopic (absorbs water from the surrounding air) and thus its weight may change. However, these may be later standardised by reacting them with other known solutions.
Prepare a standard solution of sodium hydroxide in a volumetric flask. Describe the method you used, below.
……………………………………………………………………………………
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
Characteristics of a primary standard:
Pure and free of moisture. Stable and unaffected by air during weighing. Readily soluble in distilled water. High molar weight to minimise weighing errors. Should react instantly and go to completion.
Some reagents which are used as primary standards in acid-base titration include benzoic acid, oxalic acid-2-water, potassium hydrogen phthalate, sodium
HSC Chemistry The Acidic Environment P a g e | 60

carbonate, sodium hydrogen carbonate and borax.
Question Set 241. Why is NaOH not chosen as a primary standard?
…………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
2. Name three reagents which are used as primary standards in acid-base titrations.………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………
3. When preparing our standard solution what is the correct procedure for each of the following pieces of apparatus?
Pipette
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
Burette
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
Conical flask
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
4. Why are each of these procedures important?
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
………………………………………………………………………………………………………….
HSC Chemistry The Acidic Environment P a g e | 61

I can perform a first-hand investigation to determine the concentration of a domestic acidic substance using computer-based technologies.
Identifying End Points of Titrations
Acid - base reactions are usually colourless and this makes it difficult to locate the equivalence point.
The equivalence point is the point during a titration when the quantities of the two reacting species are just sufficient to allow a complete reaction with no excess of either species.
As a result we need some device to help us determine the equivalence point. This may be use of an indicator, pH meter or conductivity meter. In practice we will generally use an indicator.
Selection of the correct indicator is important. It needs to be matched to the specific type of neutralisation reaction. Phenolphthalein and methyl orange are commonly used. The colour change range of the indicator must coincide with the range of rapid pH change at the equivalence point.
Select one of the following websites for titration simulations:
http://www.oup.com/uk/orc/bin/9780199277896/01student/activities/acidBaseLayoutf.swf
http://users.skynet.be/eddy/titratie.swf
Question Set 251. Draw the shapes of the pH change graphs for the following reactions:
a) Strong acid - strong base.
HSC Chemistry The Acidic Environment P a g e | 62

b) Strong base – weak acid
c) Strong acid - weak base
HSC Chemistry The Acidic Environment P a g e | 63

I can perform a first-hand investigation and solve problems using titrations and including the preparation of standard solutions, and use available evidence to qualitatively and quantitatively describe the reaction between selected acids and bases.
Titrations – Exploring Indicators
Method:
1. Set up the burette in the burette clamp after first rinsing the burette with the solution you intend to place in it (sodium hydroxide in this case). Note: the sodium hydroxide is placed in the burette in this experiment to highlight the colour changes. As a rule it is not wise to regularly put this solution into the burette as it can damage the burette, particularly the tap. Always rinse the burette out after use.)
2. Fill the burette with sodium hydroxide solution and bring the level to the zero mark. Make sure that the tap allows the solution to flow freely.
3. Use a pipette to add 25mL of hydrochloric acid solution to a conical flask.4. Add a few drops of phenolphthalein solution to the hydrochloric acid.5. Sit the flask on a white tile, or piece of white paper. 6. Run the sodium hydroxide solution into the flask until the solution gains a
permanent pink tinge. Record the volume of sodium hydroxide used.7. Repeat steps 2 to 6 using methyl red indicator instead of phenolphthalein
solution.8. Repeat steps 2 to 6 using universal indicator instead of phenolphthalein.
Results:
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
HSC Chemistry The Acidic Environment P a g e | 64

Titrations – Finding the Concentration of an Unknown SolutionMethod:
Prepare a standard solution of sodium hydroxide as follows.
1. Weigh out 2.0 g of sodium hydroxide into a small, clean, dry beaker. 2. Add some distilled water to dissolve the sodium hydroxide. Swirl the
solution but do not allow any to splash out of the beaker.3. Transfer the liquid by decanting into a 250 mL volumetric flask.4. Continue adding more distilled water to the beaker and transferring the
contents to the flask until all the sodium hydroxide has dissolved. 5. Use the wash bottle to rinse the contents of the beaker into the
volumetric flask.6. As the dissolution of sodium hydroxide is exothermic allow the solution to
cool to room temperature before filling the flask up to the mark with distilled water.
7. Swirl the flask, place on the stopper and invert the flask several times to ensure the contents are thoroughly mixed.
Standardising the sodium hydroxide
8. Measure out 1.0 g of tartaric acid into a conical flask.9. Add sufficient distilled water to ensure all the acid has dissolved.10.Add 5 drops of phenolphthalein solution to the flask.11.Set up the burette in the burette clamp after first rinsing the burette with
the solution you intend to place in it (sodium hydroxide in this case). 12.Fill the burette with sodium hydroxide solution and bring the level to the
zero mark. Make sure that the tap allows the solution to flow freely.13.Sit the conical flask on a white tile, or piece of white paper. 14.Run the sodium hydroxide solution into the flask until the solution gains a
permanent pink tinge. Record the volume of sodium hydroxide used.15.Repeat the procedure twice.16.Calculate the concentration of the standard sodium hydroxide solution.
Finding the acid concentration in vinegar
17.Use a pipette to transfer 5 mL of vinegar to a conical flask.18.Add 5 drops of phenolphthalein solution to the flask.19.Refill the burette with sodium hydroxide solution and bring the level to
the zero mark. 20.Sit the conical flask on a white tile, or piece of white paper. 21.Run the sodium hydroxide solution into the flask until the solution gains a
permanent pink tinge. Record the volume of sodium hydroxide used.22.Repeat the procedure twice.23.Calculate the concentration of the acid in vinegar.
Results:
HSC Chemistry The Acidic Environment P a g e | 65

………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
HSC Chemistry The Acidic Environment P a g e | 66

I can qualitatively describe the effect of buffers with reference to a specific example in a natural system.
Buffer Solutions
A buffer controls the level of acidity or basicity in a solution. If an acid or a base is added to a buffer solution, there is hardly any change in pH. A buffer solution resists changes to its pH.
A buffer solution is usually a mixture of a weak acid and its conjugate base, such as hydrogen carbonate ions, HCO3
-, and carbonate ions, CO32-.
If an acid is added to the buffer, the hydrogen ions are removed by
H+ + HCO3- H2CO3(aq)
If a base is added to the buffer, hydroxide ions are removed by
OH- + HCO3- H2O(l) + CO3
2-
The net effect is that the pH of the solution containing buffer changes only slightly.
Hydrogen carbonate ions are important in maintaining the pH of human blood at about 7.4.
There are a number of natural systems which use buffers solutions. Identify one and explain how it works.
……………………………………………………………………………………
……………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 67

I can perform a first-hand investigation to determine the concentration of a domestic acidic substance using computer-based technologies.
Comparing Concentrations using Different Methods
Use a computer-based technology to find the concentration of the vinegar (a domestic acidic substance) you used in an earlier titration. Vinegar contains acetic acid which can be titrated against standardised NaOH(aq) using a pH probe attached to a data logger. The data recorded can be used to draw a graph. The endpoint is where the pH changes most rapidly. Compare the two values and account for any differences.
Method:
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
Results:
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
HSC Chemistry The Acidic Environment P a g e | 68

I can analyse information from secondary sources to assess the use of neutralisation reactions as a safety measure or to minimise damage in accidents or chemical spills.
Chemical Spills and Neutralisation
There are a number of situations, both in a laboratory and various workplaces, where an understanding of the proper use of neutralisation reactions is useful both as a safety measure and to help minimise damage in accidents or chemical spills.
A substance containing an amphiprotic ion, such as the hydrogen carbonate ion in NaHCO3, is quite suitable for neutralising chemical spills.
If the chemical spill contains an acid; H+ + HCO3- H2O(l) + CO2(g)
If the spill contains a base; HCO3- + OH- CO3
2- + H2O(l)
Thus, NaHCO3 is suitable for neutralising chemical spills of acids, bases and unknown acidity or basicity.
Question Set 261. For the three scenarios below suggest a way in which you
could use your knowledge of neutralisation reactions to assist those involved.a) A burette filled with HCl loses its tap and the contents
flow out over the bench and onto a student.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
b) A tanker carrying concentrated sodium hydroxide overturns on a bridge and spills its contents over the road and into the waterway below.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 69

c) A worker inadvertently places their arm in a small puddle of a colourless liquid which is sitting on a bench. It irritates the skin but it is not known whether the liquid was acidic or basic.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 70

I can describe the differences between the alkanol and alkanoic acid functional groups in carbon compounds.
Alkanols and Alkanoic Acids
Recall that a functional group is a single atom or group of atoms that react in a characteristic way in different carbon compounds.
The hydroxy functional group,-OH, in alkanols provides their characteristic properties, such as high melting points and boiling points.
The carboxylic acid functional group, -COOH, in alkanoic acids can lose a hydrogen ion and behave as a weak acid.
The diagram right shows a common way to illustrate the characteristic structure of the members of a homologous series, in this case, carboxylic (or alkanoic) acids. The R group represents a carbon chain of unspecified length (for our purposes, between 0 and 7 carbons).
This is a skeletal formula as the carbon atom within the functional group (COOH) is not labelled with a C. In this type of formula the junction of bonds with no letter indicating the atom present is assumed to be a carbon and hydrogens bonded directly to carbon atoms are not shown.
However, whilst you may see this short-hand version in different text books or web sites, it is best in HSC Chemistry Exams to always draw full structural formulae.
Question Set 271. Draw the structural formula for ethanol and methanoic
acid in the space below. Circle the functional group for each compound.
HSC Chemistry The Acidic Environment P a g e | 71

I can identify esterification as the reaction between an acid and an alkanol and describe, using equations, examples of esterification.
I can describe the purpose of using acid in esterification for catalysis.
Esterification
What is an ester?An ester is an organic compound formed when an alkanol reacts with an alkanoic acid. The product, an ester, has the IUPAC form alkyl alkanoate. This is a combination produced from the reacting species.
The reaction of alcohols with carboxylic acids requires the presence of H2SO4(aq) catalyst to produce an ester.
eg. methanol + ethanoic acid methyl ethanoate + water
Hydrolysis of esters Under the influence of bases eg. NaOH, esters are hydrolysed to alcohols and alkanoate ions. The alkanoic acids may be obtained from the reaction by acidification with mineral acids, e.g. HCl.
Acid catalysis
Esterification is catalysed by the addition of a small amount of acid. Esterification is called a condensation reaction because a water molecule condenses out (recall condensation polymerisation).
Only a few drops of concentrated acid needs to be added to a mixture of alkanol and alkanoic acid to catalyse the reaction.
If concentrated sulfuric acid is added in large amounts, say 5% to 10% of the reaction volume, it can have a significant effect on the position of equilibrium. Concentrated sulfuric acid is a dehydrating agent, that is, it has a strong affinity for water. If a significant amount of sulfuric acid is present, it will shift the equilibrium position to the right by absorbing water.
alcohol + acid ester + water
This will increases the yield of ester. However, using large amounts of sulfuric acid is wasteful, uneconomic and complicates the separation of ester from the reaction mixture.
Question Set 281. Draw the structural formula for the product formed from
the reaction between ethanol and methanoic acid. Name the product. Identify any catalyst used.
HSC Chemistry The Acidic Environment P a g e | 72

I can identify the IUPAC nomenclature for describing the esters produced by reactions of straight-chained alkanoic acids from C1 to C8 and straight-chained alkanols from C1 to C8.
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
2. Complete the table showing the name for each of the following esterifications. On a separate piece of paper, draw the structural formulae for 10 different esters.
Methanoic acid
Ethanoic acid
Propanoic acid
Butanoic acid
Pentanoic acid
Hexanoic acid
Heptanoic acid
Octanoic acid
methanol
ethanol
1-propanol
1-butanol
1-pentanol
1-hexanol
1-heptanol
1-octanol
HSC Chemistry The Acidic Environment P a g e | 73

3. Write a balanced equation for two different esterification reactions from the table above. Name the products.
4. Why do we use concentrated sulfuric acid as our catalyst in this reaction?
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
HSC Chemistry The Acidic Environment P a g e | 74

I can explain the difference in melting point and boiling point caused by straight-chained alkanoic acid and straight-chained primary alkanol structures.
Physical properties of alkanols and alkanoic acids
The high melting points and boiling points in alkanols is due to hydrogen bonding between the O in one molecule and the H of an-OH in a nearby molecule, as shown below.
The ability of the-COOH group to be involved in two hydrogen bonds gives an alkanoic acid an even higher boiling point than that of a similar sized alkanol. Two hydrogen bonds can occur between a pair of alkanoic acid molecules.
Size is also important as larger molecules require additional energy for motion. So we would expect an increase in metling point and boiling point as we increase molecular weight (ie, increase the length of the chain or number of carbons).
Question Set 291. Account for the difference in the melting and boiling points
between a straight-chained primary alcohol and the corresponding straight-chained alkanoic acid, using a specific named example.
HSC Chemistry The Acidic Environment P a g e | 75

HSC Chemistry The Acidic Environment P a g e | 76

I can identify data, plan, select equipment and perform a first-hand investigation to prepare an ester using reflux.
Esterification using Reflux
Method:
Reactants chosen:
Quanitities used:
Catalyst:
Draw a diagram of your apparatus
Results:
……………………………………………………………………………………………………………
……………………………………………………………………………………………………………
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
HSC Chemistry The Acidic Environment P a g e | 77

RefluxingEsterification requires heat for the reaction to reach equilibrium quickly, ie within an hour rather than after many days.
When the reaction mixture is heated, volatile components, such as the reactant alcohol and the product ester, could escape. This problem is overcome by refluxing the reaction mixture.
A condenser is placed on top of the reaction vessel so that any volatile components pass into the condenser. The condenser can be water or air-cooled and causes the volatile components to condense back to liquid and fall back into the reaction mixture.
Refluxing also improves the safety of the operation, as the volatile components are flammable.
Question Set 301. What does the technique of refluxing involve?
………………………………………………………………………………………
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
……………………………………………………………………………………………………………
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
2. Why is refluxing needed for esterification?
……………………………………………………………………………………………………………
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
……………………………………………………………………………………………………………
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
HSC Chemistry The Acidic Environment P a g e | 78

I can outline some examples of the occurrence, production and uses of esters.
I can process information from secondary sources to identify and describe the uses of esters as flavous and perfumes in processed foods and cosmetics.
Esters
Esters occur naturally as flavouring agents and scents in plants and fruits. They are also found in animal fats and plant oils.
Synthetic esters are used in the food industry as well as for the production of cosmetics. They have a distinct aroma and some of the combinations of reactants produce esters whose aroma mimics a smell with which we are familiar. This is of great benefit to the food and cosmetic industries.
The production of esters involves either the extraction of the desired ester from its natural sources, or manufacture of the ester in an industrial environment. Often synthetic manufacture is less expensive than extraction.
Esters are used for:
Flavouring agents and scents in foods. Solvents and thinners. Medications. Plasticisers.
Octyl ethanoate is an example of a common ester. Octyl ethanoate is associated with orange flavour, as it is the main ester present in oranges. Once produced, octyl ethanoate is used as a flavouring agent in foods, such as orange-flavoured confectionary.
Question Set 311. Write an equation to represent the synthetic production of
octyl ethanoate. Include any catalysts.
2. Draw the structural formula for octyl ethanoate.
HSC Chemistry The Acidic Environment P a g e | 79

3. Identify and describe two additional uses of esters as either flavours or perfumes in processed foods and cosmetics. You should include the name and structural formula of your chosen ester and how they are obtained as well as their specific use.
……………………………………………………………………………………………………………
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
……………………………………………………………………………………………………………
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
……………………………………………………………………………………………………………
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
……………………………………………………………………………………………………………
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
……………………………………………………………………………………………………………
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
……………………………………………………………………………………………………………
………………………………………………………………………………………………………….
………………………………………………………………………………………………………….
HSC Chemistry The Acidic Environment P a g e | 80