69110174 Tablet Flow Chart
-
Upload
ekaekaekaa -
Category
Documents
-
view
11 -
download
0
Transcript of 69110174 Tablet Flow Chart
-
TORRENT PHARMACEUTICALS LTD. INDRAD
FORMULATION PLANT:
1. MANUFACTURING ACTIVITIES
Manufacturing activities proposed in the project include various processes as a part of
manufacturing Tablets, Hard-shell Capsules and Soft Shell Capsules, Injections
Ampoules and Vials, bulk drugs. The activities shall also include operation of various
utilities. The manufacturing process is described in details in following sections. The list
of products (existing as well as proposed) and their capacity is given in Table 1.2.
1.1 MANUFACTURING PROCESS, FLOW DIAGRAM & MASS BALANCE
Product wise detailed description of manufacturing process, process flow chart, chemical
reactions involved and mass balance diagram etc. are as follows.
1.1.1 TABLETS 1.1.1.1 MANUFACTURING PROCESS
1. Shift and pulverize all raw materials to be used in the product, which are
dispensed after weighing.
2. Mix the active material with diluents using mixer. If necessary do geometrical
dilution or triturating.
3. Prepare binder solution or paste using binder in appropriate solvent as per
formulation requirement.
4. Bind the mixed powder using binder in mixer.
5. Wet granulate the mix mass using communating mill fitted with perforated screen.
6. Dry the wet granules in fluid bed dryer controlling temperature of inlet air.
7. Shift the dried granules through appropriate sieve and pulverize the oversize
granules in communating mill.
8. Lubricate the shifted granules with freshly shifted lubricants using the mixer.
9. Compress the lubricated granules on suitable rotary compression machine fitted
with appropriate die-punch set.
10. If required film coat the tablets using Autocoating machine controlling spray rate
and drying temperature.
-
TORRENT PHARMACEUTICALS LTD. INDRAD
11. If required sugar coat the tablets using conventional sugar coating pan controlling
average weight of the tablet.
12. Inspect the ready tablets on inspection belt.
13. Prepack the tablets suitably in blister, strip or bottles followed by post packing in
cartons or boxes.
14. Shrinks wrap the boxes and pack them finally in corrugated boxes suitable for
dispatches.
-
TORRENT PHARMACEUTICALS LTD. INDRAD
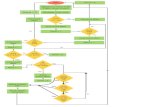
1.1.1.2 INCOMING MATERIAL FLOW CHART
Raw material Packaging material
Return to supplier/destructio
Q.C. Testing
Sampling
Verification
Receipt
Under test
For manufacturing
Approved Rejected
-
TORRENT PHARMACEUTICALS LTD. INDRAD
1.1.1.3 PROCESS FLOW CHART
Dispensing of raw material
Approval Prod. & QA
Dex, VAC
MRN Checking
Quarantine
Pulverization and sifting
Mixing Compaction
I.P.C. 1 AMP Load
Binder Preparation & Addition
Wet Mixing I.P.C 1.Amp. Load
Size Reduction
Wet Granulation
Drying
Sifting
Lubrication
Dex, VAC, Water
Steam, Air, Dex, VAC, Water
Steam, Air
I.P.C. 1. Moisture content 2. Size reduction 3. Pour volume 4. Tapped volume 5. Content uniformity
I.P.C. 1. Moisture content
Quarantine
Quarantine
Q.C.
-
TORRENT PHARMACEUTICALS LTD. INDRAD
Q.C.
CompressionI.P.C.
1. Av. Wt. 2. Diameter 3. Thickness 4. Disintegration 5. Dissolution 6. Appearance
Coating
I.P.C. 1. Av. Wt. 2. Wt. variation 3. Thickness 4. Hardness 5. Disintegration 6. Friability loss 7. Dissolution 8. Appearance
Dex, VacInspection
Quarantine
Q.A. Approval
Quarantine
Transfer to Packing
-
TORRENT PHARMACEUTICALS LTD. INDRAD
1.2.1 CAPASULES
1.2.1.1 SOFT SHELL CAPSULE
1.2.1.1.1 MANUFCTURING PROCESS
1. Dissolve the medicament in appropriate solvent by continuous stirring.
2. Filter the solution through 150-mesh screen.
3. Soak the Gelatin and melt in a Gelatin melter with controlled temperature.
Process the Gelatine to form air free, semisolid, pourable paste.
4. Encapsulation the medicament on rotary encapsulator with the Gelatine paste
using appropriate set of die roll or segment. Control the ribbon thickness and
fitted weight of capsules.
5. Degrease the capsules and dry them in the room having controlled humidity and
temperature. Degrease the waste ribbon after chopping and recycle it.
6. Inspect the capsule on inspection table.
7. Prepack the capsule in blister, strip or bottles followed by post packing the printed
cartons or boxes.
8. Shrink wrap the packs and pack finally in corrugated boxes suitably for
dispatches.
-
TORRENT PHARMACEUTICALS LTD. INDRAD
1.2.1.1.2 PROCESS FLOW CHART
Steam, Water, Air, VAC
Soft Gelatine
Gelatine Preparation
Medicament preparation
Encapsulation
Q.C.
I.P.C. 1. Fill Wt. 2. Ribbon Thickness
Semi Drying
Drying
De- Oiling
Inspection
Quarantine
Packing
Bond Room
Air
Dehumidification
Q.C.
Q.C.
-
TORRENT PHARMACEUTICALS LTD. INDRAD
1.2.2.2 HARD SHELL CAPSULE
1.2.2.2.1 MANUFCTURING PROCESS
1. Dissolve the medicament in appropriate solvent by continuous stirring.
2. Filter the solution through 150 mesh screen.
3. Soak the Gelatine and melt in a Gelatine melter with controlled temperature.
Process the Gelatine to form air free, semisolid, pourable paste.
4. Encapsulation the medicament on rotary encapsulator with the Gelatine paste
using appropriate set of dieroll or segment. Control the ribbon thickness and fitted
weight of capsules.
5. Degrease the capsules and dry them in the room temperature and temperature.
Ribbon after chopping and recycle it.
6. Inspect the capsule on inspection table.
7. Prepack the capsule in blister, strip or bottles followed by post packing the printed
cartons or boxes.
8. Shrink wrap the packs and pack finally in corrugated boxes suitably for
dispatches.
-
TORRENT PHARMACEUTICALS LTD. INDRAD
1.2.2.2.2 PROCESS FLOW CHART
Dispensing of raw material
Milling & Sieving
Blending
I.P.C 1. Fill Wt. 2. D.T. Encapsulation
Inspection & Polishing
Q.C.
Quarantine
Packing
Bond Room
Hard Gelatine
Air, VAC
Q.C
Q.C
-
TORRENT PHARMACEUTICALS LTD. INDRAD
1.3.1 INJECTION
1.3.1.1 MANUFCTURING PROCESS
1. Dissolve the medicament in appropriate solvent. Take necessary measure to
reduce the biomass. Control pH of the solution.
2. Subject the bulk medicament to sterile filtration or any other suitable method of
sterilization.
3. Wash the Ampoules, Vials or caps suitably and subject them to sterilized cycle.
4. Carryout accepting filling and sealing of bulk medicament in vials or ampouls
using appropriate injection filling machine. Control the fill volume and aseptic
condition.
5. Subject the filled ampoules to leak test.
6. Carryout visual inspection of individual unit against light.
7. Label the ampoules/ Vials suitably on labeling machine.
8. Prepack the ampoules/Vials in blister or cartons followed by packing in boxes.
9. Pack finally in corrugated boxes suitably for dispatches.
-
TORRENT PHARMACEUTICALS LTD. INDRAD
1.3.1.2 PROCESS FLOW CHART
Vials/Ampoules Dispensing of raw materials
Snap off Caps
Washing Washing Bulk manufacturing
Sterilization
Aseptic Filtration
Aseptic Filtration & Sealing
Leak test/SterilizationQ.C. 1.Bulk analysis 2.Bulk sterility
I.P.C. 1.Fill volume 2.Seal integrity
Sterilization I.P.C. 1.pH 2.Crystal Size 3.Turbidity 4.Bulk analysis
Visual Inspection
Quality Assurance
Cold Storage (Incase of vials)
Packing
Q.A. Retained Samples
Bond Room
Dispatch
-
TORRENT PHARMACEUTICALS LTD. INDRAD
1.4.1 PACKAGING OF TABLETS, CAPSULES AND INJECTION
1.4.1.1 PACKING PROCESS
1. Packaging material issued from packaging material store near packaging department.
2. Released batch issued from manufacturing department (tablet/Capsule/Injection).
3. Tablet/Capsule packed in blister/strip packing using two type of film (Printed
foil/Plain Foil/ PVC).
4. Issued packaging material from P.M. store subject to overprint B.No., Mfg Exp.,
Retail Price required matter from Export Department.
5. Released batch issued from manufacturing department subject to check it identity and
weight.
6. Packing activity divided in two, primary packing and secondary packing.
7. After primary packing meant for online checking and then secondary packing on belt.
8. Packed goods on belt for counter checking & then secondary packing on belt.
9. After checking weight on shrink pack used for final packing.
10. Packed goods, strapped and transfer to bonded store by internal transfer slip.
-
TORRENT PHARMACEUTICALS LTD. INDRAD
1.4.1.2 PACKING FLOW CHART
Packaging Flow Chart
P.M. Issue Released Batch Issue
Printed/Secondary P.M. Coding Issue
Weight Identity
Checking By Supervisor
Primary Packing
Blister/ Strip/ Ampoule/ Vial/ Labeling
On Line Checking
Secondary Packing
Counter Checking By Weight
Final Packing