534: Standardized mouse embryo cardiac evaluation using 17.6T MRI
1
Click here to load reader
Transcript of 534: Standardized mouse embryo cardiac evaluation using 17.6T MRI

ajog.org Poster Session III
534
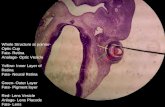
Standardized mouse embryo cardiac evaluation using17.6T MRIRinat Gabbay-Benziv1, Fang Wang1, Amnon Bar-Shir2,Ozhan Turan1, Chris Harman1, E. Reece1, Peixin Yang1,Sifa Turan11University of Maryland School of Medicine, Obstetrics, Gynecology andReproductive Sciences, Baltimore, MD, 2Johns Hopkins School of Medicine,Department of Radiology, Baltimore, MDOBJECTIVE: The mouse embryo is ideal for cardiac developmentresearch, with high correspondence to human heart and ease of genemanipulation, but current imaging induces artefacts and lacksstandardization. We aimed to standardize and optimize heart eval-uation and thereby identify heart structure using 17.6 Tesla MRI.STUDY DESIGN: After excision and preservation in 4% PFA, 17.5 daymouse embryos were imaged using 17.6 T vertical MRI (Brucker,Billerica, MA), final voxel resolution 51mmX53mmX78 mm. Imagingtime was 8h/4-embryo set, with 3D generation using Image J freesoftware (NIH). Simple anatomic landmarks were used sequentiallyto select the 4- chamber view, L and R outflow tracts. Great artery(GA) arrangement was defined by the aorta and pulmonary arteryforming a V. Two researchers, experienced/not experienced in car-diac exam, independently used a novel standardized technique on allembryos on 3 different timed occasions. Reproducibility in perfor-mance of the technique and inter-observer results were comparedwith kappa scores.RESULTS: 48 examinations of 16 different embryos (4 normal,12 Type 1 diabetics) were examined by each researcher. The 4-chamber view was obtained in all exams by both researchers. GAarrangements were visualized in 46 (96%) by non-experienced and100% by experienced researcher. Inter-observer agreement andreproducibility of the images was 0.78, SE 0.064 (95% CI 0.653-0.905) and 0.73, SE 0.078 (95% CI 0.578-0.882), respectively.Normal structure (11 embryos), VSD, anomalous GA, tetralogy ofFallot were diagnosed in 2, 2 and 1 embryos respectively. Both ob-servers identified all cardiac lesions each time. Examinations lasted<10 min per embryo regardless of operator.CONCLUSION: Standardized MRI-derived 3D imaging enables repro-ducible detailed segmental cardiac evaluation of normal andabnormal mouse embryo hearts, independent of operator experience.This technique can unify interpretation of murine-model trans-lational research in congenital heart disease.Normal embryo heart extrapolated from 3D imaging. 1- fourchamber view; 2- left ventricular outlet tract; 3- right ventricularoutlet tract; 4- great arteries arrangement. RV- right ventricle; LV-left ventricle; LA- left atria; RA- right atria; PV- pulmonary vein; TV-tricuspid valve; MV- mitral valve; Dao- decending aorta; AV- aorticvalve; SVC- superior vena cava; Ao- aorta; PA- pulmonary artery.
Supplem
17 embryonic days mouse embryo
535
Mechanism of maternal magnesium sulphate (Mg)neuroprotection may be mediated via placental ACTH andfetal brain leukemia inhibitory factor receptor (LIF-R)modulationRon Beloosesky1, Yuval Ginsberg1, Nizar Khatib1, Michael Ross2,Mordechai Hallak3, Hila Sharabi3, Zeev Weiner11Rambam medical center, Ob-Gyn, Ranana, Israel, 2Harbor UCLA MedicalCenter, Torrance, CA, 3Hillel Yaffe Medical Center, Ob-Gyn, Hadera, IsraelOBJECTIVE: Maternal Mg may protect the preterm fetus frominflammation-associated brain injury. Leukemia inhibitory factor(LIF) is a pleiotropic cytokine essential for central nervous systemdevelopment. Maternal LIF stimulates placental ACTH secretion,which subsequently induces fetal LIF secretion from nRBCs, ulti-mately promoting fetal brain development. Thus, transmission ofmaternal LIF signal to the fetus is mediated by placental ACTH. Wehypothesized that maternal infection/inflammation may influenceplacental ACTH/fetal brain LIF signal transduction pathway andthese effects may be prevented with Mg.STUDY DESIGN: Pregnant rats at day 18 (n¼6) received injections ofi.p. lipopolysaccharide (LPS) or saline (SAL) at time 0 and ran-domized to receive s.c. Mg or SAL for 2 hours prior to and followingLPS or SAL injections. At 4 hours after LPS administration, damswere sacrificed and fetal brain and placenta were collected fordetermination of ACTH, LIF and LIF-R protein levels in each group(LPS/SAL, LPS/ Mg, SAL/SAL, SAL/Mg). Relative band density wasdetermined after normalization to b-actin levels.RESULTS: LPS/SAL significantly increased ACTH, LIF and LIF-Rprotein levels in the placenta compared to SAL/SAL group(0.89�0.04 vs 0.12�0.015 u, 1.0�0.17 vs 0.12+0.02 u , 0.46�0.07vs 0.1�0.08 u, respectively, p<0.05). Mg treatment to LPS dams(LPS/Mg) significantly decreased placental ACTH and LIF-Rcompared to LPS dams (0.27�0.07 vs 0.89�0.04 u, 0.25�0.05 vsent to JANUARY 2015 American Journal of Obstetrics & Gynecology S267