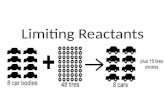
50 Days Until the Harvard Westlake Final Catalyst: 1.I increase the concentration of reactants, will...
-
Upload
hubert-owens -
Category
Documents
-
view
218 -
download
0
Transcript of 50 Days Until the Harvard Westlake Final Catalyst: 1.I increase the concentration of reactants, will...

50 Days Until the Harvard Westlake Final
Catalyst:1.I increase the concentration of reactants, will
the rate of my reaction increase or decrease? Why?
2.I decrease the temperaturewill the rate increase ordecrease? Why?
3. Define rate of reaction.End

Reading Debrief• What is decomposition?• Why don’t McDonalds fries or burgers decompose?• How can we slow the rate at which something
decomposes?• Should we be adding chemicals to our foods in order
to preserve them?• What other questions did you have during this
reading?

Post-Lab Analysis

LECTURE 8.2 – CATALYSTS, ACTIVATION ENERGY, AND REACTION COORDINATES

Elephant Toothpaste


Today’s Learning Targets• LT 8.4 – I can explain what the activation energy of a
reaction is. I can identify it on a reaction coordinate and explain how it influences the rate of a reaction.
• LT 8.5 – I can define what a catalyst is and explain how it is related to the rate of chemical reaction. I can explain how a catalyst is related to a reactions activation energy.

Today’s Focus Question
• How could we speed up the rate at which McDonald’s French Fries decompose?

What are reaction coordinates?

I. Reaction Coordinate• We can graph the energy that is associated with
a reaction using a reaction coordinate.• This can indicate exothermic and endothermic
reactions by highlighting the change in energy.
Reactants
Products

II. Activation Energy
• Reactions require a certain amount of energy in order to proceed.
• Activation Energy – The amount of energy required to get the reaction to go to completion.


Reactants
Products
Activation Energy

Quick Check• Which diagram has a lower activation energy?

What are catalysts?

I. Catalyst• A catalyst is substance that speeds up the rate of
reaction by lowering the activation energy.• Speeds up the reaction without using up the catalyst.
Ea with catalyst
Ea without catalyst


II. Catalysts and Activation Energy
• The energy difference remains exactly the same. The only change is that less energy is required for the reaction to proceed.

Quick Check• Which reaction diagram shows the effect of
using the appropriate catalyst in a chemical reaction?

Summarize
Use the following words:• Accelerate• Catalyst• Activation Energy• Reaction Coordinate

Catalyst Lab Introduction• A catalyst is anything that speeds up the rate
of a chemical reaction.• Today we will be examining the impact of
catalysts on rates of H2O2 decomposition.

Catalyst Lab
• Re-read through the lab handout.• Compare the lab instructions to the lab
checklist.

Catalyst Foundations
• Catalyst Foundations and Lab Guide• Create reaction coordinate graphs and analyze
data.

Draw Something• Let’s do some vocab
review!• Draw the picture that
best represents these terms:– Rate– Activation Energy– Catalyst– Reaction Coordinate– Concentration– Collisions

Expected Outcomes• You will complete the lab as one
group.• Roles of Group Members:1. Yeast Trials
-Don’t pipet yeast, use graduated cylinder
2. Carrot Trial 3. Tomato Trials (3 mL)
-Don’t pipet tomato, use graduated cylinder
4. Filmer/Note Taker• At the end of the lab complete the
group assessment questions 1-3

Lab Work Time

Enrichment
• Too easy? Learning Logs all 4’s?• Try out some enrichment
– Practice with 2011 ACS Chemistry Olympiad Tests– Practice with collision theory:
8.2.AP.CollisionTheory– Practice with reaction rates:
8.2.AP.ReactionRates

Lab Debrief
• Answer all of the post-lab questions.

Exit Ticket
1. Draw a general reaction coordinate. Label reactants, products, and activation energy
2. On the reaction coordinate that you drew, draw a dashed line to represent how a catalyst would impact the coordinate.


Closing Time• Lab Report Due Feb. 27th!