13.2 The Solution Process Factors Affecting the Rate of Dissolution Factors Affecting the Rate of...
-
Upload
arthur-mccarthy -
Category
Documents
-
view
221 -
download
4
Transcript of 13.2 The Solution Process Factors Affecting the Rate of Dissolution Factors Affecting the Rate of...

13.2 The Solution Process
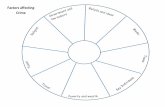
Factors Affecting the Rate of Dissolution1. Increasing the surface area2. Agitating the solution3. Heating the solventSolubility:
There is a limit to the amount of solute that is dissolved by a solvent.Saturated solutions: a solution that
contains the maximum amount of dissolved solute

• Unsaturated Solution: a solution that contains less solute than a saturated solution under the existing conditions.
• Supersaturated Solutions: a solution that contains more dissolved solute than a saturated solution under the same conditions
• The solubility of a substance is the amount of that substance required to form a saturated solution with a specific amount of solvent at a specific temperature.
Solute-Solvent Interactions:• “like dissolves like” is a good example for
determining if substances will dissolve one another.

• Ionic compounds:• The polarity of water causes the
charged ends to attract the ions of the ionic compound, and surrounds them so they separate from the other ionic molecules.
• This process with water is called hydration.
• Nonpolar Solvents:• Ionic compounds are not usually
soluble in nonpolar solvents.• Liquid solutes and solvents that are
not soluble in each other are called immiscible.
• Liquids that dissolve freely in one another are called miscible.

• Pressure and solubility:• Pressure has very little effect on the
solubility of liquids• However, an increase in pressure
increases gas solubility in liquids (CO2 in coke)
• Henry’s Law:• The solubility of a gas in a liquid is
directly proportional to the partial pressure of that gas on the surface of the liquid.

• Temperature and Solubility:• Increasing temp usually decreases
gas solubility.• Increasing temp usually increases
solid’s solubility.• Heat of Solution:• The net amount of heat energy
absorbed or released when a specific amount of solute dissolves in a solvent is the heat of solution.